Dental Hypersensitivity
Received: 06-Nov-2020 / Accepted Date: 20-Nov-2020 / Published Date: 27-Nov-2020 DOI: 10.4172/2332-0702.1000267
Abstract
Hypersensitivity to the teeth is a common phenomenon that occurs in certain conditions and is associated with the exposure of dentine. The aim of this study was to investigate the efficacy of a paste containing 8% arginine and calcium carbonate in the treatment of dental hypersensitivity (Colgate®Sensitive Pro-RelifTM). The study included 30 subjects from both genders. The respondents are dental students and residents at the Faculty of Dentistry, Ss. “Cyril and Methodius” University in Skopje. During the study all respondents used the Colgate®Sensitive Pro-RelifTM paste and Slim Soft toothbrush.
The concentration of calcium in the saliva is reduced after one month of treatment with the paste due to its absorption to the tooth surface and dentinal tubules. There is no significant difference in relation to the obtained values of the OHI-index before and after treatment among subjects with sensitive and insensitive teeth.
Keywords: Hypersensitivity; Saliva; Calcium
Introduction
Dental hypersensitivity (hypersensitive dentin) is a pathological condition in which the exposed dentin (dental tissue located under the enamel and the cement of the tooth in which the center is pulp) is attached to a vital tooth sensitive to mechanical, osmotic, chemical or thermal smears. The stimulus/sensitivity causes movement of the dentinal fluid (inside) and out (through the channels that extend from the pulp to the dentin), and it leads to stimulus/sensitivity transmission to the nerve endings of the pulp resulting in onset of pain [1-3].
The causes of hypersensitivity dentin are numerous: loss of enamel due to the action of chewing and parafunctional forces, or due to erosion caused by acidic foods. On the other hand, withdrawal of gingiva due to periodontal disease or inadequate teeth brushing technique causes the tooth root strip and loss of a thin layer of cement that protects the root. In these cases, dentinal channels are exposed to external stimuli such as chewing, consuming hot or cold drinks and food. These exposed parts of the tooth cause the appearance of pain which may be the cause of certain discomfort (difficulty) [4-6].
Two of the most important factors for the appearance of hypersensitivity to the teeth are exposed dentine and / or open dentinal tubules. Exposed dentine appears when there is enamel or peridontal tissue. Losing enamel may occur as a result of atria, abrasion and erosion [7,8]. Rarely some physical and chemical processes alone lead to loss of tissue core. In individual teeth it can act as a predisposing (destructive) factor; strong chewing forces lead to non-cervical cervical lesions, which at the same time alleviates the effect of absorption and / or erosion. Dental exhaustion as a result of atrium can lead to a pathological condition in cases of paraphonic habits (bruxism) leading to occlusal dental hypersensitivity [9,10].
In terms of abrasion, great attention is paid to brushing teeth as well as to toothpaste. If separately seen, if you do not apply excessive force while brushing your teeth, there is no loss of enamel and if the paste used has a low REA (relative enamel abrasivity) value. The problem is that the teeth are usually washed after a meal when there is a high concentration of acids in the mouth. Namely, more in vitro studies show that after a meal, the acids soften the enamel surface layer by 3-5 μm, making it sensitive to physical stimuli-in that case, only the brush (without paste) can remove the fragile layer, since the remineralization with ions of saliva occurs in a few hours. In conclusion, these studies have stated that the teeth should be brushed after the meal only when the pH of the saliva is neutralized, and not immediately after the meal [11,12].
Another factor in the occurrence of dental hypersensitivity is the gingival recession (72.5%-98% of patients with parodontopathy have sensitive teeth). Gingival recession is responsible for susceptibility solely to the cervical portion of the tooth, and erosion combined with abrasion and / or atria is the cause of exposed dentine and other parts of the tooth [13-15].
It is known that dentinal tubules that are in contact with the oral medium are compressed with a Ca3(PO4)2 deposit originating from the saliva, but it can be removed as a result of physical and chemical action. The influence of the toothbrush is almost negligible, but not on the toothpaste, either with its abrasive effect, or with its cosmetic (most often sodium laurin sulphate). Opening of the dentinal tubules may also occur as a result of vigorous brushing in combination with a pronounced abrasive paste. Similar to the aetiology of exposed dentine, erosion, carbonated drinks, acids, etc., can affect the opening of dentinal tubules.
Hypersensitive dentine can be reduced by using special tramps to reduce tooth sensitivity. Toothpaste to reduce tooth sensitivity usually contains medicaments that protect the exposed dentin and seal open dentinal tubules. However, in most cases, these pastes must be used continuously for a longer period (at least a month) to record the first results [16-18].
There are a number of therapeutic procedures for the treatment of hypersensitive dentine, including the use of medications in dental practice, the application of low-energy laser therapy, and gels for home application (sodium fluoride, sodium nitrate or strontium chloride). These compounds help to block the transmission of stimuli through the dentinal canals to the nerves in the tooth pulp. Tips are also given to avoid sour foods and daily use of special pastes with abrasive properties. In individuals with hypersensitive teeth it is necessary to avoid the use of hard toothbrushes, and it is necessary beforehand to apply the correct technique for brushing teeth (it is important to avoid vigorously brushing teeth with horizontal movements). An improper brushing technique using hard toothbrushes leads to a gingival withdrawal and stripping of the roots [19,20].
Aim
The aim of this study was to investigate the efficacy of a paste containing 8% arginine and calcium carbonate in the treatment of dental hypersensitivity (Colgate®Sensitive Pro-RelifTM).
For achieving this aim we have made:
1. Clinical trials:
-Determination of the index of oral hygiene (OHI-index) by Greene Vermillion before and after treatment;
-Determination of the index of gingival inflammation Sillnes and Loe before and after treatment.
2. Biochemical investigations:
-Determination of the calcium concentration in saliva before and after the study period;
-Determination of the concentration of saliva hydrogen ions (pH value) before and after the study period;
-Correlation of the concentration of salivary hydrogen ions (pH), calcium between the two investigated groups (with sensitive and insensitive teeth) before and after treatment.
Materials and Methods
The study included 30 subjects from both genders. The respondents are dental students and residents at the Faculty of Dentistry, Ss., Cyril and Methodius” University in Skopje. During the study all respondents used the Colgate®Sensitive Pro-RelifTM paste and Slim Soft toothbrush. The investigated period lasted for 4 weeks.
These surveys are based on a pre-made questionnaire, in which the following data were noted:
a) What kind of pain the respondents feel;
b) Have they experienced an improvement after using the Colgate® Sensitive Pro-RelifTM toothpaste and after how long did it occur?
The investigated period lasted for 4 weeks, and it was realized in two parts:
1. Clinical trials
2. Laboratory testing
Clinical trials
Intraoral status was determined during clinical trials. All data obtained from the clinical trials were noted in a pre-prepared cardboard for each respondent separately.
With dental examination, index values were determined: the condition of maintaining oral hygiene and the condition of the gingiva before and after the end of the examination.
Oral hygiene degree was determined using the index for determination of the presence of soft plaque (OHI) using the simplified method of Greene-Vermillion, where the detection of soft plaques is done by coating the surface of teeth with aniline dye, and index values range from 0 to 3:
-Index 0–indicates the absence of soft plaques;
-Index 1 -indicates the presence of soft deposits of 1/3 of the surface of the crown of the tooth;
-Index 2 -indicates the presence of soft plaques of less than 2/3 of the surface of the crown of the tooth;
-Index 3 -indicates the presence of soft deposits of more than 2/3 of the surface of the crown of the tooth.
The method of determining the index of oral hygiene (OHI) includes only six areas of six teeth, which are a representative sample for the whole dentition: the vestibular surface of the upper first molars, the upper right central incision and the lower left central incision, and the oral surface of the lower first molars (16,26,11,31,36,46). If one of these teeth is missing, then the neighboring distal tooth is used. The values obtained are collected, and the sum is divided by the number of teeth examined.
The Gingival Inflammation Index (GII) was determined by Sillnes and Loe. The condition of the gingiva was determined with the following values:
Score 0=Normal gingiva.
Score 1=Mild inflammation-slight change in color, slight edema. No bleeding on probing.
Score 2=Moderate inflammation-redness, edema, glazing. Bleeding on probing.
Score 3=Severe inflammation-marked redness and edema, ulceration. Tendency toward spontaneous bleeding.
The scores of the four areas of the tooth, vestibular, mesial, oral and distal side of six representative teeth: upper right first molar, upper right second incisor, upper left first premolar, left first molar, second left incisor and right first premolar (16,12,24,36,32,44) was be summed and divided by four to give the GI for the tooth. The assessment is based on discoloration, swelling, and bleeding of the gums, by probing gently along the wall of soft tissue of the gingival sulcus.
Laboratory examinations
For the need of the biochemical examination of the material, saliva specimen was obtained from before the beginning of the study and four weeks after treatment (use of the recommended pasta and toothbrush).
A 3-5 ml specimen of saliva was obtained without a stimulator and put in a sterile plastic tube. The specimens of saliva within one hour were centrifuged at 3,500 rpm. for 30 minutes. Part of the supernatantcentrifugate (in sterile glass tubes) was used for determining the concentration of calcium, and part of it for determining the salivary pH.
The principle for determining calcium in saliva: the cresolphthalein complex reacts in an alkaline environment with calcium and builds a red-violet complex whose absorption is measured at 570 nm.
Calcium were determined by the Chemwell Awareness Technology biochemical analyzer, USA.
Determination of the concentration of hydrogen ions (pH value)
The concentration of hydrogen ions (pH value of saliva) was determined by pH meter pH 209, Hanna instruments, Germany.
All biochemical analyses were made at the Institute of Medical and Experimental Biochemistry at the Medical Faculty in Skopje.
Statistical Analysis
The statistical analysis of the obtained data was made in the statistical program SSRS IMB 20.
Results
The results of the tests are displayed in tables and figures.
To a large extent, the respondents (86%) experienced improvement after using the Colgate Sensitive Pro-Relif TM tooth, after two weeks at most (53.3%) and at least after three weeks (3.3%), (Tables 1 and 2).
| Frequency | % | |
|---|---|---|
| Yes | 26 | 86.7 |
| No | 4 | 13.3 |
Table 1: Did you experience improvement after using toothpaste? Colgate® Sensitive Pro-RelifTM?
| Frequency | % | |
|---|---|---|
| After one week | 13 | 43.3 |
| After two weeks | 16 | 53.3 |
| After three weeks | 1 | 3.3 |
Table 2: How long have you experienced improvement?
With only one respondent was not satisfied with the paste and toothbrushes Slim Soft, and 96.7% gave a positive answer (Table 3).
| Frequency | % | |
|---|---|---|
| Yes | 29 | 96.7 |
| No | 1 | 3.3 |
Table 3: Are you satisfied with the effect of the toothpaste and toothbrushes? Slim Soft?
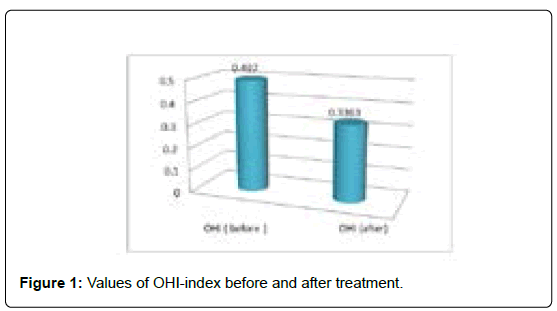
Before the start of the treatment, the minimum value of the OHI-index was 0, and the maximum value 2.16. After the 4-week-treatment, the minimum value of the OHI-index was 0, and the maximum 1.83 (Table 4, Figure 1).
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| OHI index before | 30 | 0 | 2.16 | 0.497 | 0.49724 |
| OHI index after | 30 | 0 | 1.83 | 0.3363 | 0.4245 |
* There was a significant difference
** There was a highly significant difference
Table 4: Values of OHI-index before and after treatment.
The OHI-index before and after treatment of the subjects was smaller, and its value was statistically significant (Table 5), the value of gingival inflammation index by Sillnes and Loe before and after treatment was statistically significantly reduced.
| Mean | Std. Deviation | t | df | Sig | |
|---|---|---|---|---|---|
| OHI (before/after) | 0.1607 | 0.1885 | 4.669 | 29 | 0 |
| IGI (before/after) | 0.211 | 0.19538 | 5.915 | 29 | 0 |
* There was a significant difference
** There was a highly significant difference
Table 5: Values of OHI-index and index of gingival inflammation before and after treatment.
Table 6 shows the level of pH in the saliva before and after treatment. pH levels in saliva ranged from 6 to 7 or, on average, from 6.53 in subjects before treatment, and from 5.5 to 8 or, on average, 6.43 in subjects after treatment.
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| pH (before) | 30 | 6 | 7 | 6.5333 | 0.50742 |
| pH (after) | 30 | 5.5 | 8 | 6.4333 | 0.61214 |
* There was a significant difference
** There was a highly significant difference
Table 6: Levels of saliva pH before and after treatment.
Table 7 shows calcium level in saliva before and after treatment. Calcium levels in saliva ranged from 0.9 to 2.7 or, average 1.66 in subjects before treatment, and from 0.9 to 2.8 or, average 1.49 in subjects after treatment.
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Calcium (before) | 30 | 0.9 | 2.7 | 1.66 | 0.41156 |
| Calcium (after) | 30 | 0.9 | 2.8 | 1.4967 | 0.45218 |
* There was a significant difference
** There was a highly significant difference
Table 7: Calcium levels in saliva before and after treatment.
Table 8 presents the differences in the parameters tested during the laboratory before and after-treatment. There was a statistically significant difference only in the calcium level in saliva (p<0.05).
| Mean | Std. Deviation | t | df | sig | |
|---|---|---|---|---|---|
| pH (before/after) | 0.1 | 0.59306 | 0.924 | 29 | 0.363 |
| Calcium(before/after) | 0.16333 | 0.38281 | 2.337 | 29 | .027* |
* There was a significant difference
** There was a highly significant difference
Table 8: Levels of pH and calcium in the saliva before and after treatment.
Tables 9 and 10 show the differences between the values of beforetreatment parameters in both groups of examinees (with sensitive and insensitive teeth). Between the two groups of subjects before treatment there were no statistically significant differences in the parameters tested (pH and amount of calcium in the saliva).
| Sensitivity | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| pH | Sensitive | 15 | 6.5333 | 0.5164 | 0.13333 |
| Insensitive | 15 | 6.5333 | 0.5164 | 0.13333 | |
| Calcium | Sensitive | 15 | 1.7733 | 0.49493 | 0.12779 |
| Insensitive | 15 | 1.5467 | 0.27997 | 0.07229 |
* There was a significant difference
** There was a highly significant difference
Table 9: Differences between values of before treatment parameters tested in both groups of examinees.
| F | Sig. | t | df | Sig. (2-tailed) | |
|---|---|---|---|---|---|
| pH | 0 | 1 | 0 | 28 | 1 |
| 0 | 28 | 1 | |||
| Calcium | 6.979 | 0.013 | 1.544 | 28 | 0.134 |
| 1.544 | 22.127 | 0.137 | |||
| * There was a significant difference ** There was a highly significant difference |
|||||
Table 10: Differences between values of before treatment parameters tested in both groups of examinees.
After treatment there were no statistically significant differences of the investigated parameters between the two groups of respondents (susceptible and insensitive) (Tables 11 and 12).
| Sensitivity | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| pH | Sensitive | 15 | 6.5333 | 0.74322 | 0.1919 |
| Insensitive | 15 | 6.3333 | 0.44987 | 0.11616 | |
| Calcium | Sensitive | 15 | 1.5267 | 0.54178 | 0.13989 |
| Insensitive | 15 | 1.4667 | 0.3579 | 0.09241 |
* There was a significant difference
** There was a highly significant difference
Table 11: Differences between values of the investigated parameters after treatment in both groups of examinees.
| F | Sig. | t | df | Sig. (2-tailed) | |
|---|---|---|---|---|---|
| pH | 2.101 | 0.158 | 0.892 | 28 | 0.38 |
| 0.892 | 23.045 | 0.382 | |||
| Calcium | 1.877 | 0.182 | 0.358 | 28 | 0.723 |
| 0.358 | 24.264 | 0.724 |
* There was a significant difference
** There was a highly significant difference
Table 12: Differences between values of the investigated parameters after treatment in both groups of examinees.
Discussion
Hypersensitivity to the teeth is a common phenomenon that occurs in certain conditions and is associated with the exposure of dentine. Odontoblasts as vital cells that are determined by tooth papilla change their shape and intracellular structure according to the needs of dentine and pulp. In addition to the layer of odontoblasts on the periphery of the pulp there is a layer of predentine, which is evidence that dentin is created throughout the life. The most common cause of dentine exposure is the gingival recession. Inadequate technique and inappropriate means of oral hygiene are associated with gingival damage through mechanical forces. On the other hand, periodontal disease with or without surgery is associated with a loss of gingival tissue. Mechanical and chemical processes followed by erosion and abrasion can lead to the loss of enamel, to the exposures and hypersensitivity of dentine. Excessive intake of acidic foods and beverages-a chemical erosion process can lead to significant loss of surface tooth substanceenamel and dentine exposure [21-23].
The treatment of open dentine tubules on the teeth is based on the mechanical deposition of the fluid movement by the application of agents such as strontium, fluorine, argentum nitricium.
The most commonly used agents are anti-inflammatory agents, dental closure agents (oxalate-based, sodium ions), agents that affect the depolarization of the nerve endings of the pulp. To reduce tooth sensitivity usually toothpaste contains some of the medications that protect the exposed dentine and block dentinal channels. However, in most cases, these pastes must be used continuously for a longer period (at least a month) to record the initial results [24-26].
Depositing materials in in vitro tests can be investigated by analyzing the tooth surface. The dentin has a characteristic elemental composition that derives from the mixed mineral and protein composition. After treatment of tooth cleansing or the use of a mouthwash, the composition of the dentine surface elements may change; treatments lead to the formation/accumulation of minerals with increased calcium and phosphate content and reduction of elements characteristic of proteins such as nitrogen and carbon. Treatments that contain elements that are not part of dentine, such as silicates or strontium, can be evident in post-treatment analysis [27]. Significant changes in the elemental composition of the dentine surface were observed after treatment with 8% arginine and calcium carbonate. This treatment resulted in the coverage of the dentine protein and an addition to the surface with a calcium and phosphate mineral layer. With electron microscopy it has been proven that these mineral deposits can also be seen in dentinal tubules [12]. Other studies in which dentin was treated with 8% arginine and calcium carbonate agree that this treatment caused tubulation overloading. But it was noted that it was partially lost after prolonged immersion in a solution of citric acid [28,29]. This action of acid on the tubule covered with deposit is not unexpected; because calcium phosphate-based minerals are soluble in the action of acids. Therefore, when treating dental hypersensitivity, patients should be advised to reduce the intake of acid-rich foods in order to prevent the recurrence of sensitivity symptoms [30].
Our study showed that the Colgate® Sensitive Pro-ReliefTM toothpaste reduces the sensitivity of the teeth. However, the limitation of this study is the 4-week follow-up, a time that can be considered short. In parallel with the Colgate® Sensitive Pro-ReliefTM toothpaste, other types of desensitive toothpastes as control groups should be included in the future to determine the benefits of this method in reducing dentin hypersensitivity.
In our study, we used the Colgate® Sensitive Pro-RelifTM paste containing 8% arginine, calcium carbonate and 1450 ppm F as sodium monofluorophosphate. It is assumed that arginine in combination with calcium carbonate placed on the exposed dentine blocks and seals open dentinal tubules and effectively reduces the flow of dentinal fluid. In addition to having a long-lasting effect, it also starts to act immediately after the application. Treatment with arginine calcium carbonate desensitizing paste is simple. The paste is gentle for gingival tissues; it does not cause pain when applied and has a pleasant taste. The paste can be applied in a small amount of sensitive areas of the teeth by the dentist himself, using rotary instruments at a low speed. It is important to carefully set arginine calcium carbonate desensitizing paste in all sensitive parts, focusing on the cement enamel boundary and the exposed cement and dentine. Rinsing should be avoided immediately after administration of the paste, in order to improve clinical efficacy.
It is important to note that arginine-calcium carbonate desensitizing paste is used to treat dentin hypersensitivity. It will not provide symptomatic relief to other conditions, such as fractured teeth, caries or occlusal trauma, which should be diagnosed and treated with other means/agents/procedures by the dentist. Before the treatment, the prevalence of soft padding on the teeth in the Greene-Vermillion index was 0.497, and one month after treatment a decrease was noted that amounted to 0.336. In this study period, a statistically significant reduction was also achieved regarding the values of the OHI-index.
The value of the oral hygiene index was higher among subjects with sensitive teeth compared to subjects with insensitive teeth, but without a statistical significance. This is due to a lower level of oral hygiene in subjects with sensitive teeth than in subjects with insensitive teeth.
In recent years, particular attention has been paid to saliva as one of the most important factors for maintaining oral homeostasis in the oral cavity-important for the integrity of oral tissues, especially the dental enamel. Spit plays an important role in the natural reduction of the sensitivity of teeth by supplying calcium and phosphate ions in open dentinal tubules, by gradually blocking the tubules as well as by forming a surface protective layer. Reducing salivary flow, hyposalivation, xerostomia, while increasing the risk of demineralization and dental caries, may also worsen the hypersensitivity of the teeth. From a biochemical aspect, the composition and role of organic and inorganic saliva ingredients (albumin, immunoglobulins, calcium, phosphorus, sodium, fluorine, urea, ammonia, etc.) is important.
Of the numerous features of saliva, the sensitivity of teeth is given the highest importance to calcium, as well as its buffer capacity. In our study, the pH level in saliva in the subjects at the start of the trial ranged from 6 to 7 or, on average, from 6.53, and from 5.5 to 8, or, on average, 6.43 in subjects after treatment. It should be emphasized that in the subjects with insensitive teeth, the pH level was higher than that in the group of examinees with sensitive teeth. The fall in pH of the saliva, created as a result of the breakdown of the constituent food components, whose final product is acids, is successfully overcome by activating the saliva buffer systems. This suggests that the acid medium in the oral medium persists for a long period, which can be accepted as an indicator of a greater sensitivity of the teeth among the examinees. This is consistent with the results obtained for the concentration of urea (which was statically significantly lower) in subjects prior to treatment compared to the levels obtained after completing the treatment with the paste.
Several recently produced desensitizing agents have been designed to stimulate the formation of calcium and phosphate mineral on the surface of dentine and inside the tubules [27]. The purpose of these treatments is to reduce pain caused by stimulation of the surface of dentine from acids. By actively treating with agents that close the tubules and improving the mineral content of the surface, they make it less susceptible to further damage. Many of these products, such as calcium fluoride-containing varnishes, deliver calcium directly to the surface. Arginine when combined with a source of calcium affects the reduction of susceptibility with mineral formation-an occlusion mechanism [31,32].
An analysis of the results of the calcium prevalence in saliva after the use of a paste for maintenance of oral hygiene containing calcium carbonate in its composition has shown that there is a statistically significant difference in the presence of this element in the two-time intervals of the test. The calcium levels in the saliva in the subjects prior to treatment ranged from 0.9 to 2.7 or, on average, from 1.66 and from 0.9 to 2.8 or, on average, 1.49 in subjects after treatment. Therefore, it is obvious that there is a decrease in the calcium concentration in the saliva, probably as a result of its absorption in the enamel.
Conclusion
• The following conclusions can be derived based on the results obtained in our study:
• Effective plaque control, improved salivary flow, and increased pH in the saliva may have a positive effect on preventing hypersensitive dentine.
• The obtained values for the oral hygiene index (OHI-index) before and after treatment with the paste indicate a significant improvement in oral hygiene.
• The values of the index of gingival inflammation in the subjects after one month of treatment with a paste show a significant decrease.
• There is no significant difference in relation to the obtained values of the OHI-index before and after treatment among subjects with sensitive and insensitive teeth.
• Salivary pH tests showed a gradual increase in pH after treatment compared to before treatment values.
• The concentration of calcium in the saliva is reduced after one month of treatment with the paste due to its absorption to the tooth surface and dentinal tubules.
• High percentage of the respondents experienced improvement after using Colgate Sensitive Pro-RelifTM toothpaste, after a maximum of two weeks, and at least after the third week.
Funding
No funding was received for this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cummins D (2009) Dentin hypersensitivity: From diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent 20:1.
- Bubteina N, Garoushi S (2015) Dentine hypersensitivity: A review. Dentistry 330: 2161-1122.
- West N, Seong J, Davies M (2014) Dentine hypersensitivity. Monogr Oral Sci 25:108-122
- Davari AR, Ataei E, Assarzadeh H (2013) Dentin hypersensitivity: Etiology, diagnosis and treatment; a literature review. J Dent 14:136.
- Zeola LF, Soares PV, Cunha-Cruz J (2019) Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J Dent 81:1-6.
- Porto IC, Andrade AK, Montes MA (2009) Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci 51:323-332.
- Miglani S, Aggarwal V, Ahuja B (2010) Dentin hypersensitivity: Recent trends in management. J Conserv Dent 13:218.
- Kleinberg I. SensiStat (2002) A new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today 21:42.
- Lata S, Varghese NO, Varughese JM et.al (2010) Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent 13:42.
- Merh A, Singhbal K, Parikh V, Mehta S, Kulkarni G et.al (2015) Comparative evaluation of immediate efficacy of diode laser versus desensitizing paste containing 8% arginine and calcium carbonate in treatment of dentine hypersensitivity: An in vivo study. J Evol Med Dent Sci 4:4346-4356.
- Panagakos FO, Schiff T, Guignon A et.al (2009) Dentin hypersensitivity: effective treatment with an in-office desensitizing paste containing 8% arginine and calcium carbonate. Am J Dent 22:3A-7A.
- Petrou I, Heu R, Stranick M, Lavender S, Zaidel L, et.al (2009) A breakthrough therapy for dentin hypersensitivity: How dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent 20:23-31.
- Douglas de Oliveira DW, Marques DP, Aguiarâ€Cantuária IC, Flecha OD, Gonçalves PF (2013) Effect of surgical defect coverage on cervical dentin hypersensitivity and quality of life. J Periodontol 84:768-775.
- Biagi R, Cossellu G, Sarcina M, Pizzamiglio IT, Farronato G (2015) Laser-assisted treatment of dentinal hypersensitivity: a literature review. Ann Stomatol 6:75.
- de Fátima Zanirato Lizarelli R, Miguel FA, Villa GE, de Carvalho Filho E, Pelino JE, et.al (2007) Clinical Effects of Low-intensity Laser vs. Light-emitting Diode Therapy on Dentin Hypersensitivity. J Oral Laser Applications 7: 129-136.
- Alcântara PM, Barroso NF, Botelho AM, Douglas-de-Oliveira DW, Gonçalves PF, et.al (2018). Associated factors to cervical dentin hypersensitivity in adults: A transversal study. BMC Oral Health. 18:155.
- Trushkowsky RD, Garcia-Godoy F (2014) Dentin hypersensitivity: Differential diagnosis, tests, and etiology. Compend Contin Educ Dent 35:99-104.
- Ricarte JM, Matoses VF, Llácer VF, Fernández AF, Moreno BM (2008) Dentinal sensitivity: Concept and methodology for its objective evaluation. Med Oral Patol Oral Cir Bucal 13:E201-E206.
- Shen SY, Tsai CH, Yang LC, Chang YC (2009) Clinical efficacy of toothpaste containing potassium citrate in treating dentin hypersensitivity. J Dent Sci 4:173-177.
- Pradeep AR, Sharma A. (2010) Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: A randomized clinical trial. J Periodontol 81:1167-1173.
- Prasad KV, Sohoni R, Tikare S, Yalamalli M, Rajesh G, et.al (2010) Efficacy of two commercially available dentifrices in reducing dentinal hypersensitivity. Indian J Dent Res 21:224.
- Prabhakar AR, Manojkumar AJ, Basappa N (2013) In vitro remineralization of enamel subsurface lesions and assessment of dentine tubule occlusion from NaF dentifrices with and without calcium. J Indian Soc Pedod Prev Dent 31:29.
- Ye W, FENG XP, Li R (2012) The prevalence of dentine hypersensitivity in Chinese adults. J Oral Rehabil 39: 182-187.
- Rees JS, Addy M (2004) A crossâ€sectional study of buccal cervical sensitivity in UK general dental practice and a summary review of prevalence studies. Int J Dent Hyg 2:64-69.
- Vijaya V, Sanjay V, Varghese RK, Ravuri R, Agarwal A (2013) Association of dentine hypersensitivity with different risk factors: A cross sectional study. J Int Oral Health 5:88.
- Al-Wahadni A, Linden GJ (2002) Dentine hypersensitivity in Jordanian dental attenders: A case control study. J Clin Periodontol 29:688-693.
- Cai F, Shen P, Morgan MV, Reynolds EC (2003) Remineralization of enamel subsurface lesions in situ by sugarâ€free lozenges containing casein phosphopeptideamorphous calcium phosphate. Aust Dent J 48:240-243.
- BahÅŸi E, Dalli M, Uzgur R, Turkal M, Hamidi MM, et.al (2012) An analysis of the aetiology, prevalence and clinical features of dentine hypersensitivity in a general dental population. Eur Rev Med Pharmacol Sci 16:1107-1126.
- West NX, Lussi A, Seong J, Hellwig E (2013) Dentin hypersensitivity: Pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Investig 17:9-19.
- GarcÃa-Godoy F (2009) Dentin hypersensitivity: Beneficial effects of an arginine-calcium carbonate desensitizing paste. Am J Dent 22:2A.
- Davies M, Paice EM, Jones SB, Leary S, Curtis AR, et.al (2011) Efficacy of desensitizing dentifrices to occlude dentinal tubules. Eur J Oral Sci 119:497-503.
- Zero DT, Lussi A. Erosion (2005) Chemical and biological factors of importance to the dental practitioner. Int Dent J 55:285-290.
Citation: Zabokova-Bilbilova E, Toshevska-Trajkovska K, Ivkovska A and Stefanoska T (2020) Dental Hypersensitivity. J Oral Hyg Health 9: 267. DOI: 10.4172/2332-0702.1000267
Copyright: © 2020 Efka Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3624
- [From(publication date): 0-2021 - Oct 09, 2025]
- Breakdown by view type
- HTML page views: 2764
- PDF downloads: 860