Do MRE11 Complex and Steroid Hormone Receptors Expressions Impact the Outcomes in High Grade Serous Ovarian Carcinoma?
Received: 12-Jun-2020 / Accepted Date: 17-Jun-2020 / Published Date: 26-Jun-2020 DOI: 10.4172/2161-0681.1000377
Abstract
Background: The prognosis and response of treatment of high grade serous ovarian carcinoma can be predicted by using numbers of biological markers.
Aim: The present study aimed of investigating the clinicopathological significance and prognostic value of MRE11, RAD50, ER and PR expressions in high grade serous ovarian carcinoma. The association between doublestrand breaks repair genes (MRE11-RAD50 complex) and steroid hormones receptor (ER and PR) was also studied.
Methods: An immunohistochemical study was performed on 108 high grade serous ovarian carcinoma cases. The expressions of MRE11, RAD50, ER and PR were evaluated and correlated to patients' clinicopathological data.
Results: The results showed that high MRE11, RAD50, ER and PR protein expressions were seen in 51.9%, 70.4%, 66.7% and 31.5%, of high grade serous ovarian carcinoma cases, respectively. MRE11 and RAD50 overexpression was significantly related to high-stage (p=0.012 and p=0.024, respectively), lymph node metastasis (p=0.002 and p=0.008, respectively), negative estrogen expression (p<0.001 for both) and negative progesterone expression (p<0.001 and p=0.002, respectively). MRE11 and RAD50 overexpression was a poor prognostic factor for OS compared with high ER/PR expression in high grade serous ovarian carcinoma patients.
Conclusion: High immunohistochemical expression of MRE11 and RAD50 in high grade serous ovarian carcinomas is related to poor prognosis.
Keywords: Ovarian cancer, MRE11, RAD50, ER, PR
Introduction
Ovarian carcinoma is considered the ninth most common cancer among females and the eighth leading cause of mortality among cancer-related deaths [1,2], while in USA and in Egypt as well it takes the fifth rank among the most common cancers in females [3,4]. Epithelial ovarian carcinomas comprise most cases with the predominance for the serous type with about 75% which has been defined as two different distinct types; low grade serous ovarian carcinoma (LGSOC) and high grade serous ovarian carcinoma (HGSOC) [5-7]. HGSOC is predominates and represents 20 times more common than LGSOC. Approximately 80% of HGSOC are found to be at (Stage III-IV) on diagnosis only survival rate of 20% to 30% [8,9].
The etiology of ovarian cancer is still not fully understood, among many risk factors associated with the development of ovarian cancer are genomic instability and the changes of sex hormones during a woman’s lifetime [10]. DNA repair maintains genomic stability and integrity thus any mutations may share in carcinogenesis, cancer progression and poor response to therapy [11,12]. Meiotic recombination 11 (MRE11) and RAD50 are components of MRN complex, which is consists of MRE11, RAD50 and NBS1; have major role in the phases of double stranded DNA repair. The MRN complex may have impaired function by mutations or epigenetic silencing any of the above-mentioned members. MRE11 and NBS1 germ- line mutations were lethal to experimental animals, however in man they rarely lead to an Ataxia telangiectasia-like disorder (ATLD) and Nijmegen break- age syndrome (NBS), respectively. Breast and ovarian cancer susceptibility could be attributed to certain mutations in MRN complex [12-14].
The role by which estrogen and progesterone, the main sex hormones, regulates ovarian cell proliferation has been suggested in addition taking estrogen as a hormone replacement therapy during menopause for longer periods exaggerates the risk of developing ovarian cancer [15]. Estrogen receptor (ER) and progesterone receptor (PR) mediate the effects of female steroid hormones on proliferation and apoptosis of ovarian cancer cells. The hormonal treatment for ovarian cancer has not yet been widely recommended [10].
The poor prognosis of ovarian cancer is relatively due to late diagnosis and tumor metastasis at time of diagnosis. The aim of this study was to investigate MRE11, RAD50, estrogen receptor and progesterone receptor protein expressions in high grade serous ovarian carcinoma using immunohistochemistry technique. The prognostic roles of the two DNA repair proteins MRE11 and RAD50, estrogen receptor and progesterone receptor were examined in relation to clinicopathologic factors. The correlation between the two DNA repair proteins MRE11 and RAD50 and hormone receptors as ER and PR was also studied.
Materials and Methods
Patients and clinical samples
Formalin-fixed and paraffin embedded high grade serous ovarian carcinomas of one hundred eight cases were included in this study. The cases were collected from the archive of the pathology departments in Minia University Hospital and Minia Oncology Center during the period of June 2013 to June 2018. Clinical and pathological characteristics were taken from the medical records and pathology reports. Routine hematoxylin and eosin sections were performed for histopathological evaluation. The stage of tumors was defined according to TNM staging system and the International Federation of Gynecology and Obstetrics (FIGO). Tumor grade was assessed according to the WHO classification 2014 [16]. Follow-up data were taken from the clinical databases.
Immunohistochemistry
Sections 3μm-thick were constructed. All sections were incubated for 1h at 60 ˚ C and deparaffinized in xylene, rehydrated through a graded series of ethanol solutions, and then incubated with 0.3% hydrogen peroxide for 30 min at room temperature to block endogenous peroxidase. Antigen retrieval was carried out in citrate buffer pH 6 for 20 min, and then sections were cooled to room temperature. After washing in 0.1M phosphate-buffered saline, sections were incubated with mouse monoclonal antibody to MRE11 (12D7, Abcam) at a dilution of 1:200 in PBS, mouse monoclonal antibody to RAD50 (13B3/2C6, Abcam) at a dilution of 1:100 in PBS, mouse monoclonal estrogen receptor (Roche) and mouse monoclonal progesterone receptor (Roche) at room temperature for 30 min. The reaction was visualized using the Ventana Benchmark automated system using Ventana reagents and DAB detection kit. Finally, the sections were mounted, and cover slipped.
Evaluation of immunostaining
Immunohistochemical analysis of all slides was evaluated by two pathologists (NR and MG). For ER and PR immunohistochemistry expression evaluation, two scoring systems were used. The first one was a simple system, scored as follows: <1% of tumor cells with ER/PR staining (0 points), up to 1% (1 point), 1 to <50% staining (2 point), ≥ 50% staining (3 points). The second one used is the Allred scoring system. The proportion of positive cells was scored as follows: negatively stained tumor cells (0 points), 1% (1 point), 1 to 10% (2 points), 10% to 33% (3 points), 33% to 66% (4 points), and >66% (5 points). Intensity of staining was scored as follow: absent tumor cells staining (0), weak (1), intermediate (2), and strong staining (3). The proportion and intensity scores were added to obtain a final score that ranged from 0 to 8. Using the Allred scoring system, up-to 3 was considered negative and >3 was signed as positive [15]. For evaluation of MRE11 and RAD50 immunohistochemical expression, the intensity of staining for the nuclear MRE11 and RAD50 was scored as follows: 0: no staining; 1+: weak staining; 2+: moderate staining; 3+: strong staining. The percentage of nuclear-stained cells was scored as follows: 0: no staining; 1+: 1-25%; 2+: 26-50%; 3+: 51-100%. The final score was calculated by multiplying the percentage score and the intensity score. Nuclear immunoreactivity of the MRE11 and RAD50 was scored on a range of 0 – 9+ with scores of 0 – 4+ considered as low nuclear expression and 5–9+ considered as high nuclear expression [17].
Statistical analysis
The statistical evaluation was performed using the SPSS software Version 25.0 (SPSS Inc., Chicago, IL, USA). The scoring data of MRE11 and RAD50 were categorized into “ Low Expression ” and “ High Expression”. The statistical significance of the association between these markers as well as clinicopathological parameters was assessed by Chi test and Fisher’s exact test. In addition, the probability of overall survival was determined by the Kaplan-Meier test. P-value <0.05 were considered as significant.
Results
This study was carried out on 108 patients with high grade serous ovarian neoplasms. The age of patients ranged from 30 years to 70 years with a mean (± standard deviation:SD) of 55.93 (± 9.61) years and a median of 54years. Other patients’ characteristics were shown in (Table 1).
| Clinicopathological characteristics | Carcinoma (N=108) | |
|---|---|---|
| No | % | |
| Age | ||
| ≤ 50 | 26 | 24.1 |
| >50 | 82 | 75.9 |
| Bilaterally | ||
| Negative | 30 | 27.8 |
| Positive | 78 | 72.2 |
| FIGO Stage | ||
| I | 22 | 20.4 |
| II | 32 | 29.6 |
| III | 30 | 27.8 |
| IV | 24 | 22.2 |
| LN status | ||
| Negative | 74 | 68.5 |
| Positive | 34 | 31.5 |
| Omentum Deposition | ||
| Negative | 72 | 66.7 |
| Positive | 36 | 33.3 |
| OS | ||
| Alive | 68 | 63 |
| Dead | 40 | 37 |
Table 1: Clinicopathological characteristics of the cases.
Immunoreactivity
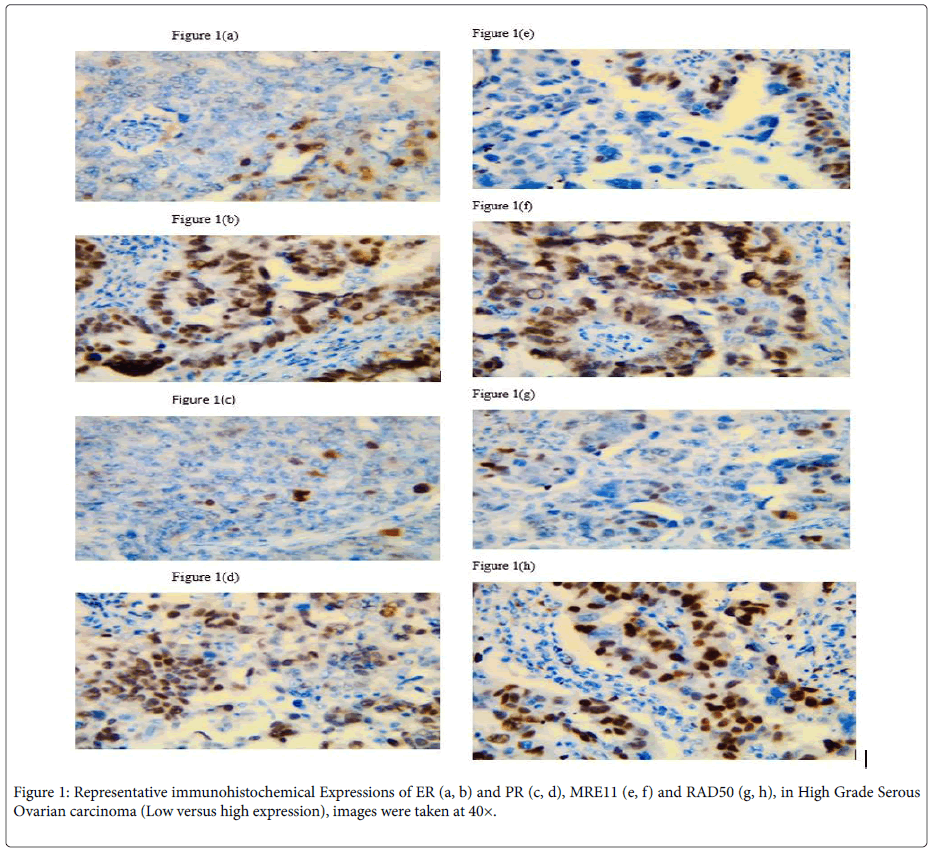
ER immunoreactivity: ER expression was observed in the nuclei of tumor cells. ER expression was negative in 36 cases (33.3%) and positive in 72 cases (66.7%). The ER immunoreactivity was inversely associated with stage (p=0.002), LN status (p=0.002), omentum deposition (p=0.013) and OS (p<0.001). No association was found between ER immunoreactivity and other clinicopathological parameters as shown in (Table 2), Figure 1a, b.
| Clinicopathological characteristics | ER | PR | ||||
|---|---|---|---|---|---|---|
| -ve (%) | +ve (%) | P value | -ve (%) | +ve (%) | P value | |
| Age | ||||||
| ≤ 50 | 20 (76.9) | 6 (23.1) | 0.293 | 18 (69.2) | 8 (30.8) | 0.617 |
| >50 | 52 (63.4) | 30 (36.6) | 56 (68.3) | 26 (31.7) | ||
| Bilaterally | ||||||
| Negative | 18 (60) | 12 (40) | 0.369 | 18 (60) | 12 (40) | 0.301 |
| Positive | 54 (69.2) | 24 (30.8) | 56 (71.8) | 22 (28.2) | ||
| FIGO Stage | ||||||
| I | 6 (27.3) | 16 (72.7) | 0.002 | 8 (36.4) | 14 (63.6) | 0.009 |
| II | 24 (75) | 8 (25) | 24 (75) | 8 (25) | ||
| III | 18 (60) | 12 (40) | 18 (60) | 12 (40) | ||
| IV | 24 (100) | 0 (0) | 24 (100) | 0 (0) | ||
| LN status | ||||||
| Negative | 26 (56.1) | 36 (47.9) | 0.002 | 48 (58.5) | 34 (41.5) | 0.003 |
| Positive | 26 (100) | 0 (0) | 26 (100) | 0 (0) | ||
| Omentum Deposition | ||||||
| Negative | 40 (55.6) | 32 (44.4) | 0.013 | 41 (58.3) | 30 (41.7) | 0.021 |
| Positive | 32 (88.9) | 4 (11.1) | 32 (88.5) | 4 (11.1) | ||
| OS | ||||||
| Alive | 36 (50) | 36 (50) | 38 (52.8)34 (47.2)0.001 | |||
| Dead | 36 (100) | 0 (0) | 36 (100) | 0 (0) | ||
| MER11 | ||||||
| Low | 18 (34.6) | 34 (65.4) | 22 (42.3)30 (57.7) | |||
| High | 54 (95.4) | 2 (3.6) | 52 (92.9) | 4 (7.1) | ||
| RAD50 | ||||||
| Low | 8 (25) | 24 (75) | 12 (37.5)20 (62.5)0.002 | |||
| High | 64 (84.2) | 12 (15.8) | 62 (81.6) | 14 (18.4) | ||
Table 2: Association of ER and PR Expressions and Clinicopathological Characteristics of High Grade Serous Ovarian Carcinoma Patients.
Test of significance: Chi-square test and Fisher’s exact test.
p-value ≤ 0.05 is considered significant.
PR immunoreactivity: PR expression was observed also in the nuclei of tumor cells. PR expression was positive in 34 (31.5%) cases and negative in 74 cases (68.5%). A statistically inverse association was noted between PR expression and FIGO stage (p=0.009), LN status (p=0.003), omentum deposition (p=0.021) and OS (p<0.001). No association was found between PR immunoreactivity and other clinicopathological parameters as shown in (Table 2), Figure 1c, d.
MER11 immunoreactivity: The MRE11 protein was mainly distributed in the nucleus, as shown in Figure 1.
MRE11 expression was low in 52 cases (48%) and high in 56 cases (51.9%). The MER11 immunoreactivity was significantly associated with high FIGO stage (p=0.012), LN status (p=0.002), omentum deposition (p=0.007) and OS (p<0.001). A statistically significant inverse association was found between MER11 and ER expression (p<0.001). A statistically significant inverse association was found between MER11 and PR expression (p<0.001). No association was found between MER11 expression and other clinicopathological parameters (Table 3), Figure 1e, f.
| Clinicopathological characteristics | MER11 | RAD50 | ||||
|---|---|---|---|---|---|---|
| Low (%) | High (%) | P value | Low (%) | High (%) | P value | |
| Age | ||||||
| ≤ 50 | 12 (46.2) | 14 (53.8) | 0.561 | 8 (30.8) | 18 (69.2) | 0.587 |
| >50 | 40 (48.8) | 42 (51.2) | 24 (29.3) | 58 (70.7) | ||
| Bilaterally | ||||||
| Negative | 18 (60) | 12 (40) | 0.215 | 10 (33.3) | 20 (66.7) | 0.477 |
| Positive | 34 (43) | 44 (56.4) | 22 (28.2) | 56 (718) | ||
| FIGO Stage | ||||||
| I | 16 (72.7) | 6 (27.3) | 0.012 | 17 (63.6) | 8 (36.4) | 0.024 |
| II | 18 (56.3) | 14 (43.8) | 10 (31.6) | 22 (68.8) | ||
| III | 16 (53.3) | 14 (46.7) | 6 (20) | 24 (80) | ||
| IV | 2 (8.3) | 22 (91.7) | 2 (8.3) | 22 (91.7) | ||
| LN status | ||||||
| Negative | 46 (62.2) | 28 (37.8) | 0.002 | 30 (40.5) | 44 (49.5) | 0.008 |
| Positive | 6 (17.6) | 28 (82.4) | 2 (5.5) | 32 (49.1) | ||
| Omentum Deposition | ||||||
| Negative | 44 (61.6) | 28 (38.5) | 0.007 | 26 (36.1) | 46 (63.9) | 0.122 |
| Positive | 8 (22.2) | 28 (77.8) | 6 (16.7) | 30 (83.3) | ||
| OS | ||||||
| Alive | 46 (67.6) | 22 (32.4) | 32 (47.1)36 (52.9) | |||
| Dead | 6 (15) | 34 (85) | 0 (0) | 40 (100) | ||
| RAD50 | ||||||
| Low | 32 (100) | 0 (0) | ||||
| High | 20 (26.3) | 56 (73.7) | ||||
Table 3: Association of MER11 and RAD50 Expressions and Clinicopathological Characteristics of High Grade Serous Ovarian Carcinoma Patients.
RAD50 immunoreactivity: RAD50 expression was observed in the nucleus as shown in Figure 2. RAD50 overexpression was low in 32 (29.6%) cases and high in 76 cases (70.4%). RAD50 high expression was significantly associated with advanced stage (p=0.024), LN status (p=0.008) and OS (p<0.001). A statistically significant inverse association was found between RAD50 and ER expression (p<0.001). A statistically significant inverse association was found between RAD50 and PR expression (p=0.002). No association was found between RAD50 high expression and other clinicopathological parameters as shown in (Table 3), Figure 1g, h.
Test of significance: Chi-square test and Fisher’s exact test.
p-value ≤ 0.05 is considered significant.
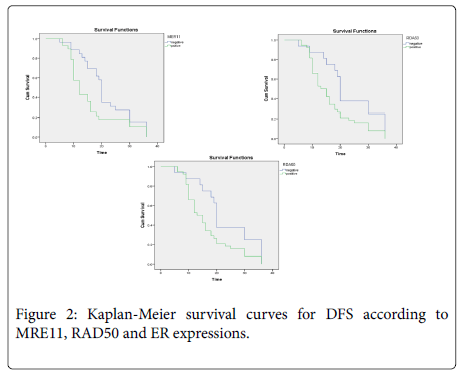
Survival Analysis: The median follow-up was 16 months (range; 5-36 months). Overall survival was not significantly associated with any prognostic clinicopathological factors. Regarding marker expressions and OS, increased expression of MER11, RAD50 and were significantly associated with lower OS (p=0.037 and p=0.026, respectively), (Figure 2).
Discussion
Ovarian carcinomas are considered one of the most complicated obstacles in onco-gynecology [18]. This complexity is due to the presence of a wide range of histological forms of ovarian carcinomas and their different etiopathogenesis [19]. Hormonal factors play the most important role in etiopathogenesis of ovarian carcinomas. Estrogens and progesterone, the same as receptors to them, are promoters of some hormone-dependent tumors, especially breast cancer, endometrial cancer and ovarian cancer [20].
Therapeutic strategy for patients with ovarian carcinomas includes surgical component and chemotherapy. Despite improvement of surgical treatment strategies and application of modern chemotherapy schemes [21], long-term results of treatment in patients with disseminated ovarian carcinomas remain unsatisfactory [22].
Hormonal therapy has been described for patients with chemoresistant ovarian carcinomas [23]. Clinical focus on hormonal treatment of ovarian carcinoma patients was increased due to the update of molecular-biological technologies [24]. However, the use of hormonal therapy in treatment of patients, as well as prognosis depending on expression of hormone receptors are not widely studied.
Prognostic value of hormonal receptor status has been studied for many years. Some studies stated high survival of ovarian carcinoma patients at expression of ER and PR in tumor [25,26]. Other studies shown that expression of PR is favorable prognostic factor, and expression of ER is associated with progression of disease and short relapse-free period [27-29].
DNA double-strand breaks (DSBs) cause base pair mismatch [30] which is directly linked with cancer susceptibility. DSBs repair occurs by three mechanisms: homologous recombination (HR), microhomology mediated end joining (MMEJ) and non-homologous end joining (NHEJ) [31]. HR requires one homologous sequence to repair breaks and repairs DSBs in the late S and G2 phases of the cell cycle when sister chromatids are readily available [32]. MMEJ requires a 5-25 base pair homologous sequence and repairs DSBs in S phase. NHEJ can directly re-ligate broken ends in the absence of a homologous template and repairs DSBs in the G0/G1 and early S phases. The stability of the genome depends on HR as it executes accurate DNA replication [33].
The MRN complex (MRE11-NBS1-RAD50), the checkpoint mediator proteins, is the main domain of HR pathway. It allows DNA repair to occur through cascade signals which arrest cell cycle progression. Therefore, it acts as a barrier to oncogene-induced neoplasia. Alterations in the HR pathway increase the risk of tumorigenesis [34,35].
The clinical significance of MRE11 and RAD50 expressions as clinical biomarkers has been studied in many cancers such breast cancer, gastric cancer, colorectal cancer, and bladder cancer [35,36]. They are involved in the poor prognosis and chemoresistance of human cancers [37].
In our study, immunohistochemical analysis showed high expression of the MRE11 complex (MRE11, RAD50) in high grade serous ovarian carcinoma. High protein expression of MRE11and RAD50 was found in 51.9% and 70.4% of cases, respectively. Our finding was in contrast with results of a previous study that demonstrated a reduced detection of MRE11, and RAD50 in ovarian carcinoma as compared to the control group of serous cystadenomas.
The findings of this study demonstrated that MRE11 and RAD50 overexpression were significantly related to lymph node metastasis and TNM stage. Of interest, we detected a significant relation between high protein expression of MRE11-RAD50 complex and steroid hormone status (ER and PR). We also found that MRE11 and RAD50 protein overexpression is associated with poor OS in high grade serous ovarian carcinoma.
Altan et al. reported that high MRE11 expression is associated with poor overall survival in gastric cancer. Some studies have suggested that high MRE11expression activates DSB repair, leading to increased possibility of local recurrence and reduced survival rates [11].
Gao et al. demonstrated that RAD50 expression is weak in colon cancer and is not associated with clinicopathological factors. On the other hand, they observed increased RAD50 expression in early stage primary colon cancer.
This study detected that ER immunoreactivity was inversely associated with tumor stage, LN status, omentum deposition and OS. No association was found between ER immunoreactivity and other clinicopathological parameters. Also, PR immunoreactivity was inversely associated with stage, LN status, omentum deposition and OS. No association was found between RR immunoreactivity and other clinicopathological parameters.
Halon et al. stated that estrogen receptor expression in ovarian cancer predicts longer overall survival [26]. Chen et al., Chakraborty et al. and Nourieh et al. discussed that estrogen and progesterone receptor expression in ovarian cancer alter the overall survival [27-29].
Our analysis indicates for the first time a putative role of the ER/PR and MRE11- RAD50 pathway as a basis for a better understanding of endocrine and target therapy in high grade serous ovarian carcinoma.
Conclusion
In conclusion, Therapeutic strategies as DNA repair interference mechanisms and endocrine therapy are of great interest to overcome treatment obstacles in high grade serous ovarian carcinoma. The endocrine treatment is based on the high ER/PR immunohistochemical expression as a predictive marker. Further studies with larger patient collective and molecular methods are needed to obtain more detailed and better insight into this research field and to predict the role of the MRE11 complex and its clinical implications for ovarian cancer treatment.
Conflict of Interest and Funding
No conflict of interest
References
- A. Sosulski (2016) CD44 Splice Variant v8-10 as a Marker of Serous Ovarian Cancer Prognosis. PLoS One 11: e0156595.
- J. Ferlay (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144: 1941-1953.
- D. C. Registry, M. C. Registry (2019) “99 375 745†399: 2018-2019.
- P. Wang (2018) Kallikrein-related peptidases 4, 5, 6 and 7 regulate tumour-associated factors in serous ovarian cancer. Br J Cancer 119: 823-831.
- N. Kenda Suster, S. Smrkolj, I. Virant-Klun (2017) Putative stem cells and epithelial-mesenchymal transition revealed in sections of ovarian tumor in patients with serous ovarian carcinoma using immunohistochemistry for vimentin and pluripotency-related markers. J Ovarian Res 10: 11.
- Sallum (2018) WT1, p53 and p16 expression in the diagnosis of low- and highgrade serous ovarian carcinomas and their relation to prognosis. Oncotarget 9: 15818-15827.
- I. Meinhold-Heerlein (2016) The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 293.
- A. J. Cole (2016) Assessing mutant p53 in primary high-grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing. Sci Rep 6.
- L J Huang, X F Deng, F Chang, X L Wu, Y Wu (2018) Prognostic significance of programmed cell death ligand 1 expression in patients with ovarian carcinoma: A systematic review and meta-analysis. Medicine (United States) 97: 43.
- Modugno, F (2012) Hormone response in ovarian cancer: time to reconsider as a clinical target?. Endocr Relat Cancer 19: 255-279.
- B Altan B, Yokobori T, Ide M, Bai T, Yanoma T (2016) High expression of MRE11-RAD50-NBS1 is associated with poor prognosis and Chemoresistance in gastric Cancer. Anticancer Res 36: 5237-5247.
- S. Brandt (2017) Lack of MRE11-RAD50-NBS1 (MRN) complex detection occurs frequently in low-grade epithelial ovarian cancer. BMC Cancer 17: 44.
- G J Williams, S P Lees-Miller, J A Tainer (2010) Mre11-Rad50-Nbs1 conformations and the control of sensing, signaling, and effector responses at DNA double-strand breaks. DNA Repair 9:1299-1306.
- J. Bartkova (2008) Aberrations of the MRE11-RAD50-NBS1 DNA damage sensor complex in human breast cancer: MRE11 as a candidate familial cancer-predisposing gene. Mol Oncol 2: 296-316.
- Llaurado Fernandez M, Dawson A, Kim H, Lam N, Russell H (2019) Hormone receptor expression and outcomes in low grade serous ovarian carcinoma. Gynecol Oncol 14: 31680-31684.
- Altan B, Yokobori T, Mochiki E, Ohno T, Ogata K (2013) Nuclear karyopherin-alpha2 expression in primary lesions and metastatic lymph nodes was associated with poor prognosis and progression in gastric cancer. Carcinogenesis 34: 23142321.
- Heintz APM, Odicino F, Maisonneuve P (2006) Carcinoma of the ovary. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 95: 161-169.
- Svintsitsky VS (2004) Ovarian Cancer: dynamics of certain endocrinologÑ–cal parameters under the complex therapy effect. Clin Endocrin Surg 3: 25-30.
- Maksimov SY, Huseynov KD (2010) Targeted therapy in ovarian cancer. Pract Oncol 11: 54-64.
- Urmancheyeva AF, Tyulyandin SA, Moiseyenko VM (2008) Practical oncogynecology: Selected lectures. St. Petersburg: Center TOMM: 400.
- Schepotin IB, Zotov AS, Anikusko NF (2011). Neoadjuvant hormonal therapy of locally disseminated breast cancer. Clin Oncol 3: 36-9.
- Burges A, Brüning A, Dannenmann C (2010) Prognostic significance of estrogen receptor alpha and beta expression in human serous carcinomas of the ovary. Arch Gynecol Obstet 281: 511-517.
- Halon A, Materna V, Drag-Zalesinska M (2011) Estrogen receptor alpha expression in ovarian cancer predicts longer overall survival. Pathol Oncol Res 17: 511-518.
- Chen S, Dai X, Gao Y, Shen F, Ding J (2017) The positivity of estrogen receptor and progesterone receptor may not be associated with metastasis and recurrence in epithelial ovarian cancer Sci Rep 7: 16922.
- Chakraborty A, Chatterjee S, Roy P (2010) Progesterone receptor agonists and antagonists as anticancer agents. Mini Rev Med Chem 10: 506-517.
- Nourieh Sharifi, Zohreh Yousefi, Shohreh Saeed (2009) Prognostic Values of Estrogen and Progesterone Expression Receptors in Ovarian Papillary Serous Carcinoma. Ir J Pathology 4: 9-12.
- Van Gent DC, Hoeijmakers JHJ, Kanaar R (2001) Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet 2: 196-206.
- Watson JD (1970) Molecular biology of the gene. Molecular biology of the gene (2nd edn).
- Haber JE (2000) Partners and pathways: repairing a double-strand break. Trends Gene 16:259-264.
- Shrivastav M, De Haro LP, Nickoloff JA (2007) Regulation of DNA double-strand break repair pathway choice. Cell Res 18: 134-147.
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A (2006) Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly (ADP-ribose) polymerase inhibition. Cancer Res 66: 8109.
- Pavelitz T, Renfro L, Foster NR, Caracol A, Welsch P (2014) MRE11-deficiency associated with improved long-term disease-free survival and overall survival in a subset of stage III colon cancer patients in randomized CALGB 89803 trial. PLoS One 9: e108483.
- Yuan SS, Hou MF, Hsieh YC, Huang CY, Lee YC (2012) Role of MRE11 in cell proliferation, tumor invasion, and DNA repair in breast cancer. J Natl Cancer Inst 104: 14851502.
- Choudhury A, Nelson LD, Teo MT, Chilka S, Bhattarai S (2010) MRE11 expression is predictive of cause specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res 70: 7017-7026.
- Ali-Fehmi R, Chatterjee M, Ionan A, Levin NK, Arabi H (2010) Analysis of the expression of human tumor antigens in ovarian cancer tissues. Cancer Biomark 6: 33-48.
- Gao J, Zhang H, Arbman G, Sun XF (2008) The different roles of hRAD50 in microsatellite stable and unstable colorectal cancers. Dis Mark 24: 127-134.
- Wang M J, Ping J (2015) Prognostic signiï¬cance and molecular features of colorectal mucinous adenocarcinomas: A strobe-compliant study. Medicine 94: e2350.
Citation: Gayyed FM, El-maqsoud NRA, Soliman MM, Abdel-Hakeem AKA (2020) Do MRE11 Complex and Steroid Hormone Receptors Expressions Impact the Outcomes in High Grade Serous Ovarian Carcinoma?. J Clin Exp Pathol 10: 377. DOI: 10.4172/2161-0681.1000377
Copyright: © 2020 Gayyed FM et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3403
- [From(publication date): 0-2020 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 2561
- PDF downloads: 842