Research Article Open Access
Utility Spectrophotometric and Chromatographic Methods for Determination of Antidepressant Drug Sulpiride in Pharmaceutical Formulations and Plasma
Mohamed Ghoneim1, Amr Lotfy Saber2,3* and Hanaa El-Desoky11Chemistry Department, Faculty of Science, Analytical & Electrochemistry Research Unit, Tanta University, Egypt
2Chemistry Department, Faculty of Science, Zagazig University, Egypt
3Chemistry Department, Faculty of Science, Umm Al-Qura University, Saudi Arabia
- *Corresponding Author:
- Amr Lotfy Saber
Chemistry Department, Faculty of Science
Umm Al-Qura University, Saudi Arabia
Tel: + 20 121430134, +966546546884
Fax: + 20 55 2306975
E-mail: alshefny@yahoo.com
Received date: February 10, 2014; Accepted date: March 09, 2014; Published date: March 11, 2014
Citation: Ghoneim M, Saber AL, El-Desoky H (2014) Utility Spectrophotometric and Chromatographic Methods for Determination of Antidepressant Drug Sulpiride in Pharmaceutical Formulations and Plasma. J Anal Bioanal Tech 5:183. doi: 10.4172/2155-9872.1000183
Copyright: © 2014 Ghoneim M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Validated spectrophotometric and chromatographic methods have been developed for determination of the antidepressant drug sulpiride (SUL) in pharmaceutical formulation and plasms. The new spectrophotometric methods were based on the formation of sulpiride yellow ion-pair complex with bromocresol green (BCG), congo red (CR) or methyl orange (MO) in Britton-Robinson universal buffer of pH 3.0, 5.0 or 2.5, respectively. The formed complexes with BCG, CR and MO were extracted with chloroform and their absorbencies were measured at 420 nm, 515 nm, and 480 nm, respectively. Beer’s law was obeyed over the concentration ranges of 2-14.0, 2-16.0, and 2-14.0 μg/ mL sulpiride with BCG, CR and MO, respectively. The molar absorpativity (ε) of the formed colored complexes with BCG, CR and MO was 4.10×104, 2.10×104 and 3.50×104 L moL-1 cm-1 and the estimated limit of detection (LOD) of sulpiride was found to be 0.044, 0.095 and 0.064 μg/mL, respectively. In the developed high performance liquid chromatographic method (HPLC), quantitation of sulpiride was carried out on C18 reversed phase column (250×4.0 mm, 5 μm) using a mobile phase of acetonitril: methanol: water: Britton-Robinson (B-R) universal buffer of pH 9 (20: 20: 40: 20, v/v/v/v) delivered at a flow rate of 0.6 mL/min with UV-detection at 225 nm. Calibration graph of bulk sulpiride was linear over the concentration range of 0.034-110 μg/mL. The described spectrophotometric and HPLC methods have been applied successfully for the analysis of sulpiride in its dosage form without interference from common excipients. Statistical comparison of their results with those obtained using a reported membrane selective electrode method showed excellent agreement and indicated no significant differences in accuracy and precision.
Keywords
Sulpiride; Dogmatil fort®; Spectrophotometry; Ion pair complexes; HPLC determination
Introduction
Antidepressant drugs are widely used for treatment of depression and they are frequently encountered in emergency toxicology screening, drug-abuse testing and forensic medical examinations [1]. Sulpiride; SUL, Scheme I, (5-(aminosulfonyl)-N-[(1-ethylpyrrolidin- 2-yl)methyl]-2-methoxybenzamide) falls into the large group of antidepressant drugs [2,3]. It possesses anti-psychotic, antidepressive, and antiulcer effects. It has peculiar affinity for the D2 and D4 brain dopamine receptors with a low frequency of extrapyramidal side-effects [4]. SUL also exhibits neuroleptic and thymoleptic properties and is used in mental disorders as a behavior regulator in the psychopathology of senescence, in depression, and in schizophrenia. It is also used in the treatment of gastric or duodenal ulcers, in the treatment of irritable colon due to psychosomatic stress, and in various vertigo syndromes.
Several methods have been developed for detection of SUL, including spectroflurometric [5,6], spectrophotometric [7-10], gas chromatography [11], liquid chromatography [2], thin layer chromatography [7-12], high performance liquid chromatography (HPLC) with fluorescence [13-17], or mass spectrometric detection [3], radioimmunoassay [18,19], voltammetric [20,21], membrane selective electrode [22], capillary electrophoresis with electrochemiluminescence (CE–ECL) and ultraviolet [23-25] and H-1-NMR spectroscopy [26].
Most of these methods involved determination of SUL in two component mixture [7,9,10,18,24,26] or in human biological fluids[2,3,5,6,11-17]. A non-aqueous titration method for determination of SUL in bulk and in pharmaceutical tablets was described in British [27] and European [28], Pharmacopoeias.
Ion-pair extractive spectrophotometry has received considerable attention for the quantitative determination of many pharmaceutical compounds [29-33], for its sensitivity and capability for offering distinct possibilities in the assay of a particular component in a complex dosage formulation. On the other side, most reported high performance liquid chromatography [3,13-17] necessitate sophisticated detection, lengthy extraction steps with organic solvents prior to assay of analyte, long run time (≈ 25 min), sensitive ion-pair reagents [34,35]. All these reported HPLC are time-consuming and not economically feasible for routine use in pharmacokinetic studies with numerous samples to be analyzed.
This paper aimed to describe a spectrophotometric method for determination of SUL in pharmaceutical formulations and plasma based on extraction of its soluble ion-pair complexes with some acid dyes in buffered solutions. Besides, a rapid HPLC-UV method without ion-pair reagent in mobile phase was also described.
Materials and Methods
Material
Bulk sulpiride (SUL) was obtained from Memphis CO. for Pharm. & Chem. Ind., Cairo, Egypt. Dogmatil fort® tablets claimed to contain 200 mg SUL per tablet (Memphis CO. for Pharm. & Chem. Ind., Cairo, Egypt) were purchased from the local market. Sodium salt of each of the acid dyes bromocresol green (3,3′,5,5′-Tetrabromo-m-cresolsulfonphthalein), congo red (Benzidinediazo-bis-1-naphthylamine-4-sulfonic acid) and methyl orange (p-Dimethylamino-azobenzenesulfonic acid) were obtained from Merck (Darmstadt, Germany).
Solutions
(a) A stock solution (200 μg/mL) of bulk SUL was prepared by dissolving 20 mg of pure drug in de-ionized water, transferring it into a 100 ml measuring flask, and diluting it with water up to the marking. Working standards (0.01 to 130 μg/mL) were prepared by serial dilutions with the mobile phase: acetonitril: methanol: water: B-R universal buffer of pH 9 (20: 20: 40: 20, v/v/v/v).
(b) Solutions of 1×10-3 mol/L of each of bromocresol green (BCG) congo red (CR) and methyl orange (MO) were prepared by dissolving accurate weight of the acid dye-sodium salt in a few drops of methanol and then in de-ionized water in 100 mL-volumetric flasks.
(c) A series of the Britton-Robinson (B-R) universal buffer of pH 2-11 were prepared [36], in de-ionized water. All the chemicals used were of analytical-reagent grade quality and were used without further purification. The pH of solutions was checked using an Orion Research digital pH-meter Model 601A (Yokohama, Japan). De-ionized water was obtained by a Purite-Still Plus de-ionizer connected to an AquaMatic double-distillation water system (Hamilton Laboratory Glass LTD, Kent, UK).
Spectrophotometric measurements
Apparatus: Adsorption spectral measurements were made using UV-Visible spectrophotometer (Jasco, Hachioji-cho, Tokyo, Japan) with 10 mm quartz cells.
General procedure: Aliquots of the standard solution of SUL were transferred into a series of reaction flasks followed by the addition of 1.5 mL, 2.6 mL, and 2.0 mL (1.0×10-3 mol/L) of BCG, CR or MO, respectively, the total volume of the aqueous phase was adjusted to 10 ml by the B-R universal buffer solution of selected pH (Table 1), then mixed well (the final concentration of SUL was in the range of 0.1 to 100 μg/mL). After 2 min vortexing the flasks were allowed to separate the two layers by centrifugation. Each of the formed yellow ion pair complexes was extracted with 10 mL chloroform. The chloroform layer was dried by running through anhydrous sodium sulfate. Absorbance of each of the yellow-colored ion pair complex was measured at λmax shown in Table 1 (after standing for 5.0 min in each case) against a reagent blank similarly prepared. A calibration graph of absorbance versus concentration of the SUL was plotted.
Chromatographic measurements
Apparatus: A liquid chromatographic pump (Bischoff, Switzerland) equipped with a UV-detector (Bischoff Lambda 1000) and a reversed phase column (Prontosil C18, 250×4.0 mm, 5 μm) were used. Data acquisition and peak integration was done with the Bischoff McDAcq integrator software v1.5. The injection volume was 20 μL with a Rheodyne 7125 injector valve.
Absorption spectra of SUL determination in pharmaceutical formulations and plasma was recorded at room temperature within the wavelength range 200-600 nm using a Shimadzu UV-visible spectrophotometer Model 160A (Kyto, Japan). From the UV spectra of the analyte, the detection wavelength was chosen as 225 nm.
HPLC procedure: Sulpiride was quantitated on a C18 reversed phase column, however the mobile composition was, acetonitril: methanol: water: B-R universal buffer of pH 9 (20: 20: 40: 20, v/v/v/v) delivered at a flow rate of 0.6 mL/min at ambient temperature of 25 ± 2°C, and with UV detection (wavelength=225 nm). The mobile phase was sonicated-well before use and the column was equilibrated with the mobile phase flowing through the system before the injection of the standard solution of the analyte. Each standard solution was injected in the chromatographic system (n=3) and mean values of peak areas (A) were plotted against concentrations (C).
Assay procedure for tablets: Ten tablets of dogmatil fort® were weighed and the average mass per tablet was determined, and then ground to fine powder. A weighed portion of the homogeneous powder equivalent to 200 μg/mL SUL was accurately transferred into a 100 mL volume calibrated flask containing 70 mL water. The content of the flask was sonicated for about 10 min and then filled up with water. The solutions were then filtered through a 0.45 μm Milli-pore filter (Gelman, Germany). Convenient concentrations of SUL were then obtained by accurate dilutions with de-ionized water (for spectrophotometric measurements) or with the mobile phase: acetonitril: methanol: water: B-R universal buffer of pH 9 (20: 20: 40: 20, v/v/v/v) (for HPLC measurements). Thereafter, the general procedure was followed.
In vitro assay of sulpiride in plasma: To 5 mL plasma contained in three separatory funnels add different volumes of SUL standard solution prepared in distilled water (0.2 mg/mL). Extract with two 5 mL portions of chloroform. After separation, collect the chloroformic extracts into a graduated measuring cylinder and evaporate in a water bath until the volume is reduced to 2 mL. Calculate the concentration of recovered drug from a calibration graph.
Results and Discussion
Spectrophotometric studies
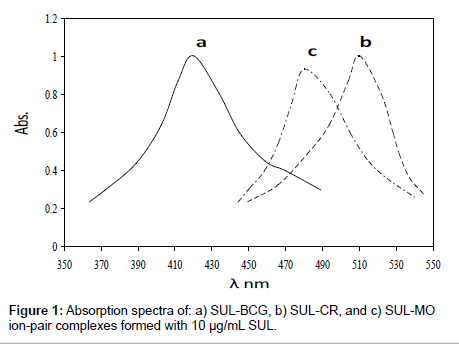
Sulpiride (SUL) was found to interact with each of the acid dyes bromocresol green (BCG), congo red (CR) and methyl orange (MO) in acidic media forming yellow ion-pair complexes. These complexes were easily extracted quantitatively into chloroform. The absorption spectra of the extracted ion-pair complexes were recorded within the wavelength range of 300-600 nm against a blank solution (Figure 1).
The formed ion-pair complexes show a maximum absorbance at λmax depends on type of the acid dye as indicated in Table 1. Hence, this wavelength was used for all subsequent measurements. The optimum conditions for these interactions were established by a number of preliminary experiments as described in the following:
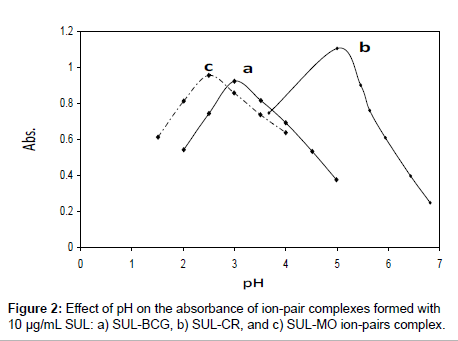
Effect of pH: Influence of pH on formation of the ion-pair complexes of SUL with the examined acid dyes has been studied in B-R universal buffers of different pH values. Maximum development of color intensities of the formed SUL ion-pair complexes with BCG, CR, and MO were achieved at the pH values 3.0, 5.0, and 2.5, respectively (Figure 2). Formation of the ion-pair complexes may be attributed to the protonation of the tertiary amino group of SUL in acid medium leading to form ion-pair complex with the anionic dye species. The formed ion-pair complexes were found to extract easily and quantitatively into chloroform. The formation reactions of the ion-pair complexes in the acidic medium can be illustrated in the following:
SUL + H+ → (SULH)+
(SULH)+ + (BCGNa) → (SULH)+.(BCG)- + Na+
(SULH)+ + (CR.2Na) → (SULH)+.(CR.Na)- + Na+
(SULH)+ + (MONa) → (SULH)+.(MO)- + Na+
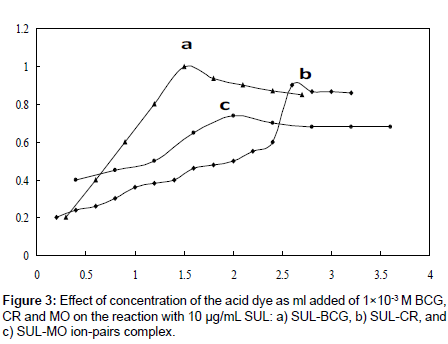
Effect of dye concentration: Effect of changing of concentration of the examined acid dyes (as mL added) on the development of the color intensity at λmax of their formed ion-pair complexes with 10 μg/mL of SUL was examined (Figure 3). The results indicated that the maximum absorbance of the formed ion-pair complexes of SUL was achieved on the addition of 1.5, 2.6, and 2.0 mL of 1×10-3 M of each of BCG, CR, and MO reagents, respectively, which were used for formation of the ion-pair complexes of SUL throughout the rest of this analytical work.
Choice of organic solvent: Quantitative extraction of the formed SUL-Dye ion pair complexes from solutions was examined using different organic solvents such as chloroform, dichloromethane, carbon tetrachloride, benzene, and toluene. Chloroform was found to be the most efficient organic solvent for this purpose. Double extraction with total volume 10 mL, yielding maximum and stable absorbance intensity for at least 24 h for studied drug and considerably lower extraction ability for the reagent blank and the shortest time to reach the equilibrium between both phases.
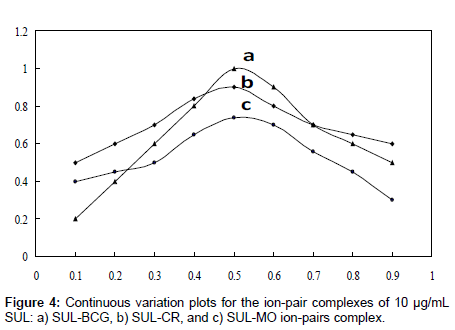
Phase ratio: Equimolar solutions was employed: a 1.0×10-3 M standard solution of drug base and 1.0×10-3 M solution of BCG, CR, and MO, respectively, were used. A series of solutions was prepared in which the total volume of drug and reagent was kept at 10 mL for BCG, CR, and MO, respectively. The absorbance was measured at the optimum wavelength. The molar ratio of the reagents (drug: dye) in the ion-pair complexes was determined by the method continuous variations (Job’s method) (Figure 4). The ratio of aqueous to organic phase was ineffective and the ratio 1:1 was chosen for extraction of the colored species. It was also noticed that the order of addition of the reagents had neither an effect on the absorbance nor the color of the complexes.
Effect of shaking and standing times (reaction time): To determine the most efficient ion-pair complex formation, shaking time of 1-5 min was studied. A constant absorbance was achieved over the examined shaking time range used and hence, 2.0 min. was chosen as an optimum shaking time throughout the experiments. Besides, the stability of the ion-pair complexes formed between the SUL and examined dyes also indicates that although the ion-pair complexes were formed instantaneously, constant absorbance readings were obtained after not less than 5 min of standing at room temperature (25 ± 2°C).
Composition of ion-pair complexes: The composition of ion-pair complexes was studied by Job’s method of continuous variations [37] which is based on the variation of both the drug and the reagent of equal molar concentrations, keeping the total volume of the drug and the dye constant. Plots of the absorbance versus molar concentration of SUL reaches a maximum value at a mole fraction of 0.5 for each of the investigated dyes (Figure 4), which indicated that 1:1 SUL-Dye ion-pairs (SULH+.D+) were formed through the electrostatic attraction between the positive protonated drug (SULH+) and anion dye (D-) species.
Conditional stability constants (Kf) of the ion-pair complexes: The stability of the ion-pair complexes was evaluated. The formations of the ion-pairs were rapid and the yellow color extracts were stable for at least 24 h at room temperature without any change of either the color intensity or the maximum absorbance. The conditional stability constant (Kf) of an ion-pair complex can be calculated from the continuous variation data using the following equation:
Kf=(A/Am)/[1−A/Am]n+1CnD
where A and Am are the observed maximum absorbance and the absorbance value when all the amount of drug is associated, respectively. C is the molar concentration corresponding to the maximum in absorbance and n is the stoichiometric constant with which dye ion associates with drug. Values of the obtained stability constant showed that the ion-pair complex of (SULH)+ with bromocrysol green is relatively much stable than those of methyl orange and congo-red species (Table 1).
| Parameter | Values | ||
|---|---|---|---|
| BCG | CR | MO | |
| pH | 3.0 | 5.0 | 2.5 |
| Extracting solvent | chloroform | Chloroform | chloroform |
| λmax | 420 | 515 | 480 |
| Molar ratio (SUL : Dye) | 1 : 1 | 1 : 1 | 1 : 1 |
| Beer’s law limits (μg/mL) | 2 – 14 | 2 – 16 | 2 -14 |
| Molar absorptivity (L mol-1cm-1) | 4.1×104 | 2.1×104 | 3.5×104 |
| Sandell’s sensitivity (ng cm-2) | 10.2 | 26.3 | 12.3 |
| Range of error % | 0.74 : -0.60 | 0.51 : 0.24 | 0.86 : -0.91 |
| Regression equation* | |||
| Intercept | 0.236 ± 0.0018 | 0.184 ± 0.0022 | 0.016 ± 0.002 |
| Slope | 0.098 ± 0.003 | 0.038 ± 0.002 | 0.081 ± 0.003 |
| Correlation coefficient (r) | 0.9990 | 0.9991 | 0.9998 |
| Limit of quantification (μg/mL) | 0.15 | 0.30 | 0.20 |
| Limit of detection (μg/mL) | 0.044 | 0.095 | 0.064 |
| pkf | 5.50 | 4.25 | 4.80 |
*A=a+bC, where C is the concentration in μg/mL
Table 1: Quantitative parameters for spectrophotometric determination of SUL as ion-pair complexes with BCG, CR, and MO.
| Procedure | Taken (μg/mL) | Within day-(n = 3) | Between days-(n = 3) | ||||
|---|---|---|---|---|---|---|---|
| Recovery % | RSD % | RE % | Recovery % | RSD % | RE % | ||
| Spectrophotometry | |||||||
| MO | 8.0 | 100.44 | 0.11 | 0.86 | 98.00 | 1.054 | -2.00 |
| 10.0 | 99.56 | 0.09 | -0.45 | 97.82 | 1.142 | -2.18 | |
| 12.0 | 99.70 | 0.10 | -0.91 | 97.95 | 0.98 | -2.05 | |
| CR | 8.0 | 99.57 | 0.07 | 0.51 | 99.23 | 1.18 | -0.75 |
| 10.0 | 99.90 | 0.08 | 0.29 | 98.41 | 0.98 | -1.59 | |
| 12.0 | 100.54 | 0.12 | 0.24 | 98.72 | 0.94 | -1.28 | |
| BCG | 8.0 | 100.46 | 0.09 | 0.74 | 98.08 | 1.02 | -1.92 |
| 10.0 | 99.80 | 0.11 | -0.60 | 97.65 | 1.12 | -2.35 | |
| 12.0 | 99.58 | 0.08 | 0.48 | 98.12 | 0.85 | -1.88 | |
| Chromatography | 5.0 | 99.40 | 0.52 | -0.60 | 98.54 | 0.84 | -1.26 |
| 10.0 | 99.83 | 0.43 | -0.17 | 99.26 | 1.07 | -0.74 | |
| 20.0 | 98.86 | 0.63 | -1.14 | 98.22 | 0.55 | -1.78 | |
Table 2: Analytical accuracy and precision of determination of bulk SUL by the described spectrophotometric and chromatographic methods (n=6).
Chromatographic studies
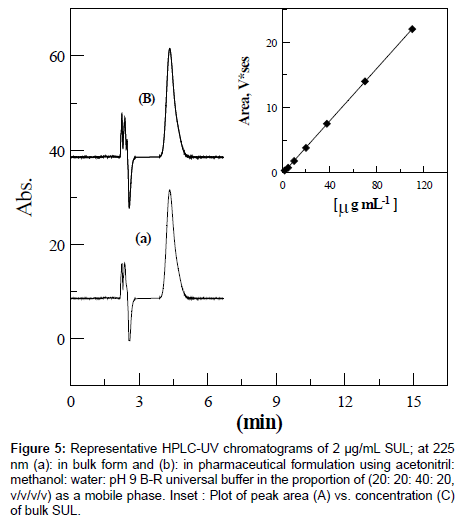
The quantification of SUL was performed using a reversed phase column (Prontosil C18, 250×4.0 mm, 5 μm). A number of variations such as detection wavelength, and nature, proportion and flow rate of the mobile phase were tested to achieve a suitable retention time and a symmetrical peak with a good resolution. The maximum absorption and good chromatographic response of SUL were found at 225 nm which was chosen for the rest of analysis. Several mobile phases of binary or ternary eluents with different buffers of various pH values were examined. However, a mobile phase consisting of acetonitril: methanol : water : pH 9 B-R universal buffer (20: 20: 40: 20, v/v/v/v) was found to be optimum with respect to peak shape, retention time , sensitivity and it was chosen in the rest of this study. Flow rates between 0.5 and 1.5 mL/min were studied and a flow rate of 0.6 mL/min was chosen since a signal-to-noise ratio with reasonable retention time (tr=4.77 min) were obtained (Figure 5a).
Figure 5: Representative HPLC-UV chromatograms of 2 μg/mL SUL; at 225 nm (a): in bulk form and (b): in pharmaceutical formulation using acetonitril: methanol: water: pH 9 B-R universal buffer in the proportion of (20: 20: 40: 20, v/v/v/v) as a mobile phase. Inset : Plot of peak area (A) vs. concentration (C) of bulk SUL.
Methods validation
Linearity, limit of detection and limit of quantitation: The Beer- Lambert law limits, molar absorptivity, Sandell’s sensitivity, regression equations and correlation coefficients obtained by linear square treatment of the spectrophotometric results are given in Table 1. The high molar absorptivities (2.10×104-4.10×104 Lmol-1cm-1) of the formed colored ion-pair complexes indicated high sensitivity of the described ion-pairs extractive spectrophotmetric methods for quantitation of SUL. Limits of detection (LOD) and quantitation (LOQ) of SUL were estimated from the calibration graphs using the expression k S.D./b [38], where k=3 for LOD and 10 for LOQ, S.D. is the standard deviation of the blank (or the intercept of the calibration curve) and b is the slope of the calibration graph. Limits of detections of 0.044, 0.095, and 0.064 μg/mL bulk SUL were achieved by means of the three described BCG, CR, and MO methods, respectively. The results reported in Table 1, indicated the reliability of the described methods for assay of bulk SUL.
For chromatographic analysis, three calibration curves with seven concentration points were constructed for bulk SUL. The method was proven to be linear over SUL concentration range of 0.034 to 110 μg/ mL with a mean correlation coefficient of 0.9996. Figure 5a shows representative chromatograms of 2 μg/mL bulk SUL in solution. A linear calibration graph A (V*s)=0.22 ± 1×10-4C (μg/mL)+0.030 ± 4×10-4, where A and C are the mean peak area and concentration, respectively, was obtained over the working concentration range (Figure 5). LOD and LOQ of 0.00555 and 0.0185 μg/mL SUL, respectively, were achieved by means of the described chromatographic method.
Precision and accuracy: The precision and accuracy of the described HPLC method were examined through intra-day, inter-day, and interlaboratory assays [39]. Accuracy; the closeness of the measured value to the true value, was expressed as percent error (relative error) (RE%), while precision; the degree of agreement among individual test results, was expressed as percentage relative standard deviation (RSD%). The mean percentage recoveries (% R), relative error (RE%), and relative standard deviations (RSD%), Table 2, indicated the high precision and accuracy of the described methods for assay of SUL.
Robustness: In regard to assay robustness [39] of the described spectrophotometric methods, influences of small variation of some of the most important procedural conditions on the recovery and the relative standard deviation of 10 μg/mL bulk SUL were studied. This included the influence of pH (± 0.2), mL reagent added (± 0.2 mL) and reaction time (5-7 min) on the ion-pair formations of SUL with BCG, CR, and MO, respectively. The obtained mean percentage recoveries and relative standard deviations (98.88 ± 0.94 to 98.45 ± 1.88) were insignificantly affected within the studied range of variation of the procedural conditions, and consequently the described spectrophotometric methods were reliable for assay of bulk SUL and they could be considered robust.
Also, the robustness of measurements by means of the described chromatographic method was evaluated by intentional minor modifications in the composition of the constituents of mobile phase (± 2%) and rate of its flow (± 0.02). Practically, insignificant effect was observed in peak area or retention time confirming the robustness of analysis by the described chromatographic method.
Ruggedness: The results obtained by described spectrophotometric methods were found reproducible using UV-Visible spectrophotometer (Jasco, Hachioji-cho, Tokyo, Japan) Lab. (1) and Shimadzu UV-Visible spectrophotometers Model 160A (Kyto, Japan), Lab. (2) (Inter laboratory precision) under the same operational conditions since there were insignificant differences between the recoveries and relative standard deviations (98.69 ± 0.19 to 98.05 ± 0.27).
Interference studies
The interference from common excipients (e.g talc, glucose, starch, sulfate, dextrose, acetate, phosphate, and magnesium stearate) usually present in formulations was examined [39] by means of the described spectrophotometric and chromatographic methods. The mean percentage recoveries (%R) and relative standard deviations (RSD%) obtained by the three described spectrophotometric methods in the absence (98.74 ± 2.66 to 99.06 ± 2.33%) and in the presence of excipients (98.16 ± 2.02 to 98.75 ± 2.25%) indicated insignificant interference from excipients. Since, the formation of the ion–pair complex with the anionic dye requires the presence of a basic functional group in the analyte molecule; therefore no possible interference is likely to occur from co-formulated drugs lacking a basic center.
On the other side, insignificant interference from excipients was found by the described chromatographic method, since recovery of SUL in the absence and in the presence of excipients were 98.48 ± 2.14 and 97.87 ± 2.25, respectively. The results suggested the specificity of the described spectrophotometric and chromatographic methods for assay of SUL. It can be seen that the proposed methods show superior selectivity behavior and exhibit a better linear response range than many of these previously suggested methods (Table 5).
Analysis of dogmatil fort® tablets
The described specrophotometic and chromatographic methods have been successfully applied to the determination of SUL in dogmatil fort® tablets using the calibration curve method (Table 3). Figure 5b shows representative chromatograms of 2 μg/mL SUL in solution of dogmatil fort® tablets which is matching well with that of 2 μg/mL bulk SUL sample (Figure 5a). For further confirmation, the standard addition method was applied to test the reliability and recovery of the described methods. This was carried out by analysis known concentrations of SUL added to a previously analyzed solution of dogmatil fort® tablets. The results obtained by the described spectrophotometric and HPLC methods were statistically compared with those obtained using a reported membrane selective electrode method [22] (Table 3). The calculated student’s t-values and F-values did not exceed the theoretical ones at 95% confidence level [40] indicating no significant difference between the described spectrophotometric and chromatographic and the reported method [22] regarding accuracy, precision and reproducibility.
| Method | Taken μg/mL | Added μg/mL | Found μg/mL | Recovery % ± R.S.D. | F-value t-test |
|---|---|---|---|---|---|
| Spectrophotometric BCG | 3.0 | 2.97 | 99.00 ± 0.90a | 1.65 2.32 | |
| 3.0 | 5.95 | 99.33 ± 0.72b | 1.06 1.93 | ||
| 6.0 | 8.95 | 99.66 ± 0.88b | 1.58 1.14 | ||
| 9.0 | 12.02 | 100.55 ± 0.83b | 0.98 0.40 | ||
| 12.0 | 15.00 | 100.25 ± 1.37b | 3.83 0.05 | ||
| CR | 3.5 | 3.52 | 100.57 ± 0.58a | 1.46 0.59 | |
| 3.5 | 7.06 | 101.14 ± 1.25b | 3.19 1.17 | ||
| 7.0 | 10.55 | 100.43 ± 0.66b | 1.12 0.27 | ||
| 10.5 | 13.98 | 99.62 ± 1.49b | 4.53 0.83 | ||
| 14.0 | 17.50 | 99.86 ± 0.88b | 1.58 0.55 | ||
| MO | 4.0 | 3.96 | 99.50 ± 0.74a | 1.12 1.57 | |
| 4.0 | 7.93 | 99.25 ± 0.68b | 1.06 2.15 | ||
| 8.0 | 11.92 | 99.50 ± 0.91b | 0.59 1.41 | ||
| 12.0 | 16.01 | 100.42 ± 0.43b | 2.78 0.29 | ||
| 16.0 | 20.10 | 100.88 ± 0.72b | 1.06 0.50 | ||
| Chromatography | |||||
| 3.0 | 2.99 | 99.67 ± 0.87a | 1.54 1.80 | ||
| 3.0 | 5.98 | 99.66 ± 0.89b | 1.62 1.30 | ||
| 6.0 | 8.95 | 99.33 ± 0.75b | 0.49 0.51 | ||
| 9.0 | 12.02 | 100.33 ± 0.92b | 1.73 0.03 | ||
| 12.0 | 15.03 | 100.33 ± 1.96b | 0.13 0.03 | ||
| Reported | 100.30 ± 0.70 |
(a) Using calibration curve method & (b) using standard addition method Theoretical F-value=6.6 and t-test=2.45 at 95% confidence limit for n1=4 and n2= 4
Table 3: Determination of SUL in dogmatil fort® tablets (200 mg/tablet) by the described spectrophotometric, and chromatographic methods compared to its determination by a reported membrane selective electrode methods [22].
Analysis of sulpiride in human plasma
The ability of the proposed method to determine SUL in plasma has been appraised through spiking plasma sampleswith the drug at different concentration levels. It was found that SUL could be estimated with good recoveries (Table 4) at the levels of 14-20 μg/mL plasma, thus indicating that there is no interference from endogenous constituents [41].
| Method | Concentration of sulpiride (μg/mL) | Recovery (μg/mL) | Recovery*, % ± R.S.D |
|---|---|---|---|
| Spectrophotometric | |||
| BCG | 14 | 13.92 | 99.43 ± 0.87 |
| 17 | 16.98 | 99.88 ± 0.76 | |
| 20 | 19.96 | 99.80 ± 0.92 | |
| CR | 14 | 13.88 | 99.14 ± 0.80 |
| 17 | 16.90 | 99.41 ± 0.86 | |
| 20 | 19.94 | 99.70 ± 0.85 | |
| MO | 14 | 13.90 | 99.28 ± 0.82 |
| 17 | 16.91 | 99.47 ± 0.66 | |
| 20 | 19.92 | 99.60 ± 0.86 | |
| Chromatography | |||
| BCG | 14 | 13.94 | 99.57 ± 0.79 |
| CR | 17 | 16.93 | 99.59 ± 0.71 |
| MO | 20 | 19.97 | 99.85 ± 0.82 |
*Average of 5 measurements
Table 4: Recovery of sulpiride added to human plasma after extraction using chloroform.
| Methods | Concentration range μg/mL | Literatures |
|---|---|---|
| HPLC and HPTLC | 5 – 60 | [10] |
| Synchronous Fluorescence Spectroscopy | 0.05–1.0 | [41] |
| Electrochemiluminescence | 0.034 – 34.14 | [24] |
| HPLC Spectrophotometry | 0.034 – 110 2 – 16 | The present work |
Table 5: Comparison of linear range for SUL with different classical methods and the present methods.
Conclusion
Three validated extractive spectrophotometric methods were described for determination of SUL in pharmaceutical formulations and plasma as ion pair colored complexes with BCG, CR and MO. Each of the ion-pair complexes was quantitatively extracted in chloroform in one step and was stable for at least 24 h. Besides a simple reversed phase HPLC with UV detection method was also developed. No significant interference from the common excipients was found by the described spectrophotometric and chromatographic methods. The high recovery and low relative standard deviation reflect the high accuracy and precision of the described spectrophotometric and chromatographic methods for assay of SUL. Moreover, the methods are simple, precise, applicable to a wide range of concentration, besides being less time consuming and depending on simple and available reagents thus offering economic and acceptable methods for the routine determination of SUL in pharmaceutical formulations and plasma. The comparative study of the molar absorptivity indicated good sensitivity of the proposed method which follow the order of BCG>MO>CR.
References
- Dawson AH (2004) Cyclic antidepressant drugs. In: Dart RC, Medical Toxicology, 3rd edition, Lippincott Williams & Wilkins, Philadelphia, 834-843.
- Shinozuka T, Terada M, Tanaka E (2006) Solid-phase extraction and analysis of 20 antidepressant drugs in human plasma by LC/MS with SSI method. Forensic Sci Int 162: 108-112.
- Kirchherr H, Kühn-Velten WN (2006) Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: a multi-level, single-sample approach. J Chromatogr B Analyt Technol Biomed Life Sci 843: 100-113.
- Reynolds JEF (1999) (Ed.), Martindale: The Extra Pharmacopoeia. 2nd edition, Royal Pharmaceutical Society of Great Britain, London, 692.
- Kleimola T, Leppänen O, Kanto J, Mäntylä R, Syvälahti E (1976) Spectrophotofluorometric method for quantitative determination of sulpiride in human plasma and urine. Ann Clin Res 8: 104-110.
- Buna M, Aaron JJ, Prognon P, Mahuzier G (1996) Effects of pH and solvent on the fluorescence properties of biomedically important benzamides. Application to determination in drugs and in human urine. Analyst 121: 1551-1556.
- El Walily AF, El Gindy A, Bedair MF (1999) Application of first-derivative UV-spectrophotometry, TLC-densitometry and liquid chromatography for the simultaneous determination of mebeverine hydrochloride and sulpiride. J Pharm Biomed Anal 21: 535-548.
- Shah J, Jan MR, Mabood F (2008) Recovery of value-added products from the catalytic pyrolysis of waste tyre. Energy Conversion and Management 50: 991-994.
- Zayed SIM (2005) Two charge-transfer complex spectrophotometric methods for the determination of sulpiride in pharmaceutical formulations. Cent Eur J Chem 4: 870-875.
- Naguib IA, Abdelkawy M (2010) Development and validation of stability indicating HPLC and HPTLC methods for determination of sulpiride and mebeverine hydrochloride in combination. Eur J Med Chem 45: 3719-3725.
- Frigerio A, Pantarotto C (1977) Quantitative determination of sulpyrid in biological samples from rats by gas-liquid chromatography and chemical ionization-mass fragmentography. J Chromatogr 130: 361-367.
- Bressolle F, Bres J, Brun S, Rechencq E (1979) [Quantitative determination of drugs by in situ spectrophotometry of chromatograms for pharmacokinetic studies. I. Sulpiride and other benzamides, vincamine, naftazone (author's transl)]. J Chromatogr 174: 421-433.
- Alfredsson G, Sedvall G, Wiesel FA (1979) Quantitative analysis of sulpiride in body fluids by high-performance liquid chromatography with fluorescence detection. J Chromatogr 164: 187-193.
- Nicolas P, Fauvelle F, Ennachachibi A, Merdjan H, Petitjean O (1986) Improved determination of sulpiride in plasma by ion-pair liquid chromatography with fluorescence detection. J Chromatogr 381: 393-400.
- Tokunaga H, Kudo K, Jitsufuchi N, Ohtsuka Y, Imamura T (1997) Sensitive determination of sulpiride in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 691: 203-207.
- Huang MC, Ho HO, Yeh GC, Ke WT, Lin LC, et al. (2001) Development of a high-performance liquid chromatographic method for bioanalytical applications with sulpiride. J Chromatogr B Biomed Sci Appl 763: 157-163.
- Chiba R, Ogasawara A, Kubo T, Yamazaki H, Umino M, et al. (2003) Direct determination of benzamides in serum by column-switching high-performance liquid chromatography. Anal Sci 19: 785-789.
- Mizuchi A, Saruta S, Kitagawa N, Miyachi Y (1981) Development of radioimmunoassay for sultopride and sulpiride. Arch Int Pharmacodyn Ther 254: 317-326.
- Cardoso MT, Pradelles P (1982) Preparation of N(1-ethyl-2-pyrrolidyl-methyl)2-methoxy-4-iodo-125I- 5-ethyl sulfonyl benzamide: A radioligand for the radioimmunoassay of sulpiride-related compounds. J Label Comp Radiopharm 19: 1103-1112.
- Farghaly OA (2000) Adsorptive stripping voltammetric determination of the antidepressant drug sulpiride. J Pharm Biomed Anal 23: 783-791.
- Ni YN, Qiu P, Serge K (2003) Determination of Sulpiride by Linear Sweep Stripping Voltammetry at a Mercury Electrode. Chem Res Chin Univ 19: 24-27.
- García MS, Ortuño JA, Albero MI, Abuherba MS (2009) Development of membrane selective electrode for determination of the antipsychotic sulpiride in pharmaceuticals and urine. Sensors (Basel) 9: 4309-4322.
- Liu J, Cao W, Qiu H, Sun X, Yang X, et al. (2002) Determination of sulpiride by capillary electrophoresis with end-column electrogenerated chemiluminescence detection. Clin Chem 48: 1049-1058.
- Li J, Zhao F, Ju H (2006) Simultaneous electrochemiluminescence determination of sulpiride and tiapride by capillary electrophoresis with cyclodextrin additives. J Chromatogr B Analyt Technol Biomed Life Sci 835: 84-89.
- Aly FA, Alarfaj NA, Alwarthan AA (2001) Flow-injection chemiluminometric analysis of some benzamides by their sensitizing effect on the cerium-sulphite reaction. Talanta 54: 715-725.
- Hassib ST, Moussa BA, Hashim HA, El-Zaher AA (2002) Determination of Certain Antispasmodic Drugs as Single Ingredient, Mebeverine Hydrochloride, and in Two Component Mixtures, Mebeverine Hydrochloride–Sulpiride and Isopropamide Iodide–Trifluoperazine Hydrochloride. Spectrosc. Lett. 35: 43-61.
- HMSO (1993) British Pharmacopoeia. Her Majesty’s Stationary Office, Pharmaceutical Press, London, 995.
- Council of Europe (1998) European Pharmacopoeia. (3rdedn), Maisonneuve, Sainte-Ruffine, France, 1566.
- Li XM, Chen ZP, Wang SP, Tang J, Liu CY, et al. (2008) Extractive spectrophotometric determination of TRODAT-1 hydrochloride in lyophilized kit. Pharmazie 63: 638-640.
- Cardoso SG, Ieggli CV, Pomblum SC (2007) Spectrophotometric determination of carvedilol in pharmaceutical formulations through charge-transfer and ion-pair complexation reactions. Pharmazie 62: 34-37.
- Onal A, Kepekçi SE, Oztunç A (2005) Spectrophotometric methods for the determination of the antidepressant drug paroxetine hydrochloride in tablets. J AOAC Int 88: 490-495.
- Al-Ghannam SM (2006) A simple spectrophotometric method for the determination of beta-blockers in dosage forms. J Pharm Biomed Anal 40: 151-156.
- Ramesh KC, Gowda BG, Melwanki MB, Seetharamappa J, Keshavayya J (2001) Extractive spectrophotometric determination of antiallergic drugs in pharmaceutical formulations using bromopyrogallol red and bromothymol blue. Anal Sci 17: 1101-1103.
- Jandera P, Churacek J (1980) Reversed-phase liquid chromatography of aromatic sulphonic acids and other strongly polar compounds without addition of an ion-pairing counter-ion. J Chromatogr A 197: 181-187.
- Kubo T, Kuroda K, Tominaga Y, Naito T, Sueyoshi K, et al. (2014) Effective determination of a pharmaceutical, sulpiride, in river water by online SPE-LC-MS using a molecularly imprinted polymer as a preconcentration medium. J Pharm Biomed Anal 89: 111-117.
- Britton HTS (1952) Hydrogen Ions. 4th edition, Chapman & Hall, London, 113.
- Christian GD, Reilly JEO (1986) Instrumental Analysis. (2ndedn), Prentice Hall, Englewood Cliffs, NJ, 186.
- Miller JC (1992) Statistics in Analytical Chemistry. (2ndedn), Ellis Horwood, Chichester, 115.
- The USA Pharmacopoeia (2003) The National Formulary Convention Inc, USP 26, 2446.
- Christian GD (1994) Analytical Chemistry. (5thedn), John Wiley & Sons Inc., USA, 36.
- Abdelal A, El-Enany N, Belal F (2009) Simultaneous determination of sulpiride and its alkaline degradation product by second derivative synchronous fluorescence spectroscopy. Talanta 15: 880-888.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15651
- [From(publication date):
May-2014 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 10885
- PDF downloads : 4766