Eco-toxicological studies of effluent of a Chlor-alkali industry on growth and photosynthetic efficiency of a cyanobacterium and possible use of this alga in reclamation of effluent waste
Received: 22-Dec-2023 / Manuscript No. JBRBD-23-123384 / Editor assigned: 26-Dec-2023 / PreQC No. JBRBD-23-123384 (PQ) / Reviewed: 10-Jan-2024 / QC No. JBRBD-23-123384 / Revised: 14-Feb-2025 / Manuscript No. JBRBD-23-123384 (R) / Published Date: 24-Feb-2025
Abstract
All forms of mercury are toxic to plant and animal life. Its use in Chlor-alkali industry as an electrolyte, its discharge into the environment along with effluent and solid waste collected from the effluent canal and dumped near water bodies is alarming. The effluent and solid waste contained significant amount of mercury. Plants around the effluent canal, effluent soaking pond and solid waste dumping site absorbed and accumulated mercury significantly. The experimental alga absorbed and accumulated significant amount of mercury by biosorption and removed significant quantity of mercury by absorption and adsorption. Mercury in the effluent significantly affected the growth (optical density and dry weight), photosynthetic efficiency (pigments) and productivity (PR, RR, GPP) of exposed alga, Westiellopsis prolifica during exposure. This toxicant, mercury contained effluent showed stimulatory effect at maximum allowable concentration and inhibitory impact at higher concentrations of the mercury contained effluent indicating dual behavior of mercury with the alga. At MAC value, the alga grows well and the toxicant could not affect any of the metabolic processes and removes significant amount of mercury from the mercury contaminated effluent. This alga removed mercury by adsorption of Hg on the outer wall and over gelatinous sheath; by absorption from the surrounding environment and accumulating in the cells. The secondary metabolite extract of 4 economically important medicinal plants did not show any reclamation value. However, this alga can be recommended for phycoremediation of mercury contained wastes.
Keywords: Chlor-alkali industry, Effluent, Mercury, Westiellopsis, Growth, Pigments, Photosynthetic efficiency, Plant extract, Productivity, Phytoremediation
Highlights
• Both the effluent collected at discharge point and effluent stocking
pond and the solid waste collected from the contaminated site
contained significant amount of mercury.
• The alga Westiellopsis prolifica, Janet found in crop fields was
resistant and tolerant. This tested alga can absorb mercury from
the environment and can retain it.
• The effluent containing mercury can affect the algal growth,
photosynthetic efficiency and gross production significantly.
• At maximum allowable concentration, the mercury contained
effluent stimulated growth and at higher concentrations of the
toxicant inhibited growth.
• Plant leaf extracts used for phytoremediation did not give any
positive sign of remediation of mercury contained waste. But
the biosorption and adhesion of mercury by alga could remove
an interesting amount of mercury from mercury contaminated
environments.
• This alga can be used as a representative in phycoremediation of
effluent for removal of mercury from the mercury contaminated
environment.
Introduction
Most of the Chlor-alkali industries in India were using old electrolysis process using mercury as cathode till 2012. These industries were causing severe mercury pollution problem in and around the industry causing contamination of all the environmental segments. Many reports including the reports of Prusti and Panigrahi indicated the intensity of mercury contamination problem in the surrounding environment. As per new guideline and direction from Department of Environment and Forest, GoI and CPCB, all the Chlor-alkali industries changed the mercury cell process to membrane technology process for manufacturing caustic soda. The present industry under study started changing its technology from Mercury cell process to membrane process from 2013 onwards and the replacement process was complete by 2014. The surrounding contaminated sites where solid waste was dumped was replaced by fresh soil and mud and effluent was removed from the effluent stocking pond and fresh mercury free effluent was stocked between 2014 to 2016. The reports of Prusti and Panigrahi clearly indicated that all the plants in and around the solid waste dumping site and effluent stocking area contained significant amount of mercury in their tissues. It is a well-established fact that all heavy metals including mercury induce serious damages to physiological and biochemical processes and cell organelles, ultimately causing death of the organism. It is also proved beyond doubt that these heavy metals including mercury bio-accumulate in independent organisms increasing the load of each and every trophic level. These heavy metals pass from one trophic level to another higher trophic level through food chain link up, leading to bio-magnification of the heavy metals in the ecosystem. Sahu reported the impact mercury contained effluent and solid waste on algae under laboratory controlled conditions. The same author also showed the possible use of BGA for phycoremediation of effluent of the Chlor-alkali industry. The present study was planned to study the impact of mercury contained effluent waste on cyanobacterium and possible use of the blue-green alga for remediation of effluent waste coming out of the Chlor-alkali industry [1].
Materials and Methods
The industry under study
The chlor-alkali industry M/s. Jayashree Chemicals Pvt. Ltd., is located at Ganjam, on the Bank of Rushikulya estuary about 1.5 km away from the Sea, Bay of Bengal, on the east and 30 km. North of Berhampur city, on the South-Eastern side of India at 84°53’E longitude and 19°16’N latitude, is under study, to assess the impact of dumped mercury contained effluent and solid waste discharged from the industry in to the environment (Figure 1) [2].
Test organism: Westiellopsis prolifica, Janet; Family: Stigonemataceae.
Toxicant: Effluent of the Chlor-alkali industry collected from effluent canal.
Physico-chemical analyses of effluent and solid waste and crop field soil samples of nearby crop fields were conducted periodically by following the procedure of APHA (1998), EC (1979), standardized field analysis kit and portable instruments. Effluent samples were brought in glass containers and stored in cold room for use in laboratory experimental work. The alga was grown in Allen and Arnon’s culture medium with trace elements, modified by Patnaik. The cultures were maintained in culture racks at an illumination of 2200 ± 200 lux and temperature was maintained at 26°C ± 2°C. The culture flasks were hand shaken twice daily to avoid clumping and adherence of the algal filaments to the walls of the culture vessels. Growth of the alga was measured by light scattering technique and dry weight of the alga in the culture flasks was estimated by filtering the culture through a pre-weighed Whatman filter paper. The filter paper with algal mass was dried in an oven at 70°C for 48 hrs, cooled and the weight was recorded in a single pan balance. The amount of total chlorophyll and phaeophytin was estimated and calculated by following the procedure and using the formula given by Vernon. The rate of oxygen evolution due to photosynthesis and oxygen consumption due to respiration was measured manometrically at 37°C and 2400 ± 200 lux light intensity in a photo-Warburg’s apparatus (New Paul, India) following the procedures of Hannan and Potouillet, Oser and as modified by Sahu. Measurement of mercury of the collected effluent, solid waste and algal samples were carried out by following the protocols of Wanntorp and Dyfverman. Samples were digested in Bethge’s apparatus in acid digestion mixture and mercury content was estimated in a Mercury Analyzer and for confirmation of data in Atomic Absorption Spectrophotometer. The obtained data was statistically calculated to find out level of significance [3].
Results
The effluent and solid waste analysis indicated presence of significant amount of mercury far higher than the prescribed limit. The pH of the effluent was highly alkaline. The amount of nitrogen and phosphorus available in the effluent supports the growth of the alga. Higher values of sodium, potassium and magnesium ions were present in the mercury contained solid waste (Table 1) [4].
| Sl. no | Parameters | Effluent storage tank, (mg /l) | Effluent from discharge point (mg/l) | Solid waste clay, grayish white. (mg/kg-1 dry wt) | SW Extract prepared from SW (mg/l) |
|---|---|---|---|---|---|
| 1 | Temperature (°C) | 29.2 ± 1.5 | 30.2 ± 1.5 | 26 ± 2 | 26 ± 2 |
| 2 | pH | 9.2 ± 0.3 | 9.3 ± 0.2 | 7.8 ± 0.6 | 7.4 ± 0.4 |
| 3 | Total nitrogen | 2.6 ± 0.5 | 2.4 ± 0.5 | - | - |
| 4 | Total phosphorus | 1.28 ± 0.18 | 0.21 ± 0.06 | 59.0 ± 10.9 | 42.6 ± 8.1 |
| 5 | Mercury | 0.49 ± 0.06 | 1.08 ± 0.32 | 728.5 ± 28.5 | 12.86 ± 1.32 |
| 6 | Potassium | - | - | 96.8 ± 10.2 | 42.5 ± 13.6 |
| 7 | Sodium | - | - | 164.2 ± 36.1 | 5.2 ± 0.8 |
| 8 | Magnesium | - | - | 56.3 ± 10.2 | 31.4 ± 16.8 |
Table 1: Physico-chemical analysis of the effluent collected from a select site-SE-3 of the effluent storage tank of the Chlor-alkali industry. Data are the mean of 4 estimations ± standard deviation.
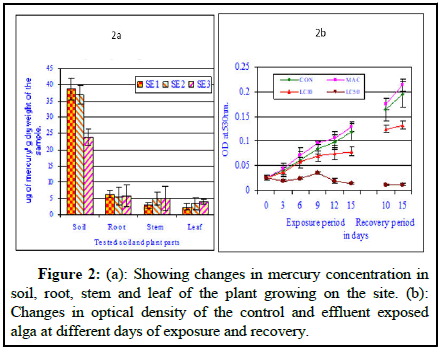
Figure 2 showed the residual mercury concentration in plant parts (root, stem and leaf) collected from the contaminated sites and mercury concentration in soil collected from the surrounding roots of the plants. The sediment collected from the effluent canal periphery contained significant amount of mercury. The amount of total mercury present was 38.65 ± 3.36 μg of mercury/g dry weight soil/sediment. The plant root collected from the same zone contained 6.33 ± 1.14 μg of mercury/g dry weight plant part; the plant stem portion collected from the same plant contained 2.95 ± 0.85 μg of mercury/g dry weight plant part and the plant leaf collected from the same plant contained 2.35 ± 0.98 μg of mercury/g dry weight plant part. The sediment collected from the effluent canal periphery contained significant amount of total mercury. The amount of total mercury present was 36.98 ± 2.82 μg of mercury/g dry weight soil /sediment. The plant root collected from the same zone contained 5.61 ± 2.85 μg of mercury/g dry weight plant part; the plant stem portion collected from the same plant contained 4.89 ± 2.14 μg of mercury/g dry weight plant part and the plant leaf collected from the same plant contained 3.37 ± 1.85 μg of mercury/g dry weight plant part (Figure 2a). The plants collected from the sides of the effluent storage pond showed significant amount of residual mercury. The sediment collected from the effluent canal periphery contained significant amount of total mercury. The amount of total mercury present was 23.88 ± 2.46 μg of mercury/g dry weight soil/sediment. The plant root collected from the same zone contained 5.85 ± 3.33 μg of mercury/g dry weight plant part; the plant stem portion collected from the same plant contained 4.85 ± 3.66 μg of mercury/g dry weight plant part and the plant leaf collected from the same plant contained 3.95 ± 0.66 μg of mercury/g dry weight plant leaf (Figure 2b). The solid waste collected from the surface of the solid waste dumping site contained significant amount of total mercury to the tune of 316.8 ± 32.4 μg of mercury/g dry weight collected from the surface of the solid waste dump site [5].
The LC00, LC10, LC50, LC90 and LC100 values of the Chlor-alkali industry effluent were deduced from toxicity testing. The maximum allowable concentration for the tested alga was found to be 0.092 ml of the effluent/50 ml culture for 15 days of exposure. The Lethal Concentration (LC10) and PS90 value was found to be 0.11 ml of the effluent/50 ml culture solution where 10% death and 90% survival was noted. The Lethal Concentration (LC50) value was found to be 0.21 ml of the effluent/50 ml culture solution where 50% death was noted. The Lethal Concentration (LC90) value found to be 0.32 ml of the effluent/50 ml culture solution where 90% death was noted. At 0.41 ml effluent solution/50 ml culture, 100% death of the alga was observed. From the toxicity data we can clued that more than 0.41 ml/50 ml culture is deadly toxic where the exposed alga is unable to survive where percent survival was zero. The death of the exposed system depends upon its age, tolerance, resistance, physiological ability to withstand stress and biochemical mechanism of the organism to defend stress [6].
Figure 2 showed the changes Optical Density (OD) of control and effluent exposed cultures at different days of exposure and at different effluent concentrations. The optical density of the homogenized culture increased with the increase in exposure period and effluent concentration except few exceptions where either decrease followed by increase or increase followed by decrease in OD value was noted. With the increase in effluent concentration no clear trend was noted. In general it can be inferred that with the increase in exposure period the optical density value increased indicating a positive growth in the control set showing a positive correlation. The optical density of the culture medium increased with the increase in exposure period up to 15 days, showing a highly significant positive correlation (0.997; P ≥ 0.001). The optical density values were much higher in case conc. APA (MAC value) compared to control values at all exposure periods and showed highly significant positive correlation (0.993; P ≥ 0.001). The optical density values were not much higher in case conc. AP.B (LC10 value) compared to control values at all exposure periods and showed a positive significant correlation (0.966; P ≥ 0.01). The optical density values were much less compared to control value and the values observed in case conc. AP.C (LC50 value) compared to control values at all exposure periods and showed a positive but insignificant correlation (0.349; P=NS) [7].
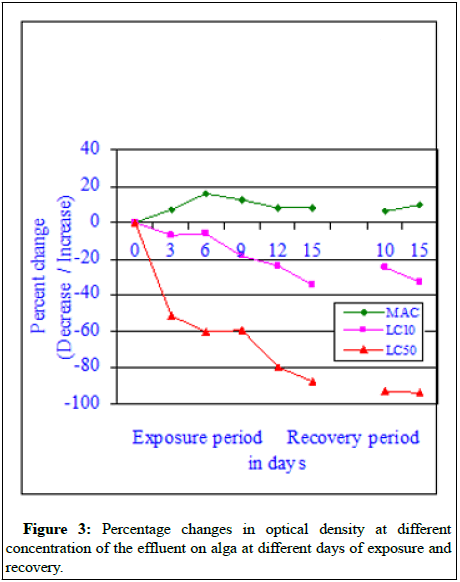
The percent changes in optical density values (Figure 3) at different exposure period and at different concentrations effluent indicated a different line of thought. In case of MAC value (sub-lethal concentration) the optical density values were much more than the control optical density values at all exposure periods and recovery periods. From the observed data it can be said that at sub-lethal concentration of the effluent, the effluent exposed alga showed better growth compared to control alga. However, with the increase in effluent concentration further the optical density values decreased compared to control values indicating the impact of effluent used for the experiment. From the observed it can be concluded that the effluent has a bad impact on the effluent exposed alga and at higher concentrations of the effluent, the impact was severe and it can be also deadly toxic. Regression analysis at each day of tested exposure indicated that all regression values were negative and significant at 0.01 (P) [8].
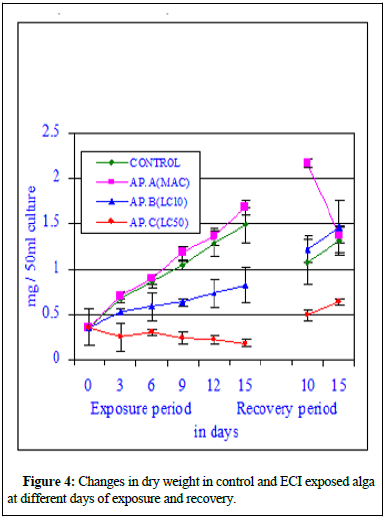
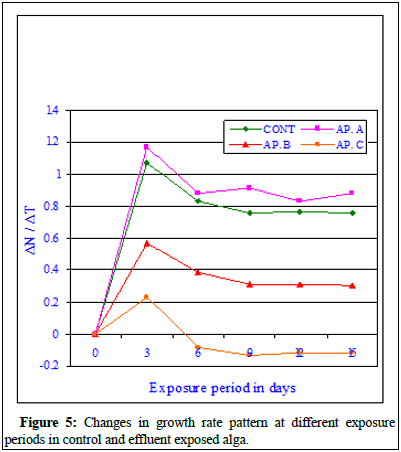
Figure 4 showed the changes in dry weight in experimental cultures, where one set served as control and the other set was exposed to toxicant (effluent). The dry weight of the control alga showed a normal sigmoid growth curve indicating increase in dry weight with the exposure period showing a positive correlation (r=0.996; P ≥ 0.001). The dry weight of the effluent exposed at sub-lethal concentration (MAC value) alga showed a significant sigmoid growth curve indicating increase in dry weight with the exposure period compared to its respective control values and also showing a positive correlation (r=0.996; P ≥ 0.001). The increase in dry weight at MAC value better than control alga indicated the stimulation induced by the toxicant at sub-lethal concentrations. The dry weight of the exposed alga at LC10 values increased significantly (r=0.983; P ≥ 0.01) similar to control set. At LC50 values, the dry weight of the exposed alga decreased insignificantly (r=-0.874; P ≥ 0.05) with the increase in exposure period and the dry weight of the exposed alga decreased insignificantly with the increase in toxicant concentration (r=-0.902; P ≥ 0.01 on 3rd day of exposure, r=-0.943; P ≥ 0.01 on 6th day of exposure, r=-0.922; P ≥ 0.01 on 9th day of exposure, r=-0.944; P ≥ 0.01 on 12th day of exposure and r=-0.930; P ≥ 0.01 on 15th day of exposure). Figure 5 indicated that the exposed alga showed better growth at maximum allowable concentration of the effluent compared to other higher concentrations of the toxicant. Figure 5 showed the Growth Rate (GR) pattern of control and exposed alga at different exposure period and at different effluent concentrations. The rate of growth of the experimental alga was very high and significant at initial periods of inoculation in control and at Conc. AP.A (MAC value). The Growth Rate (GR) depleted at higher effluent conc. AP.B (LC10) and least growth was observed in AP.C (LC50). After 9th day of exposure the growth rate steadied but higher values were marked at sub-lethal concentrations. The GR data indicated that the effluent at sub-lethal concentration is growth stimulatory and at higher concentration growth inhibitory. This data also indicated that if the effluent can be diluted sufficiently and if we can bring down the concentration of mercury and other chemical parameters to sub-lethal level the effluent mixed water can be used for growing the BGA in crop fields for production of extra-cellular nitrogenous waste which will be helpful for the crop production and can substitute the chemical fertilizers [9].
Figure 6 indicated the residual mercury accumulation by BGA during the experimental period and recovery period at different concentrations of the effluent under controlled conditions. With the increase in effluent concentration the residual accumulation increased at all exposure periods and at all effluent concentrations. The residual mercury level showed steady increase in residual mercury level with the increase in exposure period. The increase was positive and showed a positive correlation. The residual mercury concentration also increased with the increase in effluent concentration in the exposed culture indicating a positive correlation. No mercury was traceable in the alga growing in the control set. At 0.1 ml of effluent/50 ml culture, the residual mercury concentration increased to 3.9 ± 0.5 μg/g dry weight on 3rd day and the value increased to 6.1 ± 0.6 μg/g dry weight on 9th day of exposure and residual mercury further increased to 7.4 ± 0.8 μg/g dry weight on 15th day of exposure (Figure 6). At 0.2 ml of effluent/50 ml culture, the residual mercury concentration increased to 4.1 ± 0.3 μg/g dry weight on 3rd day and the value increased to 5.9 ± 0.5 μg/g dry weight on 9th day of exposure and residual mercury further increased to 7.9 ± 0.5 μg/g dry weight on 15th day of exposure. At 0.3 ml of effluent/50 ml culture, the residual mercury concentration increased to 3.8 ± 0.5 μg/g dry weight on 3rd day and the value increased to 5.1 ± 0.3 μg/g dry weight on 9th day of exposure and residual mercury further increased to 7.8 ± 0.6 μg/g dry weight on 15th day of exposure. At 0.4 ml of effluent/50 ml culture, the residual mercury concentration increased to 4.1 ± 0.4 μg/g dry weight on 3rd day and the value increased to 5.7 ± 0.5 μg/g dry weight on 9th day of exposure and residual mercury further increased to 7.6 ± 0.4 μg/g dry weight on 15th day of exposure. Figure 6 showed residual mercury accumulation at different effluent concentrations in the culture flask at different days of exposure and recovery. With the increase in effluent concentration the residual accumulation increased at all exposure periods and at all effluent concentrations and residual mercury level decreased during recovery period, mostly due to excretion of mercury. The residual mercury level showed a positive trend with the increase in exposure period and with the increase in effluent concentration. The residual mercury concentration increased with the increase effluent concentration and a maximum of residual mercury was observed in the exposed BGA on 15th day of exposure at 0.4% effluent concentration (Figure 6) [10].
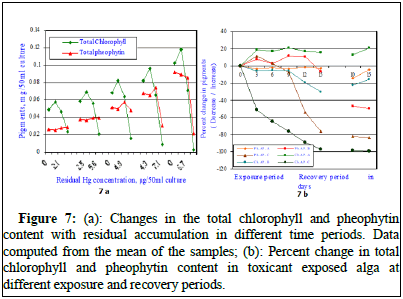
The total chlorophyll content of the exposed alga increased from 0.019 ± 0.008 mg/50 ml culture to 0.056 ± 0.014 ml/50 ml culture on 6th day; to 0.067 ± 0.015 mg/50 ml culture on 9th day; to 0.099 ± 0.014 ml/50 ml culture on 12th day and to 0.114 ± 0.012 mg/50 ml culture after 15 days of exposure in the control set. During recovery studies, the total chlorophyll content further increased to 0.131 ± 0.007 mg/50 ml culture after 10th day and increased to 0.156 ± 0.011 mg/50 ml culture on 15th day of recovery indicating significant normal growth. When the alga was exposed to sub-lethal concentration of Chlor-alkali industry effluent (MAC value; AP.A), the total chlorophyll content of the exposed alga increased from 0.019 ± 0.008 mg/50 ml culture to 0.057 ± 0.06 mg/50 ml culture on 3rd day; to 0.068 ± 0.06 mg/50 ml culture on 6th day; to 0.084 ± 0.03 mg/50 ml culture on 9th day; to 0.096 ± 0.05 mg/50 ml culture on 12th day and to 0.124 ± 0.009 mg/50 ml culture after 15 days of exposure. All the values in each exposure period are much higher than the respective control values and in set AP.A. During recovery studies, the total chlorophyll content further significantly increased to 0.151 ± 0.007 mg/50 ml culture after 10th day of recovery and increased to 0.174 ± 0.016 mg/50 ml culture on 15th day of recovery indicating significant growth much higher than the respective control values as shown in Figure 7. The second set alga was exposed to LC10 dose of Chlor-alkali industry effluent (AP.B), the total chlorophyll content of the exposed alga increased from 0.019 ± 0.008 mg/50 ml culture to 0.044 ± 0.012 mg/50 ml culture on 3rd day; to 0.052 ± 0.014 mg/50 ml culture on 6th day; to 0.062 ± 0.18 mg/50 ml culture on 9th day; to 0.065 ± 0.014 mg/50 ml culture on 12th day and decreased to 0.062 ± 0.016 mg/50 ml culture after 15 days of exposure. All the values were significantly less than the respective control values and the values shown at AP.A. All the values in each exposure period are much lesser than the respective control values and in set AP.A. During recovery studies, the total chlorophyll content increased to 0.079 ± 0.018 mg/50 ml culture after 10th day of recovery and increased to 0.093 ± 0.014 mg/50 ml culture on 15th day of recovery indicating negative impact on growth much lesser than the respective control values (Figure 7). The third set alga was exposed to LC50 dose of chlor-alkali industry effluent (AP.C), the total chlorophyll content of the exposed alga insignificantly increased from 0.019 ± 0.008 mg/50 ml culture to 0.021 ± 0.002 mg/50 ml culture on 3rd day; to 0.022 ± 0.004 mg/50 ml culture on 6th day; decreased to 0.015 ± 0.008 mg/50 ml culture on 9th day; further decreased to 0.008 ± 0.004 mg/50 ml culture on 12th day of exposure and significantly decreased to 0.002 ± 0.0008 mg/50 ml culture after 15 days of exposure to Chlor-alkali industry effluent. All the values were significantly less than the respective control values and the values shown at AP.A and B. All the observed values in each exposure period were much lesser than the respective control values and in set AP.A and B. During recovery studies, the total chlorophyll content significantly decreased to 0.0022 ± 0.0007 mg/50 ml culture after 10th day of recovery and further decreased to 0.0014 ± 0.0004 mg/50 ml culture on 15th day of recovery indicating negative severe impact on growth and the obtained values were much lesser than the respective control values (Figure 7). The regression analysis calculated between days of exposure and total chlorophyll content in control and effluent exposed alga at control and select effluent concentrations indicated positive correlations. The regression values were r=0.988, P ≥ 0.01 in control; r=0.984, P ≥ 0.01 at MAC value (AP.A); r=0.938, P ≥ 0.01 at LC10 effluent concentration and at LC50 a negative correlation was marked (r=-0.841, P ≥ 0.01) [11].
Figure 7: (a): Changes in the total chlorophyll and pheophytin content with residual accumulation in different time periods. Data computed from the mean of the samples; (b): Percent change in total chlorophyll and pheophytin content in toxicant exposed alga at different exposure and recovery periods.
The regression analysis clearly indicated better growth in maximum allowable concentration (AP.A) of the effluent indicating stimulatory effect of the effluent and significant decreases in total chlorophyll content indicating inhibitory effects of the effluent at LC10 and LC50 values of the effluent indicating the extreme poisonous nature of the toxicant tested. The total chlorophyll content decreased by 8.3% on 3rd day, 21.4% on 6th day, 7.1% on 9th day, 7.5% on 12th day and by 34.3% on 15th day of exposure in LC10 (AP.B) of the effluent toxicant. The total chlorophyll content decreased by 56.3% on 3rd day, 60.7% on 6th day, 77.6% on 9th day, 91.9% on 12th day and by 98.2% on 15th day of exposure in LC50 (AP.C) of the effluent toxicant (Figure 7a). During recovery period, in AP.A dose the total chlorophyll content increased by 15.3% on 10th day and 11.5% increase on 15th day of recovery compared to their respective control values. During recovery period, in AP.B dose the total chlorophyll content decreased by 39.7% on 10th day and 40.4% increase on 15th day of recovery compared to their respective control values. The alga showed recovery by 5.9% on 10th day and 5.2% on 15th day of recovery. However in case of conc. AP.C, no recovery was noted rather further depletion in the pigment content was seen. During recovery period further depletion to the extent 99.1% was observed indicating no recovery of total chlorophyll content (Figure 7b). The total phaeophytin content increased from 0.016 ± 0.005 mg/50 ml culture to 0.098 ± 0.011 mg/50 ml culture after 15 days of exposure in the control set. During recovery studies, the total phaeophytin content further increased to 0.152 ± 0.007 mg/50 ml culture after 10 days of recovery and increased to 0.171 ± 0.014 mg/50 ml culture after 15 days of recovery. In case of exposure to sublethal concentration of the effluent (MAC value, AP.A) the total phaeophytin content decreased from 0.098 ± 011 mg/50 ml culture to 0.078 ± 0.014 mg/50 ml culture after 15 days of exposure showing 20.4% decrease over the control value. When the effluent exposed alga was transferred to effluent free medium, the total phaeophytin content significantly increased to 0.126 ± 0.018 mg/50 ml culture on 10th day of recovery showing 17.1% increase over 15 d exposure value and to 0.172 ± 0.022 mg/50 ml culture on 15th day of recovery showing 0.6% increase over respective control value (Figure 7). In case of exposure to lethal concentration of the effluent (LC10, AP.B) the total phaeophytin content decreased from 0.098 ± 011 mg/50 ml culture to 0.074 ± 0.012 mg/50 ml culture after 15 days of exposure and decreased by 24.5% from its control value. When the effluent exposed alga was transferred to effluent free medium, the total phaeophytin content insignificantly increased to 0.084 ± 0.008 mg/50 ml culture on 10th day of recovery compared to 15 d exposure value and insignificantly increased to 0.095 ± 0.011 mg/50 ml culture on 15th day of recovery. In case of exposure to lethal concentration of the effluent (LC50, AP.C) the total phaeophytin content decreased from 0.098 ± 011 mg/50 ml culture on 15d control value to 0.019 ± 0.005 mg/50 ml culture after 15 days of exposure. But the pheophytin content insignificantly increased from 0.016 ± 0.005 mg/50 ml culture on zero day of exposure to 0.019 ± 0.005 mg/50 ml culture after 15 days of exposure. When the effluent exposed alga was transferred to effluent free medium, the total phaeophytin content insignificantly increased to 0.026 ± 0.006 mg/50 ml culture on 10th day of recovery and to 0.034 ± 0.008 mg/50 ml culture on 15th day of recovery (Figure 7). It was observed that the pheophytin content depleted at all tested exposure period. No significant recovery was noted. The regression analysis indicated all negative correlations when compared with increasing effluent concentrations. The data is very clear and at maximum allowable concentration the total phaeophytin content decreased insignificantly at all exposure and during recovery periods (Table 2) [12].
| S. no. | Plant extract | Used values as obtained from toxicity testing | Dry weight, µg/50 ml culture | Percent change (%) | Total chlorophyll content, mg/50 ml culture | Percent change (%) |
|---|---|---|---|---|---|---|
| 1 | Control | Nutrient medium | 25.6 ± 0.8 | = | 0.752 ± 0.014 | = |
| 2 | Effluent (E) | MAC value | 28.3 ± 0.4 | 10.55 | 0.814 ± 0.026 | 8.24 |
| 3 | Ocimum sanctum | 0.71%+E | 24.8 ± 0.3 | -3.13 | 0.748 ± 0.012 | -0.53 |
| 4 | Azadirachta indica | 0.62%+E | 22.6 ± 0.5 | -11.72 | 0.714 ± 0.023 | -5.05 |
| 5 | Cyanodon dactylon | 0.54%+E | 24.8 ± 0.3 | -3.13 | 0.735 ± 0.041 | -2.26 |
| 6 | Tridax procumbens | 0.48%+E | 21.4 ± 0.7 | -16.41 | 0.692 ± 0.021 | -7.98 |
Table 2: Impact of plant extracts in presence of Chlor-alkali industry effluent at MAC value on a cyanobacterium after 30 days of exposure.
Table 2 indicated the changes in optical density, dry weight, and the pigment content in control, alga exposed to effluent extract and cultures exposed to different plant extracts. The experiments were designed to study the reclamation of the toxic wastes by the plant extracts in presence of a blue-green alga, which fixes atmospheric nitrogen. The exudates of the alga help for detoxification. The data indicated that in absence of the plant extracts, the alga could not grow and the white turbid mass clearly indicated that the pigments of the inoculated alga were destroyed by the toxicant. Whereas, when leaf extract was applied in rest of the cultures, the alga showed better growth and no trace of inhibition was marked. This evidence clearly indicated that probably the plant leaf extracts helped for reclamation of the toxicants. Table 2 also supported the morphometric observation. The optical density and dry weight data did show some variation in pure toxicant medium, when compared to control and other exposed cultures but the pigment change data were clear indicative of reclamation caused by the plant extracts. However, when the exposed cultures were inoculated with plant leaf extracts, the optical density values increased significantly, indicating better growth of the alga at all exposure concentrations. The optical density value increased from 0.199 to 0.359. In all the sets where plant extracts were applied, the optical density value increased significantly. Plant extracts were applied to observe the possible reclamation in presence of the cyanobacterium. Interestingly, the dry weight data indicated that in all plant extract application, the alga survived normally but the dry weight values were less than the control value. It can be inferred that after application of the toxicants, the alga showed suppression in the parameters but in presence of plant extracts and the cyanobacterium, the parameters did not show any significant depression rather no significant variation in dry weight compared to standard control was observed after 15 days of exposure. It was observed that if the exposed alga was allowed to continue for 30 more days, better results were obtained. All the parameters increased significantly when compared to the control value and after 45 days of exposure, all the values increased but the pigment color changes from blue-green to pale yellow. The change in color might be due to increase in extra-cellular products which exuded in to the medium with strong nitrogen base. These extra-cellular products inhibit further growth of the alga. This might have shown a synergistic effect along with the plant extracts [13].
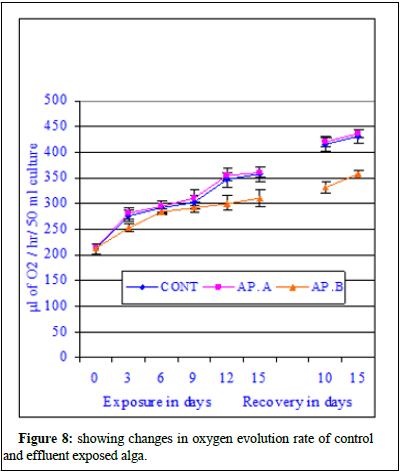
Figure 8 showed the changes in oxygen evolution rate/photosynthetic rate of control and chlor-alkali industry effluent exposed cyanobacterium at different days of exposure and recovery. The photosynthetic rate of control algal set increased from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 211.4 ± 8.2 μl of O2 evolved/hr/50 ml culture on 3rd day of exposure, from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 231.6 ± 11.2 μl of O2 evolved/hr/50 ml culture on 6th day of exposure, from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 265.8 ± 15.6 μl of O2 evolved/hr/50 ml culture on 9th day of exposure, from 165.2 ± 6.2 μl of O2 evolved /hr/50 ml culture to 288.4 ± 17.8 μl of O2 evolved/hr/50 ml culture on 12th day of exposure and increased from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 291.4 ± 11.4 μl of O2 evolved/hr/50 ml culture on 15th day of exposure. The photosynthetic rate of effluent exposed (AP.A) algal set increased from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 221.4 ± 11.4 μl of O2 evolved/hr/50 ml culture on 3rd day of exposure, from 165.2 ± 6.2 μl of O2 evolved /hr/50 ml culture to 246.2 ± 12.4 μl of O2 evolved/hr/50 ml culture on 6th day of exposure, from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 271.8 ± 9.4 μl of O2 evolved/hr/50 ml culture on 9th day of exposure, from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 294.9 ± 14.5 μl of O2 evolved/hr/50 ml culture on 12th day of exposure and increased from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 311.6 ± 8.6 μl of O2 evolved/hr/50 ml culture on 15th day of exposure. The photosynthetic rate of effluent exposed (AP.B) algal set increased from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 171.2 ± 8.4 μl of O2 evolved/hr/50 ml culture on 3rd day of exposure, from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 184.4 ± 11.3 μl of O2 evolved/hr/50 ml culture on 6th day of exposure, from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 1961.6 ± 19.2 μl of O2 evolved/hr/50 ml culture on 9th day of exposure, from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 204.5 ± 8.8 μl of O2 evolved/hr/50 ml culture on 12th day of exposure and increased from 165.2 ± 6.2 μl of O2 evolved/hr/50 ml culture to 195.1 ± 11.2 μl of O2 evolved/hr/50 ml culture on 15th day of exposure. During recovery studies no significant improvement was marked. In case conc. AP.A, little increment was noted and the obtained values were much more than the respective control values similar to the trend observed during exposure period, where an increase in photosynthetic rate value was marked in all tested exposure periods. From the data it seems at sub-lethal concentration of the mercury contained effluent an increment similar to stimulation was noticed. At higher concentrations of the effluent (AP.B) decrease in photosynthetic rate was observed when compared to their respective control values in different exposure periods. The photosynthetic rate increased with exposure period in case of conc. AP.B like the control set and also exposed set at conc. AP.A. The observed decrease in the photosynthetic rate was not highly significant and cannot be attributed to solely to inhibitory effect of the pollutant. The inhibitory effect of the effluent can be substituted by residual mercury accumulation in the algal body which might have raised the algal body burden and direct interference of mercury with cellular chemicals involved in either photosynthesis processes. The photosynthetic rate of control algal set increased from 291.4 ± 11.4 μl of O2 evolved/hr/50 ml culture to 315.4 ± 12.6 μl of O2 evolved/hr/50 ml culture on 10th day of recovery and from 315.4 ± 12.6 μl of O2 evolved/hr/50 ml culture to 345.2 ± 11.4 μl of O2 evolved/hr/50 ml culture on 15th day of recovery. The photosynthetic rate of exposed set AP.A increased from 311.6 ± 8.6 μl of O2 evolved/hr/50 ml culture to 332.4 ± 8.3 μl of O2 evolved/hr 50 ml culture on 10th day of recovery and from 332.4 ± 8.3 μl of O2 evolved /hr/50 ml culture to 361.9 ± 18.5 μl of O2 evolved/hr/50 ml culture on 15th day of recovery. The photosynthetic rate of exposed set AP.B increased from 195.1 ± 11.2 μl of O2 evolved/hr/50 ml culture to 211.2 ± 9.2 μl of O2 evolved/hr/50 ml culture on 10th day of recovery and from 211.2 ± 9.2 μl of O2 evolved/hr/50 ml culture to 225.6 ± 11.4 μl of O2 evolved/hr/50 ml culture on 15th day of recovery (Figure 8) [14].
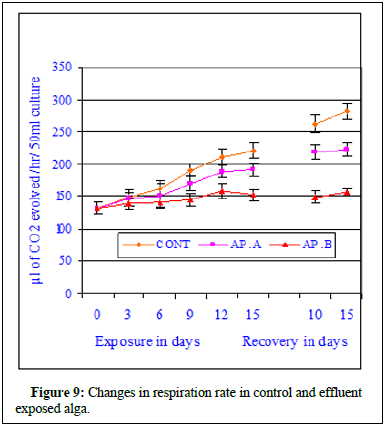
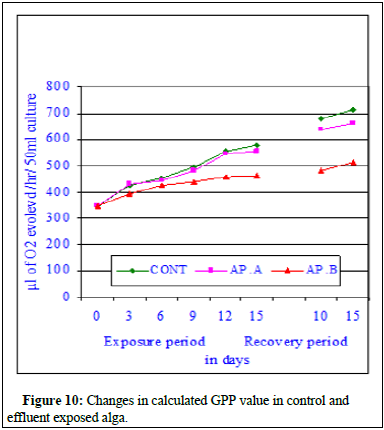
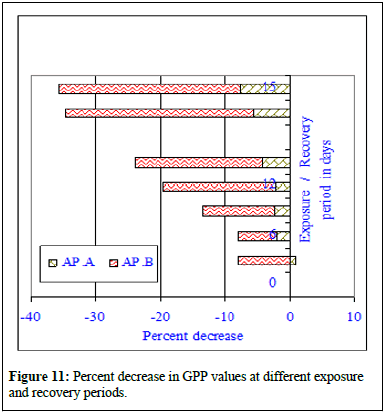
The regression analysis indicated all initial positive and then followed by negative correlations when compared with increasing effluent concentrations. The regression values were r=0.964, P ≥ 0.01 in control; r=0.960, P ≥ 0.01 at MAC value (AP.A); r=0.935, P ≥ 0.01 at LC10 effluent concentration and at LC50 a negative correlation was marked (r=-0.841, P ≥ 0.01). The ANOVA test indicated the similarity in trend between rows and non-similarity between columns. The regression analysis clearly indicated better growth in maximum allowable concentration of the effluent indicating probable stimulatory effect of the effluent and significant decreases in photosynthetic rate indicating inhibitory effects of the effluent on the alga. The data is very clear and at maximum allowable concentration the photosynthetic rate increased insignificantly at all exposure and recovery periods. The percent change in photosynthetic rate decreased insignificantly at LC10 value of the effluent. From the data it can be concluded that at LC10, there was no significant depletion in photosynthetic rate but the impact of the effluent was very clear. Significant decrease of photosynthetic rate in LC50 effluent concentration was indicative of toxicity of the effluent and the cause of photosynthetic rate depletion was due to mercury contained effluent. On 15th day of exposure at MAC value of effluent, 6.9% increase in photosynthetic rate was noted whereas at LC10 33.04% decrease in photosynthetic rate was noted compared to control value. During recovery period further depletion to the extent 34.6% at LC50 was observed indicating no recovery of photosynthetic rate. Figure 9 showed the changes in respiration rate of control and Chlor-alkali industry effluent exposed cyanobacterium at different days of exposure and recovery. The respiration rate of control algal set increased from 128.4 ± 4.2 μl to 147.6 ± 11.2 μl of CO2 evolved/hr/50 ml culture on 3rd day, from 128.4 ± 4.2 μl to 161.4 ± 8.4 μl of CO2 evolved/hr 50 ml culture on 6th day, from 128.4 ± 4.2 μl to 189.4 ± 11.6 μl of CO2 evolved/hr/50 ml culture on 9th day, from 128.4 ± 4.2 μl to 202.6 ± 12.8 μl of CO2 evolved/hr/50 ml culture on 12th day and from 128.4 ± 4.2 μl to 218.2 ± 11.4 μl of CO2 evolved /hr/50 ml culture on 15th day of exposure. The respiration rate of effluent exposed (AP.A) algal set increased from 128.4 ± 4.2 μl of CO2 evolved/hr/50 ml culture to 145.7 ± 9.4 μl of CO2 evolved/hr/50 ml culture on 3rd day of exposure, from 128.4 ± 4.2 μl of CO2 evolved/hr/50 ml culture to 151.4 ± 13.2 μl of CO2 evolved/hr/50 ml culture on 6th day of exposure, from 128.4 ± 4.2 μl of CO2 evolved/hr/50 ml culture to 172.4 ± 9.4 μl of CO2 evolved/hr/50 ml culture on 9th day of exposure, from 128.4 ± 4.2 μl of CO2 evolved/hr/50 ml culture to 187.2 ± 8.8 μl of CO2 evolved/hr/50 ml culture on 12th day of exposure and increased from 128.4 ± 4.2 μl of CO2 evolved/hr/50 ml culture to 189.9 ± 6.8 μl of CO2 evolved/hr/50 ml culture on 15th day of exposure. The respiration rate of effluent exposed (AP.B) algal set increased from 128.4 ± 4.2 μl of CO2 evolved /hr/50 ml culture to to 132.5 ± 4.6 μl of CO2 evolved/hr/50 ml culture on 6th day of exposure, from 128.4 ± 4.2 μl of CO2 evolved/hr/50 ml culture to 142.6 ± 9.8 μl of CO2 evolved/hr/50 ml culture on 12th day of exposure and increased from 128.4 ± 4.2 μl of CO2 evolved/hr/50 ml culture to 139.8 ± 14.5 μl of CO2 evolved/hr/50 ml culture on 15th day of exposure (Figure 10). During recovery studies no significant improvement was marked. In case conc. AP.A, little increment was noted and the obtained values were much more than the respective control values similar to the trend observed during exposure period, where an increase in respiration value was marked in all tested exposure periods. From the data it seems at sub-lethal concentration of the mercury contained effluent an increment similar to stimulation was noticed. At higher concentrations of the effluent (AP.B) decrease in respiration rate was observed when compared to their respective control values in different exposure periods. The observed decrease in the respiration rate was not highly significant and cannot be attributed to solely to inhibitory effect of the pollutant. The inhibitory effect of the effluent on the respiration rate can be substantiated by residual mercury accumulation in the algal body which might have raised the algal body burden and direct interference of mercury with cellular chemicals involved in either respiration processes. The respiration rate of control algal set increased from 218.2 ± 11.4 μl of CO2 evolved/hr/50 ml culture to 248.6 ± 11.4 μl of CO2 evolved/hr/50 ml culture on 10th day of recovery and from 218.2 ± 11.4 μl of CO2 evolved/hr/50 ml culture to 261.8 ± 8.4 μl of CO2 evolved/hr/50 ml culture on 15th day of recovery. The respiration rate of exposed set AP.A increased from 189.9 ± 6.8 μl of CO2 evolved /hr/50 ml culture to 226.4 ± 9.6 μl of CO2 evolved/hr/50 ml culture on 10th day of recovery and from 189.9 ± 6.8 μl of CO2 evolved/hr/50 ml culture to 228.4 ± 12.4 μl of CO2 evolved/hr/50 ml culture on 15th day of recovery. The respiration rate of exposed set AP.B increased from 139.8 ± 14.5 μl of CO2 evolved/hr/50 ml culture to 148.4 ± 14.2 μl of CO2 evolved/hr/50 ml culture on 10th day of recovery and from 139.8 ± 14.5 μl of CO2 evolved/hr/50 ml culture to 154.6 ± 14.2 μl of CO2 evolved/hr/50 ml culture on 15th day of recovery. In case of exposure to lethal concentration of the effluent (LC50, AP.C) the respiration rate decreased significantly and the values were so small that it could not measure after 15 days of exposure. When the 15d exposed alga was transferred to effluent free medium, no significant recovery was noted indicating probable death of the cyanobacteria cells. Regression analysis indicated all negative correlations when compared with increasing effluent concentrations. The correlation coefficient analysis calculated between days of exposure and respiration rate in control and effluent exposed alga at control and different effluent concentrations indicated positive correlations. The regression values were r=0.993, P ≥ 0.001 in control; r=0.984, P ≥ 0.01 at MAC value (AP.A) and r=0.917, P ≥ 0.01 at LC10 effluent concentration. The percent decrease in respiration rate increased with the increase in exposure period in both the toxicant concentrations with the exposure period. The respiration rate steadily decreased in effluent exposure at conc. AP.A and percent decrease in respiration rate increased to 1.3% on 3rd day, 6.2% on 6th day, 8.97% on 9th day, 10.4% on 12th day and 13.1% on 15th day of exposure. The respiration rate in case of conc. AP.A depleted to 8.9% on 10th day of recovery and the respiration rate value decreased by 12.8% on 15th day of recovery. The respiration rate in case of conc. AP.B decreased by 35.9% on 15th day of exposure. During recovery studies, the respiration rate further significantly decreased to 40.3% on 10th day of recovery and 40.9% on 15th day of recovery. Total decrease in respiration rate in the exposed alga was noted in conc. AP.C and hence the data for conc. AP.C was dropped from the figure. The Gross Primary Productivity (GPP) of the control alga increased with the increase in exposure period as shown in Figure 10 and the percent change in GPP value has been presented in Figure 11. The GPP value is a calculated value obtained from RR value and PR value. The GPP value showed an initial insignificant increase on 3rd day of exposure over control value and with the increase in exposure period the GPP value in conc. AP. A and B decreased at all exposure periods starting from 6th day onwards up to 15th day of exposure. During recovery period also no increase but decrease in GPP value was noted in conc. AP.A. Significant decrease in GPP value was noted at all exposure periods in effluent concentration-AP.C. The GPP value decreased to 509 μl of O2 evolved/hr/50 ml culture at conc. AP.A and to 334.9 μl of O2 evolved/hr/50 ml culture at conc. AP.B after 15 days of exposure. The GPP value was not calculated due to zero readings or insignificant fluctuating readings (Figure 10 and 11). Figure 11 was very clear to indicate the impact of mercury contained effluent on the total GPP value of the exposed alga compared to control alga. In conc. AP.A maximum insignificant 4.3% decrease was noted and in case of conc. AP. B 19.7% decrease in GPP value was marked. The effluent exposed alga was transferred to effluent free medium to test the possibility of recovery, but no recovery was noted in the GPP rate, rather further depletion in gross primary productivity value was noted. From the observed value it can be concluded that the effluent significantly influences the growth of the alga [15].
Discussion
Effluent collected from discharge point, effluent stocking pond and solid waste collected from the contaminated site contained significant amount of mercury. The dissolved oxygen of effluent was so less that aquatic plants and animals do not survive in the effluent soaking pond except the tolerant and resistant ones. The effluent at sub-lethal concentration was tested to study the impact of mercury contained effluent on the growth, pigment content and photosynthetic efficiency of the cyanobacterium was tested. The effluent is toxic and showed significant impacts on all types of plants tested. It was observed that this alga, Westiellopsis prolifica, Janet is a tolerant species and resist significant amount of mercurial stress. This alga can also volatilize mercury from the contaminated environment and can reclamate the contaminated environments where mercury nitrate was taken a single chemical. In the present investigation, it was planned to study the interaction pattern, tolerance and resistance to mercurial stress. Can the alga be a reclamator and be used for phycoremediation of mercury contaminated environments? Mercury is a persistent chemical and can remain in the environment for a pretty long time in some form or other. The temperature of the effluent is important when discharged in to aquatic water bodies. Maintenance of pH to a particular range is important since all the physiological and biochemical activities depend on pH and temperature of the environment. Suspended materials play the role of scavenger for the particulars metals in aquatic systems. The suspended particles in water bodies reduce solar energy penetration and thereby reduce photosynthetic activity of aquatic plants. They cause ecological imbalance by mechanical abrasive actions, blanketing action and sedimentation, reduction of light penetration, availability as a surface for growth of bacteria and fungi, reduction of temperature fluctuation and absorption of various chemicals, specifically the heavy metals. The pollutants can contaminate the environmental segments and ground water of the area. Sharma et al., reported that these contaminants enter the food chain and biomagnify seriously affecting human health. Mercury gets into and circulated in all segments of environment through a process known as mercury cycle. It was observed that the total mercury estimated in the effluent was high and contained mainly three types of mercury, namely-elemental, inorganic and organic mercury. These heavy metal contaminated environmental segments are to be restored for safe living of the inhabiting organisms through a planned strategy of physical, chemical and biological treatment together. Bioremediation is an emerging technology to purify the contaminated environments, where phytoremediation is blended with phycoremediation and micro remediation. The adopted technology should be efficient, non-invasive and cost effective, so that the industries should adopt to remove heavy metals and other toxicants from the waste before discharge into the natural environment. Phytoremediation involves series of events either independently or jointly by phytoabsorption, retention, phytoextraction, phytovolatilization, phytostabilization and rhizofiltration to remove the toxic Heavy Metals (HMs) from the contaminated environments. Prusti and Panigrahi, reported that secondary metabolites present in leaf extract of Azadirachta and Jatropha can be used for detoxification of mercury contaminated water and soil in presence of Westiellopsis prolifica. Plants present in and around the effluent canal absorbed mercury and accumulated in different parts by translocation [16].
Mercury can enter into the body either in the inorganic form or organic form but not in the form of metallic mercury. The quantity of elemental mercury decreased with depth and ionic mercury also increased with depth of the solid waste dump. The organic mercury also increased with the depth of the solid waste dumping site indicating high rate of conversion mediated by natural oxidation and reduction and also biological agents. The analyzed data indicated that the amounts of inorganic mercury increased with the depth whereas the elemental mercury content decreased owing to natural or biological conversions and organic mercury increased with depth indicated that more and more of inorganic mercury was converted to organic mercury with the increase in depth of the solid waste dumping site. In the effluent canal less of ionic mercury and organic mercury was observed. Most of the mercury was present in the elemental form and least of organic mercury, ionic mercury and inorganic mercury. The organic mercury content increased from the effluent canal point to effluent storage point. The increase in inorganic mercury content was probably due to higher rate of methylation of inorganic mercury to organic mercury by microorganisms and was strongly dependent on effluent retention time. From the data, it was very much clear that the plants present in and around the effluent canal and solid waste dumping site absorbed mercury and accumulated in different plant parts by translocation of mercury after absorption by the roots from the surrounding effluent or solid waste present. The amount residual accumulation was highly significant and warrants attention. The amount of mercury present in the sediment collected from the effluent canal at different sites indicated that higher amount of mercury was deposited immediately after the effluent was discharged into the canal and the residual mercury concentration of the sediment decreased with the distance. The amount of mercury present on the surface of the solid waste dumping site was the maximum compared to deeper layers of the waste dumping site. The mercury speciation study revealed that the effluent collected from the solid waste deposit site on the surface was having more amounts of inorganic mercury (less than 95.8%) and less of organic mercury (little more than 5.1%) out of which 65.3% was elemental, more than 3.4% ionic, more than 30.6% inorganic and little more than 5.1% of organic mercury. The inorganic mercury estimated was the combined value of elemental mercury and inorganic mercury Prusti and Panigrahi. The effluent of the storage pond was having 9.4% of organic mercury and 89.4% inorganic mercury. The conversion of elemental mercury to other forms of mercury was probably due to natural oxidation/reduction occurring in nature. The data clearly indicated that with the increase in retention time the organic mercury conversion increased showing a positive correlation and the amount of inorganic mercury decreased showing a negative correlation. The conversion of elemental mercury to inorganic mercury and then to organic mercury was due to the microorganisms particularly bacteria, temperature and retention time and in addition natural oxidation and reduction of chemicals and interaction of other environmental chemicals. These dynamics change with each unit of time due to regular chemical transformations occurring in the environment due to simple chemical transformation in the environment either by oxidation or by reduction, chemical reactions and mostly by biological agents like resistant bacteria and phytoplankton. It was also reported that fish and other biological agents can also transform mercury from one type to the other type once absorbed by them. Mercury can enter into the body either in the inorganic form or organic form but not in the form of elemental mercury [17].
The absorption of mercury and its retention depends on the amount of mercury present in a particular Hg contaminated site. The effluent at sub-lethal concentration was tested to study the impact of effluent on the growth, pigment content and physiological activity of the alga, Westiellopsis prolifica. It was observed that this alga, Westiellopsis prolifica, Janet is a tolerant species and resisted mercurial stress significantly. This alga can also volatilize mercury from the contaminated environment and can reclamate the contaminated environments where mercury nitrate was taken a single chemical. In the present investigation, it was planned to study the interaction pattern, tolerance, resistance and if the alga can be a reclamator and can be used for phycoremediation of mercury contaminated environments. Mercury is a persistent chemical and can remain in the environment for a pretty long time in some form or other. The temperature of the effluent is important when discharged in to aquatic water bodies. Maintenance of pH to a particular range is important since all the biochemical activities depend on pH of the environment. Suspended materials play the role of scavenger for the particulars metals in aquatic systems. The suspended particles in water bodies reduce solar energy penetration and reduce photosynthetic activity in case phytoplankton and all aquatic plants. They cause ecological imbalance by mechanical abrasive actions, blanketing action and sedimentation, reduction of light penetration, availability as a surface for growth of bacteria and fungi, reduction of temperature fluctuation and absorption of various chemicals, specifically the heavy metals. High loads of suspended solids cause a significant deterioration in the survival conditions for aquatic organisms. Salinity and hardness, which may together be represented as dissolved solids, play an important role in distribution and maintenance of many organisms in the estuary. Mercury gets into the environment through a process known as mercury cycle. It was observed that the total mercury estimated in the effluent was high and contained mainly three types of mercury. The death of the exposed system depends upon its age, tolerance, resistance, physiological ability to withstand stress and biochemical mechanism of the organism to defend stress. Mercury contained effluent is toxic and can affect the growth of the exposed alga in the exposed cultures. Mercury (Hg) is a contaminant found in ecosystems globally. Although inorganic and insoluble Hg has relatively low bioavailability, Methyl Mercury (MeHg) is a neurotoxic chemical which can bio-magnify rapidly to elevated concentrations in biota such that concentrations in top trophic predators can threaten both ecological and human health. Mercury can be biomagnified at all levels of aquatic food chains, and high levels of mercury have been detected in fish, especially in the organic form. This imposes a great threat on human health through seafood consumption. The same author also pointed out that toxic responses are specific to metals and species, but the sub-cellular basis underlying such inter-species and inter-metal differences was not clear. Wu and Wang studied the accumulation, sub-cellular distribution and toxicity of inorganic mercury and methyl mercury in marine phytoplankton. Mercury exerts its toxicity by binding with sulphydryl groups and producing oxidative stress. Significant amount of mercury was recorded in the effluent and also in the BGA as residual Hg after absorption from the culture medium. Mercury might be getting attached-SH groups, glutathiones and form a complex, thereby inhibiting chlorophyll biosynthesis in exposed BGA. A significant volume of information is available on the pollution of the surrounding biota due to the discharge of gaseous exhausts, solid waste and effluent discharged from chlor-alkali industries. The waste discharge from a caustic soda factory significantly impacted the surrounding environment as reported by Wiener et al., in the past. Singh and Singh reported pigment damage in the blue-green alga caused by a pollutant in Nostoc calicicola. Suckcharoen and Nourteva reported bio-enrichment of Hg in all living organisms growing in mercury polluted areas. de Filippis and Pallaghy reported the effects of sub-lethal concentrations of mercury and zinc on Chlorella on the growth characteristics, photosynthetic efficiency and uptake of heavy metals and also indicated that at sub-lethal concentrations of the pollutant the alga can develop tolerance and resistance. Glass et al., reported the dispersion of mercury and consequent contamination of Lake Superior region and Sorensen et al., reported in eight northern Minnesota lakes. It is time to save the existing flora and fauna at the contaminated sites, river and estuary. Due to change in technology, now no more mercury is available but the dumped mercury as solid waste needs attention. The industry people instead of recycling mercury removing all the top soil up to the depth of 10 feet and shifting to some other place and use these solid wastes for construction of roads. Now the problem is the new dumping sites, where mercury gets exposed and chances of evaporation of mercury and leaching of mercury to neighboring areas cannot be ruled out. Non availability of mercury in the area might be due to change in technology or washing of all discharged mercury into Bay of Bengal or a major portion of discharged mercury was recovered by following recycling technology [18].
The effluent is toxic and showed significant effects on all types of plants tested. It was observed that this alga, Westiellopsis prolifica, Janet is a tolerant species and resist significant amount of mercurial stress. This alga can also volatilize mercury from the contaminated environment and can reclamate the contaminated environments where mercury nitrate was taken a single chemical. In the present investigation, it was planned to study the interaction pattern, tolerance, resistance and if the alga can be a reclamator and can be used for phycoremediation of mercury contaminated environments. Mostly mercury is discharged in the elemental form along with the effluent into the environment. The elemental mercury discharged along with effluent under goes natural chemical transformations in the environment to produce different forms of mercury. Mercury gets precipitated in the effluent canal and periodically the effluent sediments were removed and dumped nearby which contained significant amount of mercury. Mercury escapes from the effluent pond and solid waste dumping site by percolation, leaching and by rain run off transportation to neighboring areas including crop fields. Mercury mobilization starts from the industrial discharge point, dumping sites, leaching areas to destination point (Crop fields, Rushikulya River, estuary, Bay of Bengal) [19,20].
Conclusion
Shaw proposed for the first time on some basic line of work on the effluent waste of the chlor-alkali industry, which lacked a detailed reclamation work either taking cyanobacteria or any other plant. Sahu for the first time indicated the stimulation of different physiological parameters of a Cyanobacterium and also clearly indicated that lower (sub-lethal) concentrations of mercury, can remove mercury from the contaminated environment by way biosorption and volatilization. Shaw and Sahu reported that the alga Westiellopsis could tolerate only 40% of the supernatant effluent concentration of a chlor-alkali industry, where the effluent contained only 1.1 mg/50 ml culture. Their work was mostly on toxicity testing and probable detoxification of waste by available environmental chemicals or the chemicals present in the nutrient medium. By altering the dose/concentration of these chemicals they were trying to find out the possibility of reclamation. There were many workers of the laboratory who worked on mercurial compounds and studied the impact on different aquatic, terrestrial and crop plants and many also worked on different types of alga belonging to family Cyanophyceae and Chlorophyceae and observed interesting results. A total review of the whole work done in our laboratory suggested that these effluents and solid wastes of the industry can be treated by biological methods to obtain a heavy metal free waste water which can be used in the agriculture. Some workers also indicated that heterocystous blue-green algae can be used for phytoremediation of the effluent waste, so that after treatment the waste water contained significant amount of nitrogenous exudates can act as bio-fertilizer and can increase the fertility of the crop field soil. These algae reclamate the waste and produce nitrogenous exudates enrich the crop fields with nitrogen.
Acknowledgement
The authors wish to thank the authorities Berhampur University, Berhampur, Odisha, for using the laboratory and library facilities. Ms. Prusti wishes to thank DST, New-Delhi for financial support in the form of INSPIRE fellowship for the whole period of work.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
References
- Allen MB, Arnon DI (1955) Studies on nitrogen-fixing blue-green algae. I. Growth and nitrogen fixation by Anabaena cylindrica Lemm. Plant Physiol 30:366.
[Crossref] [Google Scholar] [PubMed]
- American Public Health Association (1998) Standard methods for the examination of water and wastewater. American Public Health Association.
- Boudou A, Delnomdedieu M, Georgescauld D, Ribeyre F, Saouter E (1991) Fundamental roles of biological barriers in mercury accumulation and transfer in freshwater ecosystems (analysis at organism, organ, cell and molecular levels). Water Air Soil Pollut 56:807-821.
- de Filippis LF, Pallaghy CK (1976) The effect of sub-lethal concentrations of mercury and zinc on Chlorella I. Growth characteristics and uptake of metals. Z Pflanzenphysiol 78:197-207.
- Environment Canada (1979) Analytical methods manual. Inland Waters Directorate.
- Fogg GE (1949) Growth and heterocyst production in Anabaena cylindrica lemm II. in relation to carbon and nitrogen metabolism. Ann Bot 13:241-259.
- Patnaik S, Leelaveni A, Panigrahi AK (2022) Impact of lechate chemicals of red mud waste of Alumina industry: its toxicity, impact on photosynthetic efficiency and production of a crop plant. Env Cons 28:S340-S345.
- Mason RP, Reinfelder JR, Morel FM (1995) Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut 80:915-921.
- Prabha PJ, Panigrahi AK (2022) Impact of Effluent of a Chlor-Alkali Industry on the Photosynthetic Efficiency of Crop Field Inhabiting Cyanobacteria. IJBEI 21: 51-59.
- Oser BL (1965) Biuret test, determination of glycogen. Hawks Physiological Chemistry. 179-224.
- Sahu A (1987) Toxicological effects of a pesticide on a blue-green alga: III. Effect of PMA. On a blue-green alga, Westiellopsisprolifica, Janet and its ecological implications (Doctoral dissertation, Ph. D. Thesis, Berhampur University, India).
- Sahu A (2017) Distribution of mercury contained leached chemicals of solid waste of a chlor-alkali industry on BGA and possible reclamation. Life Sci Bull 14:133-139.
- Sahu A (2017) Impact of mercury contained leached chemicals of solid waste of a Chlor-alkali industry on BGA and its Ecological implications. Life Sci Bull 14:115-118.
- Sharma JK, Kumar N, Singh NP, Santal AR (2023) Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front Plant Sci 14:1076876.
[Crossref] [Google Scholar] [PubMed]
- Shaw BP, Sahu A, Panigrahi AK (1986) Mercury in plants, soil, and water from a caustic chlorine industry. Bull Environ Contam Toxicol (United States) 36.
[Crossref] [Google Scholar] [PubMed]
- Shaw BP (1987) Eco-physiological studies of the waste of a chlor alkali factory on biosystems. Ph. D. Thesis, Berhampur University, Orissa, India.
- Singh SP, Singh RP (1984) Bisulphite-induced pigment damage in the blue-green alga Nostoc calcicola bréb. New Phytologist 98:277-283.
- Sorensen JA, Glass GE, Schmidt KW, Huber JK, Rapp Jr GR (1990) Airborne mercury deposition and watershed characteristics in relation to mercury concentrations in water, sediments, plankton, and fish of eighty northern Minnesota lakes. Environ Sci Tech 24:1716-1727.
- Vernon LP (1960) Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal Chem 32:1144-1150.
- Wanntorp H, Dyfverman AR (1955) Identification and determination of mercury in biological materials. Arkiv for Kemi 9:7-27.
Citation: Prusti, Prabha J, Panigrahi AK (2024) Eco-Toxicological Studies of Effluent of a Chlor-Alkali Industry on Growth and Photosynthetic Efficiency of a Cyanobacterium and Possible Use of This Alga in Reclamation of Effluent Waste. J Bioremediat Biodegrad 16: 658.
Copyright: © 2025 Prusti AK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1042
- [From(publication date): 0-0 - Dec 14, 2025]
- Breakdown by view type
- HTML page views: 879
- PDF downloads: 163