Development of Novel Folate Conjugated Nanoparticles Based Skin Lotion for Skin Cancer Treatment: In vitro Characterization and in vivo Study
Received: 22-Oct-2021 / Accepted Date: 05-Nov-2021 / Published Date: 12-Nov-2021
Abstract
Quercetin a natural flavonoid has been extensively investigated for its anti-cancer activity. However, the major disadvantage remained its poor aqueous solubility. In the present research, we investigated the efficacy of folate conjugated quercetin nanoparticles in vitro and further formulated its lotion and studied its skin permeation ability with in vivo activity. The in vitro cellular uptake and antiproliferative potentials of folate conjugated quercetin nanoparticles was evaluated using HaCaT, KB and A431 cell lines. The results showed robust cellular uptake of folate conjugated quercetin nanoparticles by cell lines. ROS study and intracellular GSH study revealed that folate conjugated QuNPs remarkably reduces the intracellular reactive oxygen species generation and release of GSH. Cells viability studies showed that folate conjugated quercetin nanoparticles were found to exert profound effect at a concentration of 20 μM on all three cell lines as compared to non-conjugated quercetin nanoparticles, in a shorter span of time. The skin permeability of the formulation was compared with the marketed drug and was estimated by CLSM study. In vivo studies supported the in vitro activity of folate conjugated quercetin nanoparticles by reducing the epidermal hyperplasia, inflammation and lesion formations.
Keywords: HaCaT cell lines; KB-cell lines; A431 cell lines; Reactive oxygen species; Intracellular GSH; CLSM
Introduction
Cancer is a progressive cellular disorder. It is characterized by uncontrolled cell growth and proliferation [1]. Various remedies have been developed in due course of time to fight against this disease but, the lack of specificity has still kept the appropriate drug, out of site. Different natural bio actives possess anticancer property and hence are under investigation too [2]. Quercetin, one such polyphenol flavonoid, has shown several biological effects like anti-inflammatory, anticancer, antiproliferative, antimutagenic and apoptosis induction but its use is limited due to its low aqueous solubility [3,4]. The aim of our research was to design and develop such a Nano-formulation which can overcome the limitation of poor solubility profile of the drug and can improve cytotoxicity.
In order to achieve this, we have conjugated the nanoparticles of quercetin with folic acid by applying EDC-NHS chemistry. Folic acid is a vitamin involved in various cellular metabolic pathways. It is an essential element of the nucleotide biosynthesis. Various researches have proved that the entry of folic acid is governed by receptor mediated endocytosis [5]. The prominent feature of this process is that the internalization of folate conjugates is free of size specificity that means, they are ideal carriers for small molecules delivery. Further, Folate receptor is a glycopoly peptide membrane anchored protein derivative having high affinity towards folic acid also known as folate binding protein. These are highly expressed on like ovarian, lung, breast, kidney, brain, endometrial and colon cancer cells. This folate receptor on cellular uptake of folic acid undergoes cellular endocytosis by stimulating clathrin-independent pathway (polocytosis) which is a characteristic for macromolecule drug delivery [6]. Thus, the major goal of our research was to develop such a delivery system of quercetin that can deliver the drug specifically at the site of action.
Materials and Methods
Quercetin was purchased from Sigma Aldrich, USA, Poly (lactideco- glycol ide) copolymer (PLGA) (Mw ~ 17 000-22000,50:50) was obtained with thanks from Resomer, Evoniks, Germany, Folate, N-(3-dimethylaminopropyl)-N’-Ethylcarbodiimide (EDC) and N-Hydroxysuccinimide (NHS) was purchased from Sigma-Aldrich, USA and D(+)Mannose was purchased from HIMedia. Phosphate buffered saline-PBS (pH 5.5 and pH 5.6) used for drug release. All other reagents used were of analytical grade.
For cell culture chemicals
Quercetin was purchased from Sigma, USA. All solvents were HPLC grade. Water was purified using a Millipore Super Q water system. All the three cell lines were obtained from the National Center for Cell Science, Pune, India. 3-(4,5-Dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Aldrich Ltd. (India), HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 HAM (DMEM F-12 HAM) with 2 mM L-glutamine supplemented with 10% fetal bovine serum (FBS), 45 IU/ml penicillin and 45 IU/ml streptomycin (HaCaT media) at 37°C in 5% CO2. A commercial kit ThiolTracker™ Violet, Invitrogen, India. For A431 cells were cultured in DMEM supplemented with 10% heat inactivated calf serum, 100 U of penicillin G and 100 U of streptomycin sulfate under 5% CO2 and 95% humidified atmospheric air All the experiments on animals were also performed after taking approval from Institutional Animal Ethical committee.
Synthesis of QuNP
Modified Nano precipitation technique was employed to prepare quercetin nanoparticles. Briefly, 50 mg of PLGA and 20 mg of quercetin was dissolved in acetone and injected in Millipore distilled water and was subjected to sonication with additional volume of water Then the organic phase was removed by using Rota evaporator (IKA® RB 10) at 37°C under reduced pressure and then it was centrifuged repeatedly (Remi, C-24 BC) at 15000 rpm for 30 minutes and finally lyophilized (Thermo fisher, Germany & Thermo Heto LL 3000) to obtain dry sample [7].
Bi conjugation with folate
The attachment of folate to the quercetin nanoparticles was done by applying EDC-NHS conjugation chemistry. For this, the previously suspended quercetin nanoparticles (1 mg ml-1) in PBS (pH 5.6) were incubated with EDC in dark for half an hour and then immediately mixed with NHS for at least 6 hours [7,8] The obtained product is washed with water several times, filtered and mixed with folate at a concentration of 100 μgml−1 in PBS at pH 5.5 for overnight duration. Again, the product is washed with 1 ml PBS, pH 5.5 to remove the excessive reagents and further lyophilized. Finally, the FA-QuNPs were re-dispersed in water [9].
Treatment procedure
HaCaT cells, A431 cells and KB Cells were cultured separately in DMEME F-12HAM media supplemented with 10% FBS (fetal bovine serum) 100 U of streptomycin and 100 U of penicillin G at 37°C in 5% CO2 Supply. All the cell lines were seeded at a density of 1×105 cells well. After an exposure of 24 hours, the medium was replaced with DMEM containing FA-QuNP ranging from 0-75 μm in concentration [10]. All the samples were prepared in stock solution of DMSO. After the treatment, cells were collected using a hem cytometer. Typhon Blue was dye was used for staining the cells.
Reactive oxygen study
The test was employed to measure the intracellular oxidative stress generated as a result of administration of FA-QuNPs, QuNPs and Free Quercetin by using 2’,7’-Dichlorofluoresin Diacetate (DCFH-DA) which quantifies intracellular hyper oxides [11,12]. The healthy cells were grown and experiment was carried out in eight sets consisting of eight replicate cells comprising of controls positive and test sample. A 25 μM DCFH-DA in PBS was prepared and all the samples were prepared in this stock solution only. The cells were left under exposure till 24 hours for attachment and then treated with negative, positive control and test sample and incubated for 30, 60,120,180, 240 and 300 min. The oxidative stress generated was measured by monitoring the emission at 529 nm of the DCFH-DA.
Cellular uptake study
The cellular uptake of FA-QuNPs, QuNPs and Free Quercetin were analyzed in HaCaT cells, A431 cells and KB Cells by fluorescence method. Briefly, all the three cell lines were incubated with Hoechst dye (50 μg/mL) for half an hour separately and then were washed thrice with PBS. Then the cells were subjected to QuNPs, FA-QuNPs and Free Quercetin for different time intervals. Cells were then analyzed under a fluorescence microscope and images were captured using a Nikon Eclipse E600 microscope fitted with a RT spot digital camera [13-17]. For control readings, cells were incubated with the dye for 30 minutes, washed thrice with PBS and again suspended in medium and further incubated with PBS for half an hour.
Apoptosis study
In order to estimate the apoptotic activity of FA-QuNPs, QuNPs and Free Quercetin in HaCaT cells, A431 cells and KB Cells, an assay was carried out using Live Dead Assay kit (Invitrogen, India) which works on the activity of intracellular esterase and also specifies the plasma membrane integrity. Briefly, 1 × 105 cells/well were kept under incubation with FA-QuNPs, QuNPs and Free Quercetin for 24 hours, then the cells were subjected to dyes Calcein (green fluorescent dye) which is retained by living cells and Ethidium homodyne dye (red fluorescent dye) which enters the damaged cell membranes and get bind to nucleic acid [18-24]. These two colored dye’s cells were observed under the fluorescence microscope by counting their numbers on the basis of color emission.
Intracellular glutathione assay
To estimate the reduction in intracellular glutathione level in HaCaT cells, A431 cells and KB Cells upon FA-QuNP, QuNP and Free Quercetin exposure, a commercial kit (ThiolTracker™ Violet) was used. In order to quantify the intracellular glutathione levels in the three cell lines and their respective controls fluorescence micro plate reader was employed. The study was carried out in 96 well micro-plates were cells were grown at a density of 1 × 105 cells/well in DMEM with 10% FBS [25-27]. The cells were left for attachment for 24 hours and then washed twice with fresh PBS (100 μl/well) and then exposed to FA-QuNP, QuNP and Free Quercetin, in the concentration range of 20-200 mg/ml supplemented with 10% FBS. After the exposure, again the cells were washed with fresh PBS (100 μl/well) and exposed to the commercial dye (ThiolTracker™ Violet) prepared in PBS at a concentration of 20 μM. The incubatory conditions were maintained at 37°C with 5% CO2 for half an hour [28,29]. After incubation, the cells were washed thrice to remove the dye with 100 μl/well PBS and the cells were analysed under the reader to measure the wavelength at 405 and 535 nm respectively in Bio-Rad micro plate reader.
Preparation of Novel Lotion (NL-4)
The measured quantity of cetyl alcohol, zinc oxide, stearic acid, glycerin and hydroxyl propyl methyl cellulose (HPMC) were added to Luke warm water slowly with constant stirring until a smooth paste is formed. Then it is kept aside for cooling. Lotions were prepared using different concentration of folate conjugated quercetin nanoparticles; free drugs as well as non-conjugated quercetin nanoparticles (2%, 2.5% and 5%).Only optimized products were further preceded for animal studies. Then weighed quantity of folate conjugated quercetin nanoparticles (about 5%) is added and kept under stirring condition for uniform mixing to get the novel targeted lotion (NL-4). In the same way base lotion (NL-1), lotion containing free quercetin (NL-2) and lotion containing non-conjugated quercetin nanoparticles (NL-3) were prepared (Table 1).
| S No | Name of ingredients | Quantity used |
|---|---|---|
| 1 | HPMC | 15 |
| 2 | Zinc oxide | 15 |
| 3 | Glycerin | 5 |
| 4 | Stearic acid | 5 |
| 5 | Propyl paraben | 1.5 |
| 6 | Cetyl alcohol | 2 |
| 7 | FA-Qu-NPs | 5% |
| 8 | Distilled water | 50.5 |
Table 1: Ingredients for the preparation of novel targeted lotion.
Physiochemical characterization
Different types of lotion formulations were developed and the morphology of the Nano cargoes present in the formulations were observed by transmission electron microscopy, then all the physicochemical parameters were evaluated to check the safety of the product and along with it in vitro. The pH, thermal stability, fatty content and non-volatile content of the prepared formulations were determined according to the Indian standard guideline (IS: 6608- 1978B-1, IS: 6608-1978B-2, IS: 6608-1978B-3). All evaluations were carried out in triplicate presented as mean ± standard deviation (SD).All parameters were statistically analysed at 99% confidence level in the column. Analysis of variance ANOVA and Student’s paired t-tests were performed. Differences were considered statistically significant if p<0.01.
Ex vivo permeation studies
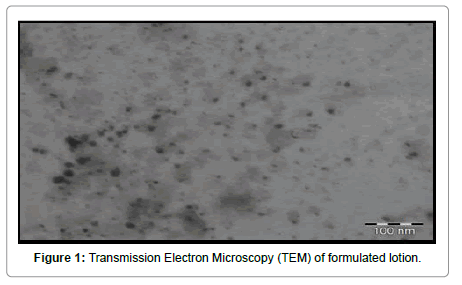
For Ex vivo permeation study full-thickness goat skin was used for locally fabricated Franz diffusion cell. The skin was clamped between the donor and the receptor chamber of diffusion cell with an effective diffusion area of 2.5 cm2. The receptor chamber was filled with freshly prepared phosphate buffer pH 5.5. The diffusion cell was maintained at 32ºC and the solution of the receptor chamber was stirred continuously at 350 rpm by using magnetic stirrer with hot plate (Remi, Mumbai, India). The formulation (4.0 g) was gently placed in the donor chamber. At 1,2,3,4,5,6,12,18 and 24 h, 5.0 ml of the solution in the receptor compartment was removed and analysed using UV-spectrophotometer (Syntonic, UV-Double beam spectrophotometer) and replaced immediately with an equal volume of fresh buffer. All experiments were performed in triplicate. The results of drug release are reported in Table 2 and graphically shown in Figure 1.
| Formulation Code | pH | Non-volatile (% ) | Saponification value | Acid value | Fatty concentration (% w/w) | Spread ability (% ) | Layer thickness (µm) | Ash value | Viscosity (Cps) |
|---|---|---|---|---|---|---|---|---|---|
| NL-1 | 5.54 ± 0.03 | 23.20 ± 0.2 | 20.30 ± 0.3 | 6.750 ± 0.5 | 11.84 ± 0.4 | 92 ± 1 | 3.57 ± 0.3 | 0.02 ± 1 | 5880 ± 10 |
| NL-2 | 5.60 ± 0.01 | 23.14 ± 0.2 | 20.12 ± 0.1 | 7.82 ± 0.3 | 13.53 ± 0.3 | 94 ± 2 | 3.35 ± 0.4 | 0.02 ± 2 | 5870 ± 10 |
| NL-3 | 5.24 ± 0.02 | 18.98 ± 0.4 | 19.88 ± 0.4 | 8.92 ± 0.2 | 12.32 ± 0.3 | 93 ± 2 | 4.24 ± 0.2 | 0.03 ± 1 | 5720 ± 15 |
| NL-4 | 7.34 ± 0.03 | 17.94 ± 0.1 | 18.43 ± 0.3 | 8.34 ± 0.3 | 11.78 ± 0.5 | 95 ± 2 | 5.54 ± 0.3 | 0.03 ± 2 | 5900 ± 20 |
Note: *NL-1= Novel Lotion containing base lotion; **NL-2= Novel Lotion containing free quercetin ; ***NL-3= Novel Lotion containing quercetin nanoparticles; ****NL-4= Novel Lotion containing folate conjugated quercetin nanoparticles
Table 2a: General physiochemical evaluated for the prepared novel formulations.
| Formulation code | Appearance | Fragrance | Lathery | Softness | Smoothness | After effect | Overall ranking |
|---|---|---|---|---|---|---|---|
| NL-1 | 2.8 ± 2.1 | 2.42 ± 1.9 | 5.3 ± 2.4 | 5.4 ± 1.6 | 5.1 ± 1.6 | 5.2 ± 2.9 | 26.6 ± 12.5 |
| NL-2 | 3.4 ± 3.1 | 3.25 ± 2.9 | 5.5 ± 2.2 | 7.3 ± 1.3 | 6.5 ± 1.2 | 6.3 ± 1.1 | 33.10 ± 11.8 |
| NL-3 | 4.9 ± 2.3 | 4.20 ± 2.6 | 6.3 ± 2.2 | 7.4 ± 0.5 | 6.7 ± 0.9 | 7.4 ± 0.9 | 35.2 ± 9.4 |
| NL-4 | 5.35 ± 3.7 | 3.5 ± 2.9 | 8.6 ± 1.0 | 8.1 ±0.3 | 7.2 ± 0.9 | 7.6 ± 1.2 | 38.4 ± 10.0 |
Note: *NL-1= Novel Lotion containing base lotion; **NL-2= Novel Lotion containing free quercetin ; ***NL-3= Novel Lotion containing quercetin nanoparticles; ****NL-4= Novel Lotion containing folate conjugated quercetin nanoparticles
Table 2b: General psychological parameters were evaluated for the prepared novel formulations.
Skin retention study
The skin retention studies of different formulations were performed in order to analyse the content of quercetin in the skin after 24 h of diffusion. At the end of the experiment the skin samples were washed up with water and methanol on both sides and carefully dried. After this procedure a definite amount of methanol was added to each piece of skin. The samples were vortexes for 10 min and stirred overnight. After vortexing, the samples were analysed by UV spectrophotometer. The result of drug retention studies are reported in Table 3 and graphically presented in Figure 2.
| Formulation | Jss (µg/ch-2/h) | P (Ch/h) | LT (hr) | Dd (Ch2/h) |
|---|---|---|---|---|
| NL-1 | 5.30 ± 1.02 | 0.250 ± 1.42 | 2.7 | 6.3 |
| NL-2 | 5.68 ± 0.59 | 0.280 ± 1.2 | 2.4 | 6.7 |
| NL-3 | 5.44 ± 1.34 | 0.280 ± 1.64 | 2 | 7.2 |
| NL-4 | 6.65 ± 0.46 | 0.238 ± 1.56 | 1.6 | 8.6 |
| Marketed product | 5.57 ± 0.63 | 0.220 ± 1.59 | 2 | 7.7 |
Note: Where, Jss: Transdermal flux; P: Permeability coefficient; LT: Lag time; Dd: Diffusion coefficient.
Table 3: Permeation parameters of folate conjugated quercetin loaded different formulations across abdominal goat skin.
In vivo study in mice
All the experiments on animals were performed after taking approval from Institutional Animal committee. Animals were caged properly and had free access to food and water until use. The animal study was carried out according to the procedures carried out by Kaur with slight modifications. The animals were exposed to UV radiation by the help of a UV lamp (200-400 nm) in a chamber designed specifically in the laboratory to induce photo carcinoma. Anaesthesia was given to the animals before exposing them to radiations to maintain homogenous exposure. The process of irradiation was done with an average irradiance of 3.6 ± 0.4 m W/cm2 to achieve 5 J/cm2 intensity of exposed light radiation for a specific duration of time. The time of light exposure was calculated according to OECD guidelines by following formula. The animals were exposed four times a week for 6 weeks. The dorsal hairs of the mice were removed with a razor and the nude skins were washed with physiological solution. About 0.5 mL (drug content 0.5%, w/w) of each lotion formulations were applied to the dorsal surface of the skin at an area of 3.14 cm2 approx. and free quercetin lotion and Fivocil®, Alkem, India. The lotion was applied until it disappears from into the skin.
Macroscopic and histopathological evaluation of skin for photo damage
The animal skin was constantly examined for 6 weeks for the slow progress of photo carcinoma. The evaluation criteria were adopted from Kaur 2010 with slight modifications. A evaluator scale was developed according to the skin conditions from 0-5 representing normal skin at 0 and severally damaged skin at 5 scale (Table 4). Further Skin biopsy was taken every week from each group of animals and was embedded in paraffin after fixing in 10% buffered formalin. Then the sections were sliced with a semi-Automated Rotary Microtome (Leica®, Germany) and stained accordingly using Hematoxylin and Eosin (H and E) staining dyes. Finally, the stained specimens were observed under Fluorescence microscope (Nikon®, India), in order to determine the extent of damage caused to skin components.
| Grading scale | Evaluation parameters |
|---|---|
| 0 | No Wrinkle formation, No Inflammation, No lesion |
| 1 | Fine wrinkles, formation of fine lines |
| 2 | Shallow wrinkles , clear visible lines |
| 3 | Deep multiple wrinkles |
| 4 | Inflammation, lesion formation |
| 5 | Lesion/Tumor formation |
Table 4: Macroscopic observance of the mice’s skin conditions from 0-5 on a grading scale.
Statistical analysis
Data are shown as means ± standard deviation (n=5). Statistical data were analyzed by the Student’s t-test at the level of P=0.05.
Results
Characterization of targeted nano systems (FA-Qu-NPs)
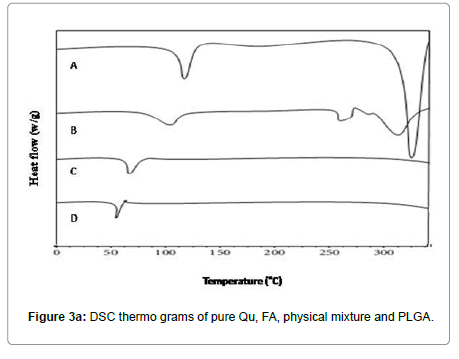
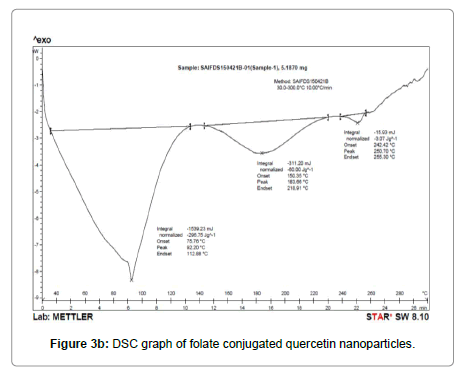
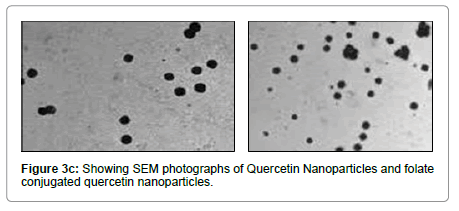
Average size and size distribution of the drug-loaded nanoparticles were measured by the laser light scattering technique using a particle size analyzer (Malvern®,Zetasizer,ZF-90). Samples for measurement were prepared by diluting the material suspension with Milli Q water. Differential scanning calorimeter (DSC) is a technique of employed to investigate the melting and recrystallization behavior of crystalline materials. The DSC thermo grams of pure Qu, FA, and physical mixture are shown in Figure 3A. The melting point of pure Qu was found to be 316°C. The DSC thermo gram of Qu and FA showed a sharp endothermic peak at 316°C and 250°C respectively. Physical mixture showed two mild peak changes in the position of endothermic peaks. Thus, there was a chemical interaction between Qu and FA. FAQu- NPs showed two endothermic peaks in DSC thermo gram, one at 250°C for ligand and another at around 155°C for mannitol (cry protectant used in lyophilization of FA-Qu-NPs) the peak for PLGA was not visible showing its amorphous nature, the peak of Qu and FA was clearly visible in FA-Qu-NPs. Although, the final peaks of drug and ligand in FA-Qu-PLGA-NPs formulation showed a negative shift towards temperature. This may be due to lowering in particle size and increase in surface area; leading to decrease in melting enthalpy as compared with heat flow through larger crystals, which require more time. The SEM study showed the structural morphology of quercetin nanoparticles and folate conjugated quercetin nanoparticles. The result of the study suggests that Qu was successfully loaded with in polymeric nanoparticles and conjugated with folate in the form of FA-Qu-NPs and existed in crystalline state. Entrapment efficiency was also estimated for both. Table 5 summarizes the results of the above stated experiments (Figures 3A-3C) (Table 6).
| PD ratio (w/w) | Entrapment efficiency (%) | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|
| Plain Qu-PLGA nanoparticles | 55.6 ± 4.2 | 120.4 ± 10.5 | 0.208 ± 0.23 | -20.98 ± 2.2 |
| FA-Qu-PLGA nanoparticles | 71.48 ± 3.2 | 100.3 ± 7.32 | 0.287 ± 0.19 | -23.20 ± 3.5 |
Note: Each batch was prepared by homogenous Nano precipitation method. S.E.M: Standard Error of Mean; PDI: Polydispersity Index
Table 5: Summarizes the physiochemical characteristic of the prepared Nano formulation before and after incorporation of folate.
| Formulation code | % Drug retention after 24 hrs. |
|---|---|
| NL-1 | 0.43 ± 0.23 |
| NL-2 | 0.56 ± 0.47 |
| NL-3 | 0.69 ± 0.30 |
| NL-4 | 0.78 ± 0.05 |
| Marketed product | 0.58 ± 0.30 |
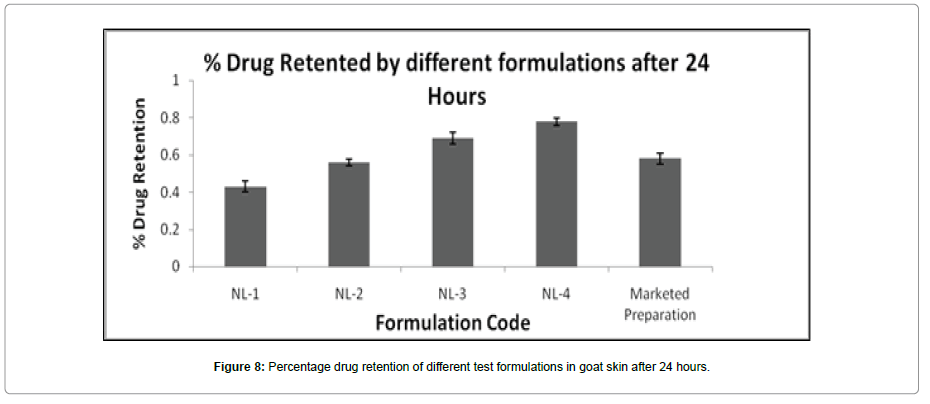
Table 6: Percentage drug retention of different formulations in goat skin after 24 hours.
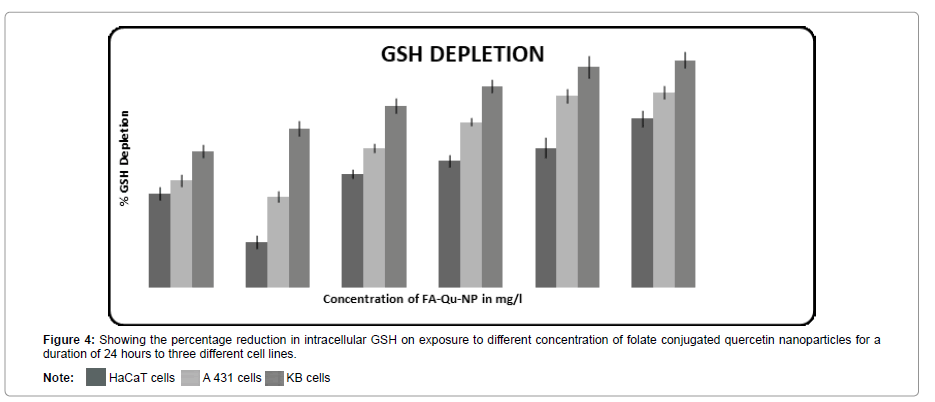
Intracellular GSH study
The intracellular GSH measurement reduces significantly when folate conjugated quercetin nanoparticles was administered, in comparison to the non-conjugated quercetin nanoparticles and free quercetin. The rate of intracellular GSH depletion was found to be significant at a concentration of 150 mg/l in both A431 and KB cell lines in 24 hours (Figure 4).
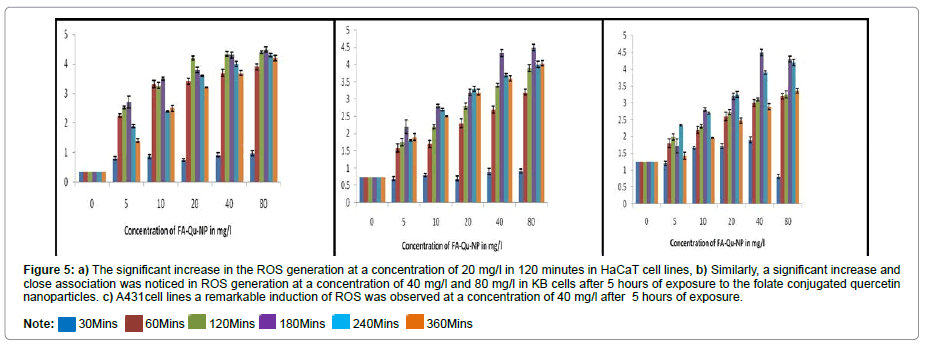
Reactive oxygen species
The ROS Study has shown that time exposure to folate conjugated quercetin nanoparticles increases the production of reactive oxygen species generation in all the three cell lines. In case of FA-Qu-NPs, there was tremendous increase in ROS production in all three cell lines. The conjugation of folate with quercetin potentiate the generation of reactive oxygen species leading to cell death (Figures 5A-5C).
Figure 5: a) The significant increase in the ROS generation at a concentration of 20 mg/l in 120 minutes in HaCaT cell lines, b) Similarly, a significant increase and close association was noticed in ROS generation at a concentration of 40 mg/l and 80 mg/l in KB cells after 5 hours of exposure to the folate conjugated quercetin nanoparticles. c) A431cell lines a remarkable induction of ROS was observed at a concentration of 40 mg/l after 5 hours of exposure.

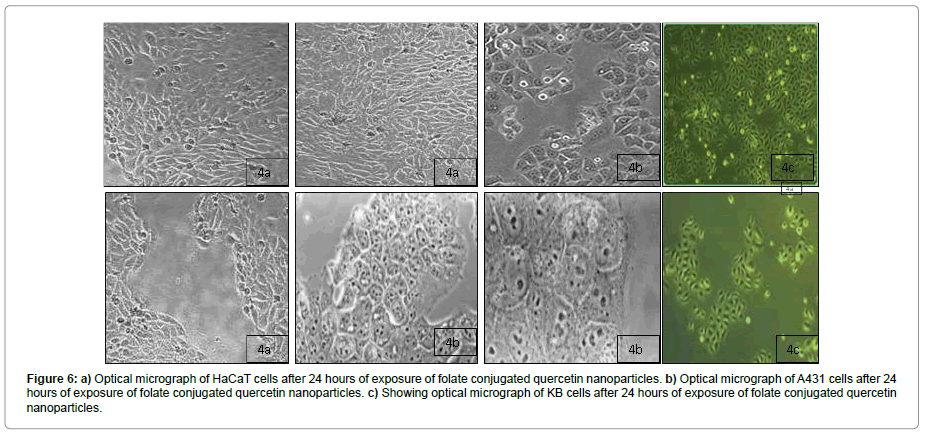
Apoptosis study
The apoptotic activity was also investigated for FA-Qu-NP, Qu-NP and Free Quercetin in all the three cell lines. In our investigation, we found that the FA-Qu-NP, Qu-NP and Free Quercetin showed a dose dependent apoptotic effect. We finally found that FA-Qu-NP showed a significant apoptotic activity at a concentration below than 20 μM while Qu-NP showed apoptotic activity at a concentration of 30 μM and while that of Free quercetin was observed at a concentration ranging from 4-50 μM (Figures 6A-6C).
Figure 6: a) Optical micrograph of HaCaT cells after 24 hours of exposure of folate conjugated quercetin nanoparticles. b) Optical micrograph of A431 cells after 24 hours of exposure of folate conjugated quercetin nanoparticles. c) Showing optical micrograph of KB cells after 24 hours of exposure of folate conjugated quercetin nanoparticles.
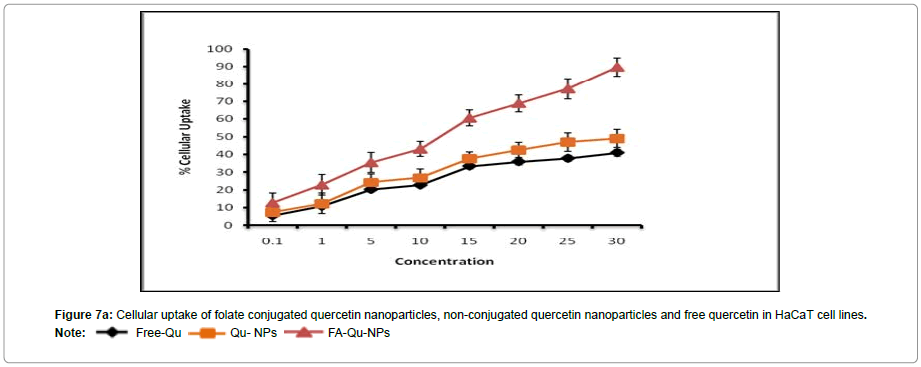
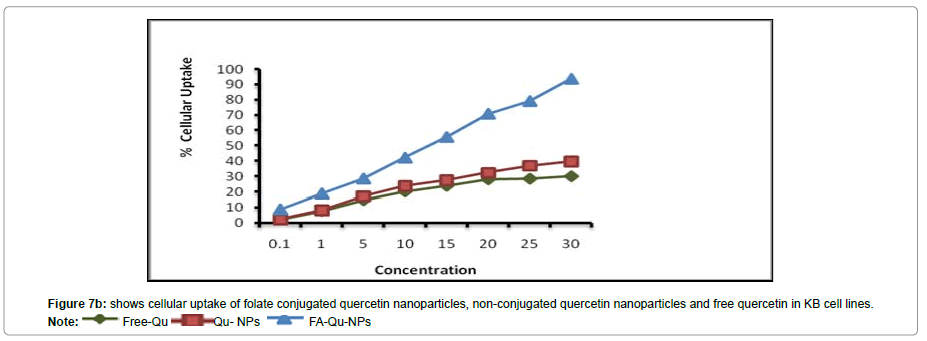
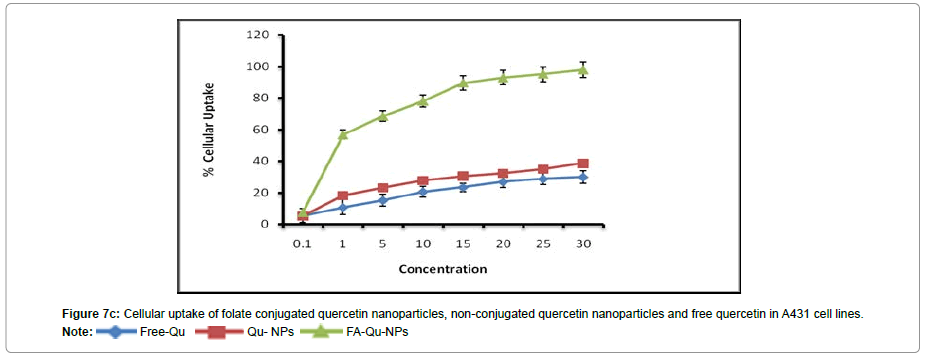
In vitro cellular uptake study
To corroborate with the above results we also carried out the experiment to investigate the targeting capability of FA-Qu-NP on all the three cell lines using fluorescence microscope. The results of In vitro cellular uptake of FA-Qu-NP, Qu-NP and Free Quercetin were remarkably significant. The cellular uptake response of FA-Qu-NP and Qu-NP was observed relatively slower than free quercetin for early 5 minutes of exposure. The response pattern was relatively similar in HaCaT cells and that of A341 cells while a slightly different cellular uptake pattern was observed for FA-Qu-NP, Qu-NP and Free Quercetin in KB cells. Here, the cellular uptake of FA-Qu-NP started at an initial exposure of 5 minute and lasted for 45 minutes. Moreover, the overall pattern cellular uptake of FA-Qu-NP, Qu-NP and Free Quercetin for all the three cell lines remained in an exponential order i.e. FA-Qu-NP > Qu-NP > Free Quercetin. The reason behind the variations in the cellular uptake response exerted by these cell lines may be the availability of folate receptor at the cellular surface of KB cells which facilitates the cellular uptake of folate conjugated quercetin inside the cell, while in case of A341 cells the role of macrophage and MIF could be anticipated. In HaCaT cell lines no significant changes were observed and it may be due to the lack of folate receptors, on the contrary the role of activated macrophages is also debatable (Figures 7A-7C).
Characterization of novel lotion
Various parameters like, microscopy, physiochemical evaluation, skin permeation and skin retention studies were carried out in order to characterize the Novel Targeted Lotion (NTL).
Microscopy
The morphological characterization of folate conjugated quercetin nanoparticles incorporated in the lotion was done using Transmission Electron Microscopy (TEM) (Morgagni 268-D) at an accelerating voltage of 100 kV (Figure 1). The lotion sample was diluted with distilled water and a drop of the sample was placed on a carbon-coated copper grid to form a thin film and negatively stained by adding a drop of 1% w/v phosphor tungstic acid. The grid was allowed to air dry and the samples were viewed and photographed.
Physicochemical evaluation of lotion
The general physiochemical and psychological parameters were evaluated for the prepared novel formulations. These physicochemical parameters provided information regarding formula stability and skin compatibility. Based on the physicochemical parameters shown in Tables 2A and 2B lower acid and saponification values, high thermal stability; resulted in stable formulations. The determination of physicochemical evaluation parameters is essential as they predict the safety as well as stability of the formulations.
Ex vivo skin permeation studies
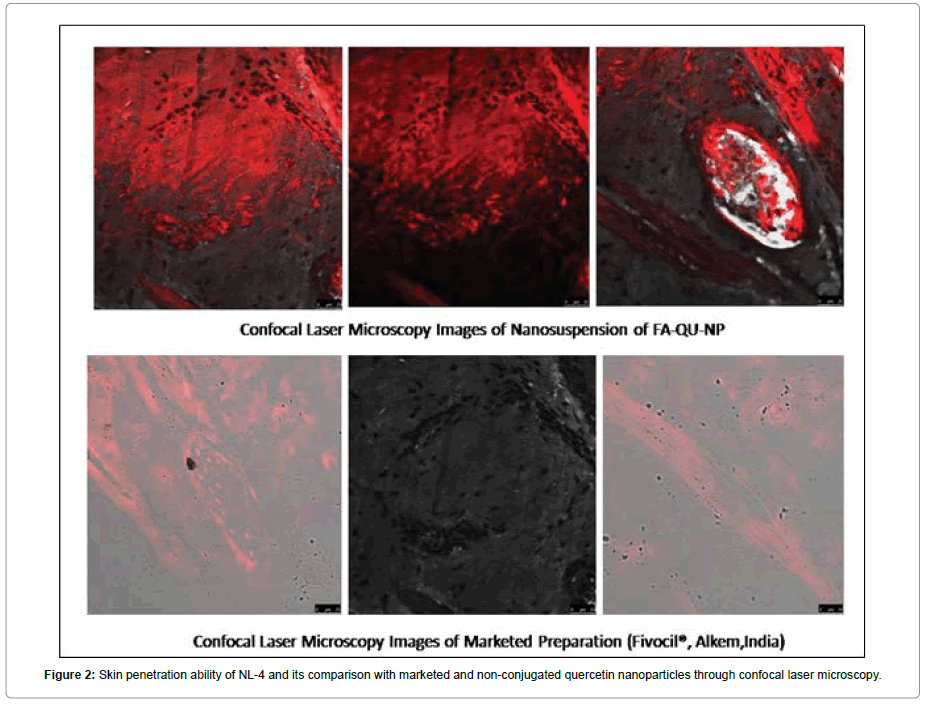
Skin penetration study of NL-4 by CLSM
The confocal laser microscopy study was employed to study the skin penetration effect of NL-4 using goat skin in vitro. Goat skin was employed for the purpose of evaluating skin penetrating ability of formulated conjugates because the goat’s skin is anatomically and physiologically similar to that of human skin. The skin penetration of NL-4 was assessed using Rhoda mine 123. Briefly, the test samples and the probe containing 0.03% of Rhoda mine were applied homogeneously and non-occlusive to the skin. The experiments were carried out employing Franz diffusion cells with the receiver chamber filled with phosphate buffer pH 5.5 solutions. After 24 h, the skin was removed and washed with phosphate buffer. The skin was then rapidly frozen by liquid nitrogen and a skin surface perpendicular rectangular piece was taken from the site of drug application with the help of a sharp blade. This tissue was fixed on the sample holder with the help of a Tissue frozen medium gel. (Gung, Leica, Germany). The skin perpendicular sections (dermis to horny layer) of (250 μm) full thickness were cut with the help of cry microtome (Leica, Germany). The treated area was cut out and tested for probe penetration. The full skin thickness was optically scanned at 15-30 nm increments through the Z-axis of a Leica DMIRE2 confocal laser scanning microscope (Germany) attached to a Leica TCS SP2 fluorescence microscope. The figures are shown in Figure 8.
Skin retention study
The skin retention studies of different formulations were performed in order to analyse the content of quercetin in the skin after 24 h of diffusion (Table 3). The study showed that percentage drug retention of formulations was found higher for FA-Qu-NPs loaded novel lotions (NL-4) (Table 6). The % retention was near about similar for Marketed formulation, 0.58 ± 0.30 and that of FA-Qu-NPs loaded novel lotion 0.78± 0.5 (Figure 8).
In vivo study
The main objective of our study was to determine the targeting ability of folate conjugated quercetin nanoparticles (NL-4) dermally and to evaluate its topical and targeted effect on skin. The macroscopic effects of UV radiation on the animal’s skin were highly distinguishing for evaluation (Table 4). The non-irradiated skin was free from any type of lesion formation while the irradiated groups developed lesion from the fourth week onwards in nearly 50% of the test animals. At the end of week 6 about 85% of animals developed photo carcinoma lesions (extensive degradation). The cellular components like collagen, elastin, matrix proteins network was found damaged. The animals treated with free quercetin lotion developed lesser skin lesions in comparison to the irradiated group. The animals treated with market formulation Fivocil®, Alkem, India, showed minimal lesion formation at the last week of the irradiation. On discontinuation of the UV irradiations the formulation showed healing effect. The test sample showed nominal skin degradation and negligible lesion formation (Table 4).
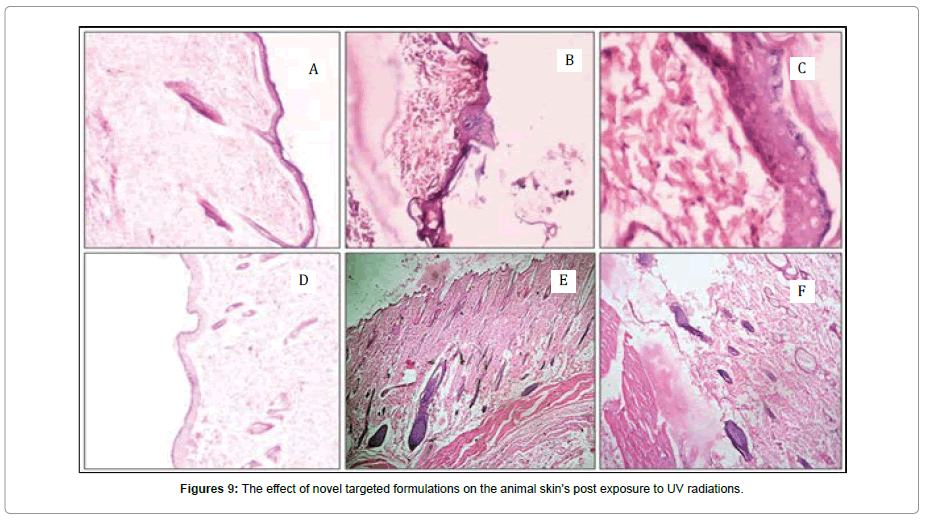
In above study, Figure 9 shows the skin histology of that group of animal which received irradiation. There is a complete loss of epidermal lining and accumulation of elastic materials with complete degeneration of collagenous meshwork. The generation of epidermal cyst was also observed on continuing the exposure. In Figure 9a shows that there is denaturation of elastic and collagen fibers in the dermis region. There was Inflammation in the dermal and epidermal region due to cell necrosis. The hair follicles were also found to be damaged. Figure 9d shows the histological skin section of the group of animals which were treated with free quercetin containing lotion. This slide is identical in histological characteristics to the histological slide of UV irradiated group. The free quercetin lotion does not exert the desired effect in protecting the skin against radiations. Figure 9e shows the effect of marketed formulation (Fivocil, Alkem, India) on the irradiated animal skin. The skin showed inflammation in deeper layer of the skin with the formation of wrinkles. Although the formulation was found to be very effective in restoring the dermal components of the skin but the skin was found to be rough. The epidermal hyperplasia and dermal elastics were remarkably reduced in the skin of the animals receiving the application of marketed formulation. In Figure 9f the animal skins were treated with the test sample (targeted formulation containing folate conjugated quercetin nanoparticles). The result of the application showed no cyst formation and lesser inflammation. The skin was smooth with no sign of wrinkle formation. The collagen and elastin bundle was regular and the hair follicles were also found to be regenerative.
Discussion
Quercetin is a natural flavonoid exhibiting range of pharmacological activities. It has been proven to be a potent anticancer agent in various studies, despite of several research findings over quercetin against various cancer cells, its mode of action in treating skin cancer is not well addressed [30]. The major issue with quercetin is its polyphenol nature which reduces its availability due to poor solubility. Hence, we developed folate conjugated quercetin nanoparticles as targeted drug delivery system and formulated them into a lotion to estimate the targeting ability of these Nano cargoes in penetrating skin barrier and reaching the desired site of action. The average particle size was found to be 100.3 ± 7.32 which indicates that the particles were in Nano range. The zeta potential was found to be -23.20 ± 3.5 with a poly disparity Index less than 1. The entrapment efficiency was found to be 71.48 ± 3.2. Further the SEM analysis confirms the uniform morphology of folate conjugated quercetin nanoparticles [31]. While the DSC study clearly indicates that the compatibility of FA-Qu-NP. The anticancer potential of folate conjugated quercetin nanoparticles was evaluated in HaCaT and A431 cell lines due to a close association between keratinocytes and melanocytes while KB cells were used to measure cellular viability because KB cells highly express folate receptor [32]. The results of our study potentiate the role of folate receptor mediate targeting of novel (natural) bioactive for the treatment of skin cancer. The results showed that cellular uptake by the A431 and KB cell lines were higher in comparison to the HaCaT cell lines. While the folate conjugates quercetin nanoparticles showed an equilibrium effect on all the three cell lines. The reason for this effect may be the migration of the Nano carries across the cellular membrane was estimated higher in leaky and ruptured cells in comparison to the normal cells. In a similar study, Zhang et.al synthesized folate mediated poly (3-hydroxy butyrated-CO-3-hydroxyoctonate) nanoparticles of doxorubicin and found that the nanoparticles were highly effective against HeLa cell lines and showed a remarkable cellular uptake response. Further, they supported their study by evaluating the therapeutic efficacy in vivo too [26,33].
In cancer cells, the rate of mutagenicity is greatly governed by the presence or absence of ROS which further leads to DNA damage and chromosomal instability and ultimately causing cancer progression. Secondly, ROS also promote cell survival and proliferation, thus contributing to cancer development [34,35]. Cancer cells having higher concentration of ROS are more susceptible to quercetin, one of the main dietary flavonoids. Quercetin depletes intracellular glutathione and increases intracellular ROS to a level that can cause cell death [36]. In Reactive Oxygen species generation study, the results revealed that on FA-Qu-NP exposure, the level of antioxidant present was changed. There was a considerable difference in the ROS level indicating a clear interference of FA-Qu-NP, Qu-NP and Free Quercetin on normal and cancerous cells. The study parameters selected were dependent on the activity of cellular components on targeting. 2’,7’-dichlorodihydrofluorescencein diacetate is a cell per meant commonly used to detect ROS generation.
Glutathione is a major component of the intracellular antioxidant defenses. It is present at mill molar concentrations [37]. The GSH system helps in maintaining an appropriate intracellular redox homeostasis. Various studies have demonstrated that Qu can modify ROS metabolism by directly lowering the intracellular pool of GSH. Taking into consideration the biological and biochemical differences between cancerous and normal cells, the folate conjugated Quercetin nanoparticles plays a crucial role in internalizing the quercetin inside the cells [38]. The difference in the level of ROS in all the three cell lines can be due to nature of normal, transformed and non-transformed cells. Similarly, in our study we have taken three cell lines in order to comparatively evaluate the binding, internalization and therapeutic efficacy of the nano-conjugates and further confirm their specificity with the targeting moieties. The aim of our study was to enhance the cellular internalization of the conjugate for effective targeting. A similar study by Changli in 2010, in which they formulated a folate bovine serum albumin cis aconitic anhydride doxorubicin pro-drug (FA-BSA-CAD) to enhance the specificity of targeting moiety towards a large number of reactive sites [39]. They claimed in their study that folate conjugated DOX showed good therapeutic activity than non-conjugated DOX. In the lieu, Kim also evaluated the targeting ability of 99 mTc labeled FR-PEG conjugate against KB and A549 cell lines [29]. They showed that folate conjugated moiety specifically bounded to the cancer cells irrespective of the normal cells. Finally, the results of the series of experiment draw our attention towards the fact that at a concentration of 100 μg/ml, The FA-Qu-NP exerted significant antiproliferative activity, causing 90% of cell death in 24 hour of incubation. The IC50 values for KB, A431 and HaCaT cell lines were reported between 30- 40 μg/ml indicating a profound dose dependent effect of FA-Qu-NP. It was found that folate conjugated quercetin nanoparticles exhibited a threefold increase in activity as compared to non-conjugated and free quercetin.
In addition to this, the skin penetrating effect of the targeted novel formulation was studied using CLSM. The result showed that the targeted moieties embedded in the lotion has the ability to penetrate deeper in to the dermal layer and exert its effect. The enhanced penetration effect may be due to the attached folate group which is readily taken up by the cells for their normal cellular functions. The polymeric shell also facilitates its entry into the cells. The percent drug retention of the formulation after 24 hours was satisfactory in comparison to the marketed formulation. Through histological studies, it was found clear that the novel targeted formulation was better than the marketed formulation in restoring the skin vitality as well as in the safe guarding of the skin against UV radiations.
Conclusion
The novel lotion was found to significantly improve the collagen and elastin network, decrease inflammation, regenerate hair follicles and decreases the signs of photo aging. The novel formulation was found to be efficient against UV radiation caused skin cancer, not only in reducing oxidative stress in vitro but also as anti-photo aging formulation in vivo. Although extensive studies are left to confirm the overall therapeutic potentials of FA-Qu-NPs conjugates and its formulation, we expect that our study will be small step towards the targeting of natural bio actives in the form of Nano formulations for efficient drug delivery for skin cancer.
References
- Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R (2009) Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol Rev 61: 198-223.
- Zhao X, Li H, Lee RJ (2008) Targeted drug delivery via folate receptors. Expert Opin Drug Deliv 5: 309-319.
- Ganta S, Devalapally H, Shahiwala A, Amiji M (2008) A Review of stimuli-responsive nano-carriers for drug and gene delivery. J Control Rel 126: 187-204.
- Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, et al. (2005) Nanoparticles targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res 65: 5317-5324.
- Lu Y, Sega E, Leamon CP, Low PS (2004) Folate receptor-targeted immunotherapy of cancer mechanism and therapeutic potential. Adv Drug Deliv Rev 56: 1161-1176.
- Lu Y, Low PS (2012) Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev 64: 342-352.
- Narayanan S, Binulal NS, Mony U, Manzoor K, Nair S, et al. (2010) Folate targeted polymeric 'green' Nano therapy for cancer. Nanotechnology 21:16-21.
- Anindita M, Vishwanatha JK (2009) Formulation, characterization and evaluation of curcumin-loaded PLGA Nano spheres for cancer therapy. Anticancer Res 29: 3867-3876.
- Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, et al. (2010) Design of curcumin-loaded PLGA nanaoparticless formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol 79: 330-338.
- Lu Y, Ding N, Yang C, HuangL, Liu J, et al. (2012) Preparation and in vitro evaluation of a folate-linked liposomal curcumin Formulation. J Liposome Res 22: 110-119.
- Zhang L, Zhu W, Yang C, Guo H, Yu A, et al. (2012) A Novel folate-modified self-micro emulsifying drug delivery system of curcumin for colon targeting. Int J Nanomedicine 7: 151-162.
- Dosio F, Milla P, Cattel L (2010) EC-145, A Folate-targeted vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers. Curr Opin Investig Drugs 11: 1424-1433.
- Mukherjee SG, O'Claonadh N, Casey A, Chambers G (2012) Comparative in vitro cytotoxicity study of silver nanoparticles on two mammalian cell lines. Toxicol In vitro 26: 238-251.
- Kaur M, Agarwal C, Singh RP, Guan X, Dwivedi C, et al. (2005) Skin cancer chemo preventive agent, a-santalol, induces apoptotic death of human epidermis carcinoma A431 cells via caspase activation together with dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis 26: 369-380.
- Agrawal R, Kaur IP (2010) Inhibitory effect of encapsulated curcumin on ultraviolet induced photoaging in mice. Rejuvenation Res 13: 397-410.
- Aggarwal BB, Sung B (2009) Pharmacological basis for the role of curcumin in chronic diseases: An Age-old spice with modern targets. Trends in Pharmacological Sciences 30: 85-94.
- Shishodia S, Sethi G, Aggarwal BB (2005) Curcumin: Getting back to the roots. Ann N Y Acad Sci 1056: 206-217.
- Goel A, Kunnumakkara AB, Agarwal BB (2008) Curcumin as ‘‘curecumin’’: From kitchen to clinic 75: 787-809.
- Strimpakos AS, Sharma RA (2008) Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10: 511-545.
- Kunnumakkara AB, Anand P, Aggarwal BB (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269: 199-225.
- Singh S, Aggarwal BB (1995) Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane). J Biol Chem 270: 24995-5000.
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB (2006) Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol 69: 195-206.
- Huang TS, Lee SC, Lin JK (1991) Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci USA 88: 5292-5296.
- Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, et al. (2006) Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncology Rep 15: 1557-1562.
- Jaiswal AS, Marlow BP, Gupta N, Narayan S (2002) Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 21: 8414-8427.
- Kim SH, Kim JK, Lim SJ, Park JS, Lee MK, et al. (2008) Folate-tethered emulsion for the target delivery of retinoids to cancer cells. Europ J Pharmac Biopharm 68: 618-625.
- Hou Z, Zhan C, Jiang Q, Hu Q, Li L, et.al. (2011) Both FA- and mPEG-conjugated chitosan nanaoparticless for targeted cellular uptake and enhanced tumor tissue distribution. Nanoscale Res Lett 6: 563-566.
- Caliceti P, Salmaso S, Semenzato A, Carofiglio T, Fornasier R, et.al. (2003) Synthesis and physicochemical characterization of folate-cyclodextrin bioconjugate for active drug delivery. Bioconjug Chem 14: 899-908.
- Kim SL, Jeong HJ, Kim EM, Lee CM, Kwon TH, et al. (2007) Folate receptor targeted imaging using poly (ethylene glycol)-folate: In vitro and In vivo studies. J Korean Med Sci 22: 405-411.
- Stella B, Arpicco S, Peracchia MT, Desmaële D, Hoebeke J, et al. (2000) Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci 89: 1452-1464.
- Gupta Y, Jain A, Jain P, Jain SK (2007) Design and development of folate appended liposomes for enhanced delivery of 5-FU to tumor cells. J Drug Target 15: 231-240.
- Tse C, Zohdy MJ, Ye JY, O'Donnell M, Lesniak W, et al. (2011) Enhanced optical breakdown in KB cells labeled with folate-targeted silver-dendrimer composite nanodevices. Nanomedicine 7: 97-106.
- Zhang C, Zhao LQ, Dong YF, Zang XY, Lin J, et al. (2010) Folate-mediated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur J Pharm Biopharm 76: 10-16.
- Jain NK, Mishra V, Mehra NK (2013) Targeted drug delivery to macrophages. Expert Opin Drug Deliv 3: 1-15.
- Gibellini L, Pinti M, Nasi M, Biasi SD, Roat E, et.al. (2010) Interfering with ROS metabolism in cancer cells: The Potential role of quercetin. Cancers 2: 1288-1311.
- Ramos S (2007) Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem 18: 427-442.
- Bravo L (1998) Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev 56: 317-333.
- Du C, Deng D, Shan L, Wan S, Cao J, et al. (2013) A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials 34: 3087-3097.
Citation: Gupta A, Kaur CD, Saraf S, Saraf S (2021) Development of Novel Folate Conjugated Nanoparticles Based Skin Lotion for Skin Cancer Treatment: In vitro Characterization and in vivo Study. J Oncol Res Treat 6: 169.
Copyright: © 2021 Gupta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 2986
- [From(publication date): 0-2021 - Nov 18, 2025]
- Breakdown by view type
- HTML page views: 2362
- PDF downloads: 624