Development and Validation of LC-MS/MS Method for Determination of an Antimigraine Naratriptan HCl in Human Plasma: An Application to a Pharmacokinetic Study
Received: 26-Feb-2020 / Accepted Date: 10-Mar-2020 / Published Date: 18-Mar-2020 DOI: 10.4172/2155-9872.1000421
Abstract
A rapid sensitive and validated liquid chromatography/tandem mass spectrometry (LC-MS/MS) method is developed for determination of an antimigraine drug naratriptan (NAR) in human plasma using (alomtriptan) as internal standard (IS). a Liquid-liquid extraction with diethyl ether was used. The chromatographic separation was carried out using reversed phase C8 analytical Column (5 μm, 50 mm × 4.6 mm i.d.) with a simple isocratic mobile phase composed of methanol : 0.02 M ammonium formate (pH 3.5) (40:60 v/v) and flow rate 0.6 mLmin-1. Detection was performed on a triple quadrupole mass spectrometer employing electrospray ionization (ESI) technique, operating in multiple reaction monitoring (MRM), with the transitions of 336.3 → 98.2 and 336.3 → 201.0 m/z for NAR and IS, respectively in the positive ion mode. The analysis was carried out within 2.4 min over a linear concentration range of 0.05-20 ngmL-1. The method was validated according to the FDA bio-analytical method validation guidance for industry. The developed LC-MS/MS method was successfully applied to a pharmacokinetic study of the studied drug after being orally administered by Egyptian healthy volunteers.
Keywords: Formigran; Naratriptan HCl; Mass spectrometry; Pharmacokinetics; Bio-analytical Validation ; Liquid-Liquid extraction; Antimigraine
Introduction
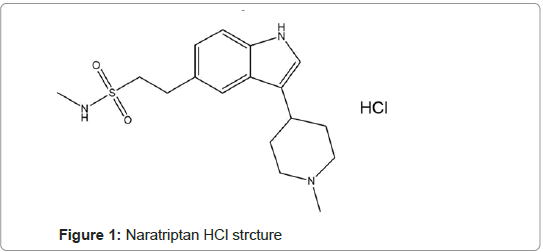
Naratriptan hydrochloride(NAR)[N-Methyl-3-(1-methyl- 4-piperidinyl)-1H-indole-5-ethanesulfonamide monohydrochloride] [1,2] (Figure 1). It is a white to pale yellow solid soluble in water. NAR is a selective serotonin (5-HT1) agonist; it is used for the acute treatment of the headache phase of migraine attacks. It should not be used for prophylaxis. It is given orally as the hydrochloride salt and doses are expressed in terms of the base; NAR HCL 1.11 mg is equivalent to about 1 mg of NAR base [3,4]. The recommended dose of NAR in the UK is 2.5 mg, and in the USA it is 1 or 2.5 mg [3].
The USP describes high-performance liquid chromatographic method with UV detection for determination of NAR [2]. Reviewing the literature has demonstrated some reported analytical techniques for the determination of NAR including voltammetry [5], spectrophotometry [6-9], densitometry [10], high-performance liquid chromatography (HPLC) with UV detection [11], spectrofluorimetry [12].
The key characteristic of (LC- MS/MS) is its high sensitivity and selectivity. Mass spectrometry can achieve limits of detection several orders of magnitude lower than those of most other techniques. Because of the low detection limits, Mass spectrometry is widely used for quantification of trace constituents in biological and environmental samples [13]. Surveying the literature has revealed different LC-MS/MS methods published for determination of NAR in biological fluids [14-17]. By studying these methods for their application to the analysis of NAR in biological fluids, it was found that one method was applied to rabbit plasma [14], another one was applied to human serum [15] other methods were applied to human plasma , but they suffer a serious drawback [16,17]. The mobile phase utilized by methods [16,17] contained high organic phase ratio that resulted in severe ion suppression effect on NAR peak when applied to human plasma matrix obtained from local source [Egypt], which affected the response and accuracy of the results. Also it was difficult to obtain isopods to be used as internal standard. So in our proposed method we tried to use different members of the triptan family (almotriptan and zolmitriptan ) due to their structure similarity to NAR. We found that both gave good recovery and response but almotriptan gave more accurate and precise results than zolmitriptan. All the above mentioned factors motivated us to study and validate our proposed method to achieve more reliable and applicable method for determination of NAR in human plasma.
Experimental
Instruments
Chromatographic analysis was performed using (Exion LC, USA) HPLC system equipped with ABDXR5370003, ABDXR5370002 pump, ABCXR5370001 autosampler, AB2CT5370010 Column oven, ABCBM5370044 Controler and ABDG5370039 Degaser. Mass spectrometric detection was carried out using a triple quadrupole API 4500 (ABSciex, Canada) operated in postive electrospray ionization and multiple reaction monitoring (MRM) mode. Hardware control, data acquisition and treatment were carried out using (Analyst® 1.6.3.) Software (ABSciex, Canada). Licensed and validated (kinetica® 5.1 SP1) software was applied for all pharmacokinetic (PK) calculations, and data plotting.
A Vcuume concentrator Eppendorf (Memmert, Germany). Cooling Centrifuge, model (K 2015R, Centurion scientific, UK). Deep freezer, model (NU-9483E), NuAire laboratory, USA. Multi vortex (VWR Scientific, USA). pH-meter, model (Jenway 3510), Jenway, UK, calibration was done daily using standardized buffers of different pH- values (4.00-10.00). Water Purification System, model Lab Conco, USA. Ohaus Analytical balance, Model (Discovery DV215CD).
Materials
Naratriptan. HCl (NAR), was purchased from SIGMA pharmaceutical industries (Osmopharm, Swittazerland), its percent purity was certified to be 99.6%, whereas Almotriptan Malate (IS) was obtained from SMS pharmaceutical limited, India. Formigran®2.5 mg Film Coated Tablets produced by GlaxoSmithKline Consumer Healthcare GmbH & Co.KG, Germany. Human blank plasma was obtained from the holding company for biological products and vaccines (VACSERA), Egypt. All other chemicals and solvents were of HPLC grade and were obtained from sigma aldrich (Germany).
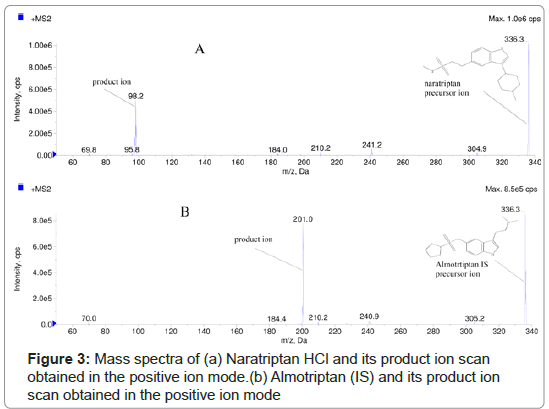
Liquid chromatographic and mass spectrometric conditions: Separations were carried out using a phenomenix kinetex C8 column (5 μm, 4.6 × 50.0 mm) and a mobile phase of methanol : 0.02 M ammonium formate (pH 3.5) (40:60 v/v). Isocratic elution with a flow rate of 0.60 mLmin-1 for 2.4 min and injection volumes of 3.0 μL were employed. A mixture standard solution of NAR and IS (50.00 ngmL- 1 each in methanol) was directly infused into the mass spectrometer, and the operating conditions were optimized. The following transitions 336.3>>98.2 and 336.3>>201.0 m/z were used to monitor NAR and IS, respectively, as summarized in Table 1. The nebulizer gas was air (zero grade), whereas nitrogen was used as the auxiliary curtain and collision gas. The source gas-dependent parameters for NAR determination were as follows: curtain gas, 20 psi; collision gas, 8 psi; medium temperature, 500°C; ion spray voltage, 2000 V; ion source gas one 45 and gas two, 30 psi.
| Analyte | Q1a (m/z) | Q3b (m/z) | DPc (V) | EPd (V) | CEe (V) | CEPf (V) |
|---|---|---|---|---|---|---|
| Naratriptan | 336.3 | 98.2 | 80 | 10 | 30 | 9 |
| Almotriptan IS | 336.3 | 201 | 67.8 | 10 | 23.1 | 19 |
a. Q1, precursor ion b. Q3, product ion
c. DP, declustering potential d. EP, entrance potential
e. CE, collision energy f. CEP, cell exit potential
Table 1: LC-MS/MS parameters selected for the quantification of Naratriptan hydrochloride and ALmotriptan internal standard.
Procedures
a. Preparation of Calibration Standards and Quality Control (QC) samples: Stock solutions of NAR and IS (100.00 μgmL-1 each) were prepared in methanol and stored at 2-8°C. Spiked calibration standards were prepared using blank plasma spiked with NAR at a concentration range of 0.05-20.00 ngmL-1. Quality control samples of NAR were prepared in blank plasma at five levels:LLOQ (0.05 ngmL-1 ) low QC (0.15 ngmL-1), medium QC-A (2 ngmL-1), medium QC-B (6 ngmL-1), and high QC (15 ngmL-1). All prepared samples were stored in aliquots of 3.0 mL plasma at ≤ -70°C until analysis.
b.Plasma Sample preparation: Plasma samples were stored frozen at ≤ -70°C, and all analytical procedures were carried out at room temperature. For the determination of NAR, aliquots of 0.50 mL plasma were spiked with 50 μL of 200.00 ngmL-1 IS and 50 ul of (amonia solution 0.3% ) then vortex mixed for 10 Sec. liquid liquid extraction was then carried out by adding 3.50 mL Diethyl ether followed by vortex mixing at 2000 rpm for 3 min, and samples were centrifuged at 4000 rpm (1789 × g) at 5°C for another 10 min, 3 ml of The upper clear layer was carefully separated, evaporated under vacuum at 45°C for 30 min then reconstituted with 300 μL (methanol:water 7:3 v/v). The resulted solution was vortex mixed and 3 μL was injected into the LC-MS/MS system.
c.Bio-analitical method validation: Validation of the proposed method was carried out according to the FDA bio-analytical method validation guidance for industry (may 2018) and EMA guideline on bio-analytical method validation [18,19] for the bio-analytical method validation, the following parameters were calculated: selectivity, linearity and range, accuracy, precision, recovery, matrix effect, dilution integrity and stability. The validation was further extended to the analysis of incurred samples and incurred sampls reanalysis.
d.Application to volunteers: This study was approved by the ethics committee of (zi-diligence Research Center, Cairo, Egypt ), and a written informed consent was obtained. An oral dose equivalent to 2.5 mg NAR was administered to six healthy male volunteers after fasting for 8 h. Fasting of the volunteers has removed the possible interaction from food or caffeine consumption. Plasma samples were withdrawn from a forearm vein at zero time, 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, 12.0, 18.0 and 24.0 h into heparinized tubes. Samples were prepared as described under 2.4.2. Sample preparation, and the concentrations of NAR were determined. The plasma concentration- time curves were constructed for NAR, and various pharmacokinetic parameters were calculated.
Results and Discussion
Development of Liquid chromatography and mass spectrometric conditions
Coupling of LC with tandem MS/MS detection is a highly selective technique that results in minimal interference from endogenous impurities. This could be explained on the basis that in the MRM mode, only the ions derived from the target analytes are monitored. Initially, several trials using different columns (C18 kinitex, Phenomenex and C8 kinitex, phenomenex) and mobile phase compositions using methanol and different ratios of acetonitrile (70, 60, 50 and 40% ) were studied in order to optimize the chromatographic separation conditions from matrix and ion supperssion effect.
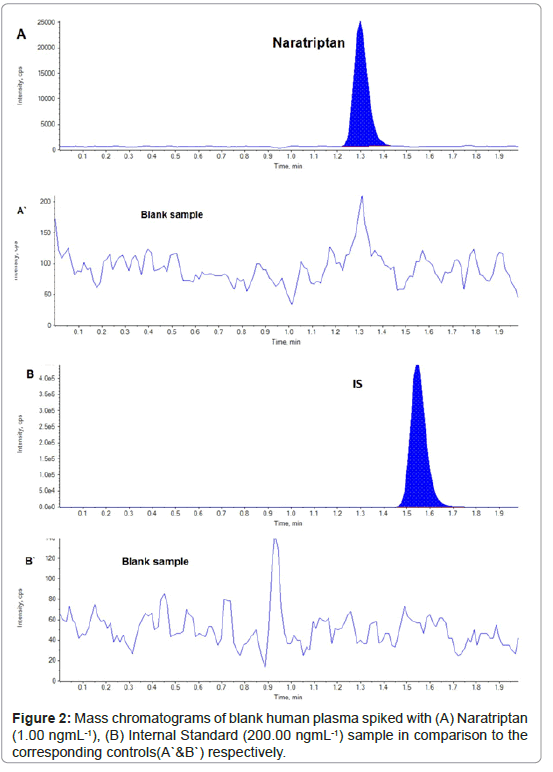
Optimum performance was obtained using phenomenex kinetex C8 column and a mobile phase consisting of methanol : 0.02 M ammonium formate (pH 3.5) (40:60 v/v) with a flow rate of 0.6 ml min-1 (Figure 2). The protonated precursor ions [M+H]+of NAR, were detected in the full scan mass spectra at m/z 336.3 (Figure 3). The collision energy was optimized, and the following MS/MS transitions were selected 336.3 to 98.3 and 336.3 to 201.0 for the determination of NAR and IS, respectively, as summarized in Table 1.
Development of sample preparation techniques
Initially, various sample preparation techniques were studied such as liquid-liquid extraction using diethyl ether, tert-butyl methyl ether, ethyl acetat, N-hexan and dichloromethane. Protein precipitation was also employed using methanol and acetonitrile with neutral and acidified or alkalinized sample in each solvent. Results of this study indicated that liquid liquid extraction using diethyl ether after alkalinization of plasma sample wih 0.3% ammonia solution followed by centrifugation and evaporation under vacume then reconistitution with (methanol:water 7:3 v/v) was the optimum approach in terms of simplicity, cost and recovery of the studied drugs. Extraction of NAR from alkalinized plasma samples gave the best results due to the fact that the predominant species of NAR in alkaline medium is the nonpolar form which is highly soluble in diethyl ether, while in acidic medium the predominant form of NAR is the ionic protonated form with lower solubility in the extracting organic solvent.
Bio-analytical method validation
Selectivity: Results obtained from the analysis of six batches of blank plasma in addition to three batches one of them is heamolysed, the other is lipemic and the third one is spiked with general co- adminstered drugs as (triptans, paracetamol, pseudoephedrine, ketoprofen, Caffeine and chlorpheniramine maleate) indicated the absence of endogenous interference with the studied drugs and IS. Representative chromatograms showing the results of analysis of blank plasma are shown in Figure 2. This confirmed the high selectivity of the proposed assay towards the studied drugs in the presence of matrix components.
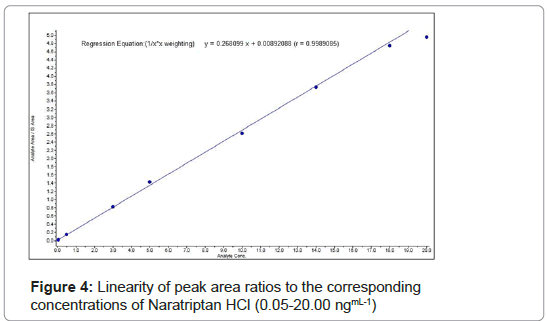
Linearity and lower limit of quantitation: Linearity was evaluated using the determinations at eight concentration levels, covering the range of 0.05-20.00 ngmL-1 for NAR. Figure 4 shows Linearity of peak area ratios to the corresponding concentrations of Naratriptan HCl. Linear relationships were obtained between peak area ratios of NAR to the internal standard and corresponding concentrations of NAR. Blank and zero samples were included in the analysis in order to verify the absence of interference. The LLOQ was determined as the lowest concentration of analyte that could be quantitatively determined with acceptable precision , where coefficient of variation (CV.% )<20% and accuracy ( 80%-120%) and was included while studying various assay validation parameters.
Accuracy, precision and recovery: Analysis of the spiked plasma samples at five concentration levels (LLOQ, low QC, medium QC-A, medium QC-B and high QC). Within run accuracy has varied between 97.20-104.50% with a precision (CV.% ) in the range of 4.17%-12.74%, while between run accuracy has varied between 99.43%-102.31% with (CV.% ) in the range of 5.51%-13.70%. Obtained results presented in Table 2 clearly shows that the proposed assay possesses adequate accuracy and precision.
| Within run | Between run | |
|---|---|---|
| NAR | NAR | |
| Mean Accuracy% ± CV* | ||
| LLOQ | 103.82 ± 12.74 | 99.59 ± 13.7 |
| Low QC | 97.20 ± 8.09 | 99.43 ± 8.83 |
| Medium QC-A | 102.20 ± 5.81 | 101.43 ± 6.83 |
| Medium QC-B | 101.14 ± 6.3 | 101.58 ± 5.51 |
| High QC | 104.50 ± 4.17 | 102.31 ± 5.95 |
| N | 6 | 18 |
| *Mean percentage Accuracy and RSD were calculated using six determinations | ||
Table 2: Accuracy, precision and recovery for the determination of Naratriptan hydrochloride by the proposed LC-MS/MS method.
Recovery: Liquid-liquid extraction using diethyl ether was found to be the optimum approach to achieve consistent recovery of analyte and IS. The recoveries of NAR and IS were measured at four QC levels in six replicates as shown in Table 3.
| Within run | ||
|---|---|---|
| NAR | IS | |
| Low QC | 78.2 ± 4.63 | 64.2 ± 2.63 |
| Medium QC-A | 76.2 ± 3.83 | 63.62 ± 3.03 |
| Medium QC-B | 72.06 ± 3.64 | 65.06 ± 2.46 |
| High QC | 77.62 ± 8.17 | 62.62 ± 4.17 |
| N | 6 | 6 |
Mean percentage recovery were calculated using six determinations.
Table 3: Recovery for the determination of Naratriptan hydrochloride by the proposed LC-MS/MS method.
Matrix effect: The matrix effect was investigated in order to reveal possible ionization suppression or enhancement caused by matrix components. The matrix effect was examined, and the mean peak areas of NAR and IS in the two QC leveles (low QC and high QC) prepared in extracted plasma were compared to those obtained from analysis of neat standard solutions of equivalent concentrations. Normalized factor (CV.% ) was calculated. Satisfactory results within the stated limits were obtained for Mean percentage recoveries and for normalized factor (CV.% ) as summarized in Table 4.
| Naratriptan (MF)* | IS (MF)* | NF* CV% | |
|---|---|---|---|
| Low QC | 107.0 ± 6.0 | 101.0 ± 2.0 | 4.17 |
| High QC | 103.0 ± 4.0 | 105.0± 6.0 | 4.78 |
| N | 8 | 8 | 8 |
*Mean percentage recovery and CV were calculated using eight determinations.
MF (Matrix Factor). NF(Internal standard Normalized Factor)
Table 4: Matrix effect for the determination of Naratriptan hydrochloride by the proposed LC-MS/MS method.
Stability: According to FDA bio-analytical method validation guidance for industry (may 2018), the following are considered: (i) conditions used in stability experiments should reflect situations likely to be encountered during actual sample handling and analysis, (ii) samples should be prepared from a freshly made stock solution of the analyte in the appropriate analyte-free, interference-free biological matrix. Initially, stock solution stability during bench top storage at room temperature for 12 h and 1 week storage at 2°C-8°C was assessed. The response obtained using the LC-MS/MS assay was compared to that of the freshly prepared solution and demonstrated adequate stability of the stock solution. Results confirmed that three freeze-thaw cycles of the (Low QC and high QC) samples did not affect the quantification of NAR. Thawing of the frozen samples and keeping them at room temperature for short term stability assesment 24 h did not result in any notable degradation of the analytes. The QC samples were stored frozen at (-70°C) and remained stable for at least 160 days of long- term stability. The extracted samples had been analysed also after storage in the auto-sampler (15°C) for at least 24 h. the dry extract after evaporation of the extraction solvent were kept at room temperature for 24 h. . Results presented in Table 5 prove that That NAR is stable in dry extract form for 24 h. All stability samples concentrations were calculated using freshly prepared calibration curve and QCs. All mean% nominal values of the analyte were found to be within ± 15% of the predicted concentrations at their low and high QC levels. Results summarized in Table 5 indicate that human plasma samples containing NAR can be handled under normal laboratory conditions without any significant degradation of the studied drugs.
| Naratriptan | ||
|---|---|---|
| LowQC (n=6) | HighQC (n=6) | |
| Freez and thaw stability | ||
| Mean Recovery% ± RSD* | 99.17 ± 2.18 | 101.46 ± 1.18 |
| Benchtop stability | ||
| Mean Recovery% ± RSD* | 99.42 ± 2.69 | 102.52 ± 4.38 |
| Dry extract stability | ||
| Mean Recovery% ± RSD* | 99.00 ±4.69 | 102.92 ± 4.51 |
| Long term stability | ||
| Mean Recovery% ± RSD* | 100.33 ± 3.06 | 99.79 ± 2.40 |
| Processed sample stability | ||
| Mean Recovery% ± RSD* | 98.50 ± 2.59 | 101.42 ± 3.30 |
*Mean percentage recovery and RSD were calculated using three determinations
Table 5: Summary of stability data of Naratriptan hydrochloride in human plasma by the proposed LC-MS/MS method.
Dilution integrity: by spiking the matrix with an analyte concentration above the ULOQ (factor 2 QC High *2 And factor 4=QC High *4) and diluting this sample with blank matrix to reach the concentration of High QC (6 replicates determinations per dilution factor ). Accuracy and precision should be within the set criteria, i.e. within ±15%.
Application to volunteers
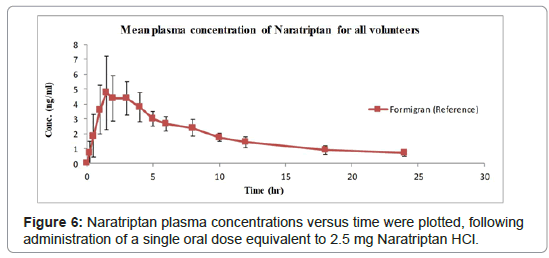
The proposed LC-MS/MS method was successfully applied to study phrmacokinitecs parameters of NAR after an oral dose equivalent to 2.5 mg was administered by six healthy male volunteers (Figure 5 ). The study was conducted according to FDA bio-analytical method validation guidance for industry (may 2018) [18]. The pharmacokinetic parameters results of six volunteers showed that The maximum plasma concentration (Cmax) for NAR was 5.53 ± 1.86 ng/mL and it was achieved at (Tmax ) 2.25 ± 1.07 h. The elimination half-life (T1/2) for NAR was found to be 8.8 ± 2.02 h, as summerized in Table 6. Mean plasma concentration-time curve for NAR is shown in Figure 6 (Scientific TF Licensed and validated Kinetica® 5.1 SP1 software was applied for all pharmacokinetic (PK) calculations, and data plotting, Thermo Fisher Scientific; 2018).
| Parameters | naratriptan |
|---|---|
| C max (ng/ml) | 5.53 ± 1.86 |
| T max (h) | 2.25 ± 1.07 |
| T1/2 (h) | 8.87 ± 2.02 |
| AUC 0-tng h /ml | 44.81 ± 8.12 |
| AUC 0-∞ng h /ml | 53.25 ± 10.85 |
| Kel (h-1) | 0.08 ± 0.02 |
The values Cmax and Tmax were obtained directly from the concentration versus time curve of naratriptan.
The terminal elimination rate constant (Kel or λz) was estimated for naratriptan and for each treatment via linear regression of the last points (at least three points will be used) at the terminal phase of the log-concentration versus time curve of naratriptan.
Thalf will be calculated from 0.693/ λz. AUC0-t was calculated by trapezoidal rule .
AUCt-∞ (Extrapolated Area, AUCExtrapolated) which is the Area under the plasma concentration-time curve from t-∞, calculated as Clast/λz. It is also called residual Area (AUCResidual) or tail Area (AUCtail).
AUC0-∞ was calculated from the sum of AUC0-t and AUCt.
Table 6: Results of Pharmacokinetic parameters of Naratriptan hydrochloride (result ± SD) by the proposed LC-MS/MS method
Incurred sample re-analysis (ISR): Sample matrix is largely the same in both QC samples and incurred samples. However, QC samples do not contain various drug metabolites, isomers and co-administered drugs along with their metabolites. In this study, incurred sample re-analysis was carried out using incurred samples as well as plasma samples collected from a volunteer at different time intervals around Cmax and around elimination phase. The percent difference between the initial concentration and the concentration measured during the repeat analysis should not be greater than 20% of their mean for at least Two-thirds (67% ) of the repeated sample results according to the following equation [19].
Equation 1: % difference= [(Repeat value–Original value)/mean value] *100
In this study, incurred sample stability was investigated in order to verify the stability of the target analytes in real sample matrix and thus to demonstrate the validity of the assay. Analysis results of incurred plasma samples, when compared to an original sample that was analysed in the beginning of analysis is presented in Table 7. Results clearly indicated that the developed assay is valid for the determination of NAR in incurred plasma samples.
| Incurred samples reanalysis | Initial sample conc. | Reanalysis samples conc. | %differance | Pass/failed |
|---|---|---|---|---|
| (ngmL-1) | (ngmL-1) | |||
| Around cmax | 3.380 | 3.231 | 4.508 | pass |
| 4.180 | 4.513 | 7.661 | pass | |
| 3.300 | 3.151 | 4.619 | pass | |
| 5.394 | 4.983 | 7.921 | pass | |
| 2.540 | 2.763 | 8.410 | pass | |
| 5.720 | 5.435 | 5.110 | pass | |
| Around elimination | 0.832 | 0.798 | 4.17 | pass |
| 1.001 | 1.122 | 11.40 | pass | |
| 0.883 | 0.712 | 21.44 | failed | |
| 0.101 | 0.085 | 17.20 | pass | |
| 0.585 | 0.431 | 20.35 | Failed | |
| 0.210 | 0.245 | 15.35 | pass | |
| Total no. of ISR | 12 | |||
| No of passed* samples | 10 | |||
| ISR % | 83% | |||
| Passed samples have˂20% difference | ||||
Table 7: Incurred sample re-analysis for the determination of Naratriptan hydrochloride in human plasma samples by the proposed LC-MS/MS method.
Conclusion
A fast and accurate LC-MS/MS assay is developed and validated for determination of NAR in human plasma. A liquid-liquid extraction procedure is employed for sample preparation. Results of the validation studies shows that the developed assay is selective, accurate and precise over a concentration range that covers the Cmax of the drug. Results also confirmed appropriate extraction recovery and lack of matrix interference with the determination. Application to incurred samples confirmed the superior performance of the extraction method. This could be attributed to the ability of the suggested sample preparation protocol to maintain the integrity of the studied drugs. Integrating incurred sample re-analysis and incurred sample stability into the assay validation protocol should help ensure the validity of the results obtained from bioequivalence studies as well as clinical investigations employed in this assay.
References
- Moffat AC, Osselton MD, Widdop B, Watts J (2011) Clarke's analysis of drugs and poisons in pharmaceutical , body fluids and postmortem material (4th Edn), Pharmaceutical press, London, UK.
- United States Pharmacopeial Convention (2017) The United States Pharmacopia 40-NF 35. North Bethesda, Maryland, United States.
- Sweetman SC (2009) Martindale the complete drug reference (36th Edn), pharmaceutical press, london,UK.
- Moffat AC, Osselton MD, Widdop B, Watts J (2011) Clarke's Analysis of Drugs and Poisons. London: Pharmaceutical press, UK.
- Velasco-Aguirre C, Ãlvarez-Lueje A (2010) Voltammetric behavior of naratriptan and its determination in tablets. Talanta 82:796-802.
- Shelke S, Shahi S, Patil V, Kale S (2015) Development and validation of UV spectrophotometric method of Naratriptan Hydrochloride in bulk and pharmaceutical formulation. Asian J Biomed Pharm Sci 5(47):36-39.
- . Kumara SG, Kumar JM, Kumar UA (2011) Spectrophometric determination of naratriptan hydrochloride in bulk and pharmaceutical dosage form. IAJPR 1:253-256.
- .Borse JS, Shirkhedkar AA (2012) Estimation of Naratriptan Hydrochloride in Bulk and Formulation by First Order Derivative UV-Spectrophotometric Methods. J Appl Pharm Sci 6:227-229.
- Reddy RBD, Charitha A, Sowjanya GN, Kumar GS, Gananadhamu S, et al. (2013) Spectrophotometric estimation of naratriptan hydrochloride with iron reagents. J Gbl Trd Pharm Sci 4:1107-1110.
- Prajapati PB, Chotalia J, Bodiwala KB, Marolia BP, Shah SA (2016) Development and validation of stability-indicating HPTLC method for estimation of Naratriptan Hydrochloride in its pharmaceutical dosage form and its content uniformity testing. J Chromatogr Sci 54:1129-36.
- Ramu G, Babu AB, Kumar MS, Rambabu C (2012) Assay of naratriptan hydrochloride in pharmaceutical formulations by RP-HPLC method. J Pharm Res 5:2627-2630.
- Rizk M, Sultan M, Elshahed M, Ali M (2018) Development of spectrofluorimetric stability indicating method for determination of naratriptan hydrochloride in pharmaceutical dosage form. Eur J Chem 9:251-7.
- Watson JT, Sparkman OD (2007) Introduction to mass spectrometry: Instrumentation, applications, and strategies for data interpretation. John Wiley & Sons, Chichester, England.
- Dulery BD, Petty MA, Schoun J, David M, Huebert ND (1997) A method using a liquid chromatographic-electrospray-mass spectrometric assay for the determination of antimigraine compounds preliminary pharmacokinetics of MDL 74,721, sumatriptan and naratriptan in rabbit. J Anal Pharm Biomed Sci 15:1009-20.
- Vishwanathan K, Bartlett MG, Stewart JT (2000) Determination of antimigraine compounds rizatriptan, zolmitriptan, naratriptan and sumatriptan in human serum by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 14:168-72.
- Yadav M, Patel C, Patel M, Mishra T, Singhal P, et al. (2011) Development and validation of a sensitive and rapid method to determine naratriptan in human plasma by LC-ESI-MS-MS: application to a bioequivalence study. J Chromatogr Sci 49:101-7.
- Challa BR, Awen BZS, Chandu BR, Shaik RP (2011) Method development and validation for naratriptan determination in human plasma by HPLC with tandem mass spectrometry detection, and its application to bioequivalence study. Braz J Pharm Sci 47:13-22.
- U. S. Department of Health Human Services, Food and Drug Administration (2018) Bioanalytical Method Validation, Guidance for Industry.
- European Medicines A (2011) Guideline on bioanalytical method validation. Committee for Medicinal Products for Human Use (EMEA/CHMP/ EWP/192217/2009).
Citation: Ali M, Rizk M, Sultan MA, Elshahed MS (2020) Development and Validation of LC-MS/MS Method for Determination of an Naratriptan HCl in Human Plasma: An Application to a Pharmacokinetic Study. J Anal Bioanal Tech 11: 421. DOI: 10.4172/2155-9872.1000421
Copyright: © 2020 Ali M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3702
- [From(publication date): 0-2020 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 2813
- PDF downloads: 889