Effect of Low Frequency Noise on Fundic Mucosa of Adult Male Albino Rats and the Role of Vitamin E Supplementation (Histological and Immunohistochemical Study)
Received: 08-Oct-2015 / Accepted Date: 28-Oct-2015 / Published Date: 31-Oct-2015 DOI: 10.4172/2161-0681.1000256
Abstract
Low frequency noise (LFN) is an environmental problem particularly to sensitive people. Stomach is one of the main targets of noise stress. Our aim was to detect histological and immunohistochemical changes that may occur in fundic mucosa of adult wistar male albino rats exposed to LFN and the role of vitamin E administration. Fifty adult wistar male albino rats were classified into three groups: control group, experimental group subdivided into two subgroups: [LFN exposed and LFN+vit.E] and recovery group. Results: fundic mucosa of LFN exposed group showed shedding of surface epithelial cells, glandular cells with pyknotic and karyolitic nuclei, parietal cells had pyknotic nuclei and vacuolated cytoplasm, Chief cells appeared with pyknotic nuclei and separated basal lamina, mononuclear cell infiltration and thick muscularis mucosa. Highly statistically significant increase in the mean area percent of collagen fibers of fundic mucosa of LFN exposed group as compared to the control group. Highly statistically significant decrease in the mean thickness of mucous film in PAS-alcian blue stained sections of the fundic mucosa was detected in the LFN exposed group as compared to the control group and LFN+vit.E exposed group. Highly statistically significant decrease in the mean optical density of chromogranin-A immunoreaction in LFN exposed group as compared to the control group. Scanning electron microscope (SEM) examination revealed dilated gastric pits and loss of the normal mucous sheet covering the surface mucous cells with some mucous patches. LFN+vit.E group revealed marked improvement. To conclude, LFN induced alteration in fundic mucosa and its mucous barrier. Marked improvement after vitamin E supplementation was detected. So, vitamin E may be beneficial for at risk people
Keywords: Chromogranin-A, Fundic mucosa, Low frequency noise, SEM, Vitamin E
323724Abbreviations
LFN: Low Frequency Noise; Vit.E: Vitamin E; SEM: Scanning Electron Microscope; ROS: Reactive Oxygen Species; O2-: Superoxide Radical; OH: Hydroxyl Radical; H2O2: Hydrogen Peroxide; DAB: Diamiobenzidine
Introduction
Noise is one of the main components of modern society that has become an important environmental problem. It is not only an irritating sound but also a stress factor leading to serious health problems. It could affect people both psychologically and physiologically. Exposure to noise has a significant impact on a variety of lab animals and human [1,2].
Noise consists of sounds with broad frequencies. Low-frequency noise (LFN) is continuously generated from many occupational and daily sources including transportation systems, industrial devices, air movement devices as wind turbines, compressors, ventilation and airconditioning units and household appliances (washing machines, refrigerators and freezers). Thus, we are routinely exposed to LFN generated from different devices [3,4].
LFN is less attenuated by walls and enclosures. Moreover, because of its band width, LFN can spread across large distances with low attenuation, passing through walls and windows and making protection very difficult [5].
There are many considerations regarding noise effects on immune function, hormonal levels, mental illness, sleep rhythm, cardiovascular and respiratory systems [6].
Most of the published medical researches on noise-induced diseases are related to hearing disorders. In the last three decades, non-auditory effects of noise were related to different systems as neurologic, cardiac, vascular, respiratory and gastrointestinal disorders [7].
Gastric mucosa is continuously exposed to harmful factors. Surface epithelium forms a physical barrier between the lumen and the underlying mucosa. An increase in the epithelial cell loss or a decrease in the cell renewal may lead to mucosal damage [8,9]. LFN not only affects cellular structure and organization, but also leads to the development of tissue fibrosis and abnormal collagen fibers proliferation [10].
Several studies suggest the involvement of oxidative stress in the etiology of stress-induced gastric lesions [11]. Increasing plasma concentrations of epinephrine and cortisone suggest that noise induces stress response in rodents [12].
Vitamin E is a powerful antioxidant. It is effective in preventing oxidation of polyunsaturated fatty acids. It is a fat soluble vitamin that is divided into two subgroups; tocopherols and tocotrienols. Tocopherol is the most abundant and active form of vitamin E homologues in vivo. Vitamin E also acts as a scavenger of reactive oxygen species (ROS) such as superoxide radical (O2-), hydroxyl radical (OH), hydrogen peroxide (H2O2), hypochlorus acid and singlet oxygen. It exerts an anti-inflammatory action by inhibiting the production O2- in the activated neutrophils, adhesion of neutrophils to endothelial cells and transendothelial migration of neutrophils [13,14].
Although gastrointestinal complaints are common among individuals exposed to noise, limited literatures were available regarding the gastric morphological alterations of fundic mucosa induced by LFN exposure. Therefore, the goal of the present study was to investigate fundic mucosal lesions in adult male albino rats exposed to LFN. Moreover, to evaluate the possible protective effect of vitamin E supplementation.
Materials and Methods
Animals: Fifty healthy adult Wistar male albino rats (4-6 months) weighing 200-250 gm were used in this study. The animals were obtained from the Animal House, Faculty of Medicine, Zagazig University, Zagazig, Egypt. They were fed standard balanced diet and allowed water ad-libitum. They were housed in hygienic cages in 12 h light/12 h dark cycle at room temperature according to the guidelines for animal research issued by the National Institute of Health and approved by Animal Ethics Committee, Zagazig University, Zagazig, Egypt.
Chemicals: Vitamin E 400 mg soft gelatin capsules were purchased from Pharco Pharmaceuticals, Alexandria, Egypt.
Anti- chromogranin-A antibodies, (Novacastra Laboratories Ltd, UK) were purchased from Sigma office (Egyptian International Center for Import, Cairo, Egypt).
LFN Exposure: Noise generator in the Physics Department, Faculty of Science, Zagazig University, Egypt, produced an amplified and frequency filtered signal was used creating an acoustic environment rich in low frequency components. The generator was put near the position of the exposed rats (Experimental group) [7].
Experimental procedure
The rats were classified into three groups:
Group1 (Control group): included 20 animals kept in a quiet place and classified equally into two subgroups:
Subgroup 1a: received no treatment.
Subgroup 1b: received vitamin E by gavage at an oral dose of 60 mg/kg body weight for 5 weeks.
Group 2 (Experimental group): comprised 20 animals and classified equally into two subgroups:
Subgroup 2a (LFN exposed group): rats exposed to LFN 24 hours per day for 5 weeks with band width 200 HZ and amplitude 100 dB [7].
Subgroup 2b (LFN+vit.E group): rats exposed to LFN for 5 weeks as subgroup 2a after that vitamin E supplementation at an oral dose of 60 mg/kg body weight for 5 weeks [14].
Group 3 (Recovery group): included 10 animals, they were exposed to LFN for 5 weeks as in group 2a and after that they kept in a quiet place for 5 weeks [14].
At the end of the experiment, the rats were fasted overnight. They were sacrificed with intraperitoneal injection of pentobarbitone sodium 60 mg/kg body weight [15] and their stomach were dissected out, rinsed and cut along the greater curvature. Specimens from the fundic region were prepared for light and SEM examination.
Histological study: Specimens for light microscopic examination were fixed in 10% neutral formol saline, processed for paraffin block preparation, cut into 5 μm sections, and subjected to H&E [16]. Mallory trichrome stain for detection of collagen fibers, PAS-alcian blue histochemical method was used to differentiate neutral mucin from acidic mucin [17].
Immunohistochemical analysis of chromogranin-A: Immunohistochemical reaction was carried out using the avidin-biotin complex immunoperoxidase system. Serial sections of paraffinembedded specimens were deparaffinized on charged slides. The sections were incubated in 0.1% hydrogen peroxide for 30 min to block the endogenous peroxidase and then incubated with the primary antibody. The primary antibody used for chromogranin A was a readyto- use rabbit polyclonal antibody (CAT-No. RB-9003-R7; Thermoscientific Laboratories, Rockford, IL, USA). The slides were then incubated with the secondary anti-rabbit antibody versal kits (Zymed laboratories) diluted 1:200 for 30 min. Staining was completed by incubation with a substrate chromogen called diamiobenzidine (DAB). Mayer’s hematoxylin used as a counterstain. For negative control, the primary antibody was replaced with phosphate buffer solution [18,19].
SEM examination: For SEM, the specimens were washed in phosphate buffer saline, fixed at room temperature in an aldehyde mixture made up of 4% formaldehyde, 1.25% glutaraldehyde and 10 nmol/L CaCl2 in 0.05 mol/L cacodylate buffer. The samples were dehydrated in ethanol and critical point-dried in a Balzer’s apparatus using carbon dioxide as the transitional fluid. The preparations were mounted on metal stubs with conductive carbon paste. The specimens were coated with Au/Pt under vacuum and examined in a [JEOL (Japan) JSM 6510 lv] SEM at Electron Microscope Unit, Faculty of Agriculture, Al Mansoura University, Egypt [20].
Histo-morphometrical analysis: The image analyzer computer system Leica Qwin 500 (Leica Ltd, Cambridge, UK) at the Image Analyzing Unit of the Pathology Department, Faculty of Dentistry, Cairo University, Egypt, was used to evaluate area percentage (%) of collagen fibers and optical denisty for chromogranin-A immune reaction. It was measured using the interactive measure menu. Measuring frame of a standard area equal to 118 476.6 mm2 was chosen so that collagen fibers and the brown positive immune reaction for chromogranin-A could be seen and masked by blue binary colour to be measured. Ten readings from five non-overlapping sections from each rat of all groups were examined.
Statistical analysis: All data were expressed as mean ± SD. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software, version 13.00 (Chicago, Illinois, USA). Statistical significance was determined by one-way analysis of variance for differences between the means of different groups. Further analysis was carried out using the post-hoc test to compare the parameters between the different groups with each other. Probability of P less than 0.05 was considered statistically significant.
Results
Histological results
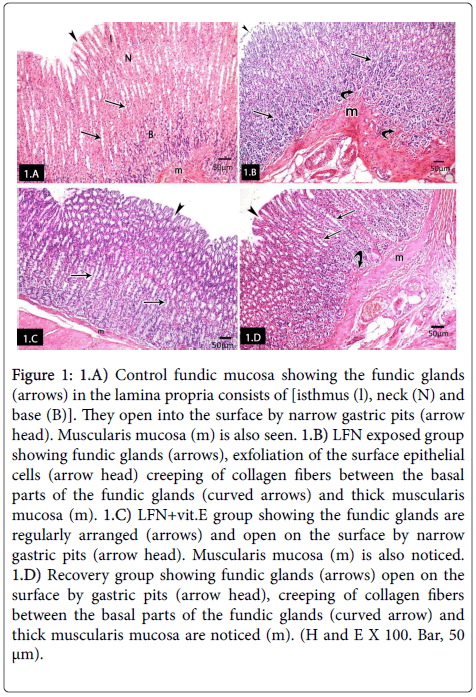
Group I (control group): Histological examination of subgroups 1a and 1b showed similar histological results. So, we used the negative control subgroup 1a as the control group. H and E stained sections of control albino rats' fundic mucosa showed the fundic mucosal layers; epithelium, lamina propria containing fundic glands and muscularis mucosa. Fundic glands were formed of isthmus, neck and base regions. They were long, straight, tubular and perpendicular to the surface occupying the whole thickness of the lamina propria and opened into the surface by narrow gastric pits (Figure 1). LFN exposed group showed exfoliation of the surface epithelial cells, creeping of collagen fibers between the basal parts of the fundic glands and thick muscularis mucosa. Notice, fundic glands (Figure 1). Fundic glands of LFN+vit.E group were regularly arranged and opened on the surface by narrow gastric pits. Musculais mucosa was also noticed (Figure 1). Fundic glands of the recovery group opened on the surface by gastric pits, creeping of collagen fibers between the basal parts of the fundic glands and thick muscularis mucosa were noticed (Figure 1).
Figure 1: 1.A) Control fundic mucosa showing the fundic glands (arrows) in the lamina propria consists of [isthmus (l), neck (N) and base (B)]. They open into the surface by narrow gastric pits (arrow head). Muscularis mucosa (m) is also seen. 1.B) LFN exposed group showing fundic glands (arrows), exfoliation of the surface epithelial cells (arrow head) creeping of collagen fibers between the basal parts of the fundic glands (curved arrows) and thick muscularis mucosa (m). 1.C) LFN+vit.E group showing the fundic glands are regularly arranged (arrows) and open on the surface by narrow gastric pits (arrow head). Muscularis mucosa (m) is also noticed. 1.D) Recovery group showing fundic glands (arrows) open on the surface by gastric pits (arrow head), creeping of collagen fibers between the basal parts of the fundic glands (curved arrow) and thick muscularis mucosa are noticed (m). (H and E X 100. Bar, 50 μm).
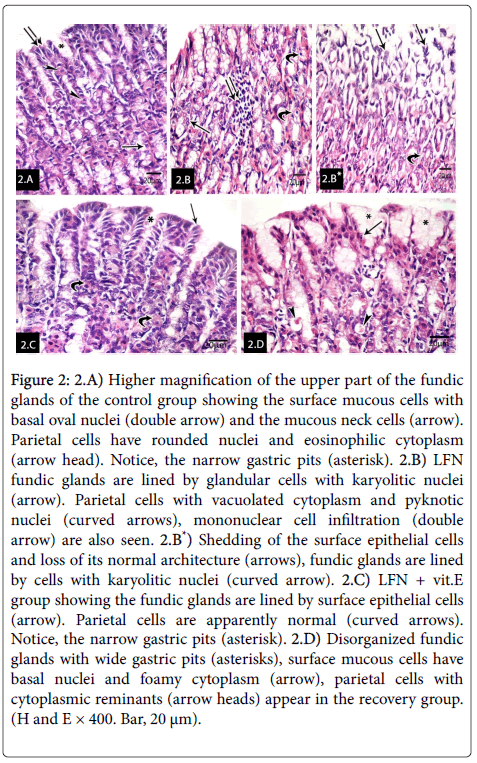
Higher magnification of the upper part of the fundic glands of the control group revealed the surface mucous columnar cells with basal oval nuclei and the mucous neck cells. Parietal cells had rounded nuclei and eosinophilic cytoplasm. Notice, the narrow gastric pits (Figure 2). LFN fundic glands were lined by glandular cells with karyolitic nuclei. Parietal cells had pyknotic nuclei and vacuolated cytoplasm. Mononuclear cell infiltration was also seen. Other sections of LFN exposed group showed shedding of the superficial epithelial cells and loss of its normal architecture, fundic glands lined by cells with karyolitic nuclei (Figure 2*). LFN+vit.E group, fundic glands were lined by surface mucous cells and opened into the surface by narrow gastric pits. Parietal cells had rounded nuclei and eosinophilic cytoplasm (Figure 2) however, in the recovery group disorganized fundic glands with wide gastric pits were noticed. Surface mucous cells had basal nuclei and foamy cytoplasm. Some parietal cells with cytoplasmic reminants were noticed (Figure 2).
Figure 2: A) Higher magnification of the upper part of the fundic glands of the control group showing the surface mucous cells with basal oval nuclei (double arrow) and the mucous neck cells (arrow). Parietal cells have rounded nuclei and eosinophilic cytoplasm (arrow head). Notice, the narrow gastric pits (asterisk). 2.B) LFN fundic glands are lined by glandular cells with karyolitic nuclei (arrow). Parietal cells with vacuolated cytoplasm and pyknotic nuclei (curved arrows), mononuclear cell infiltration (double arrow) are also seen. 2.B*) Shedding of the surface epithelial cells and loss of its normal architecture (arrows), fundic glands are lined by cells with karyolitic nuclei (curved arrow). 2.C) LFN + vit.E group showing the fundic glands are lined by surface epithelial cells (arrow). Parietal cells are apparently normal (curved arrows). Notice, the narrow gastric pits (asterisk). 2.D) Disorganized fundic glands with wide gastric pits (asterisks), surface mucous cells have basal nuclei and foamy cytoplasm (arrow), parietal cells with cytoplasmic reminants (arrow heads) appear in the recovery group. (H and E × 400. Bar, 20 μm).
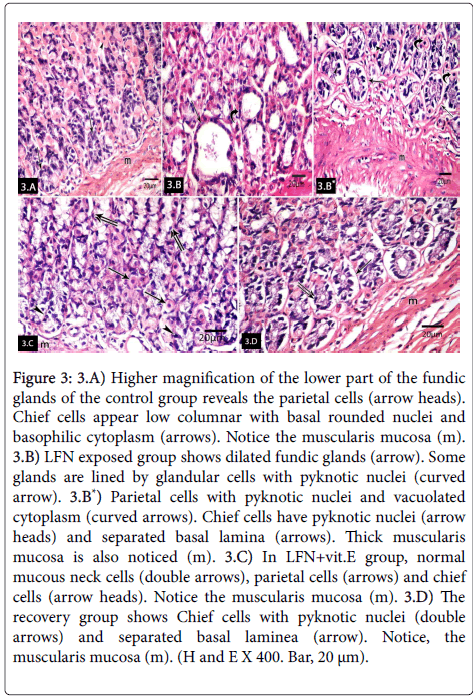
Higher magnification of the lower part of the fundic glands of control group revealed the parietal cells with central rounded nuclei and eosinophilic cytoplasm. Chief cells appeared low columnar with basal rounded nuclei and basophilic cytoplasm. Musculais mucosa was also noticed (Figure 3) however LFN-exposed group revealed dilated fundic glands. Some glands were lined by glandular cells with pyknotic nuclei. Parietal cells had pyknotic nuclei and vacuolated cytoplasm. Chief cells appeared with pyknotic nuclei and separated basal lamina. Muscularis mucosa was noticed (Figure 3*). LFN+vit.E group showed normal mucous neck cells, parietal cells and chief cells. Musculais mucosa was also noticed (Figure 3). The recovery group showed chief cells with pyknotic nuclei and separated basal lamina. Musculais mucosa was also noticed (Figure 3).
Figure 3: 3.A) Higher magnification of the lower part of the fundic glands of the control group reveals the parietal cells (arrow heads). Chief cells appear low columnar with basal rounded nuclei and basophilic cytoplasm (arrows). Notice the muscularis mucosa (m). 3.B) LFN exposed group shows dilated fundic glands (arrow). Some glands are lined by glandular cells with pyknotic nuclei (curved arrow). 3.B*) Parietal cells with pyknotic nuclei and vacuolated cytoplasm (curved arrows). Chief cells have pyknotic nuclei (arrow heads) and separated basal lamina (arrows). Thick muscularis mucosa is also noticed (m). 3.C) In LFN+vit.E group, normal mucous neck cells (double arrows), parietal cells (arrows) and chief cells (arrow heads). Notice the muscularis mucosa (m). 3.D) The recovery group shows Chief cells with pyknotic nuclei (double arrows) and separated basal laminea (arrow). Notice, the muscularis mucosa (m). (H and E X 400. Bar, 20 μm).
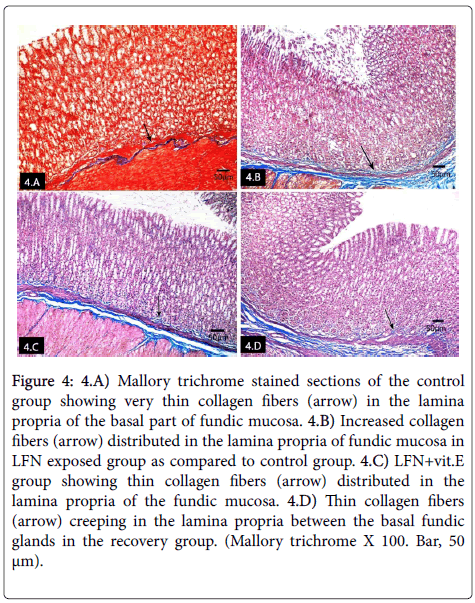
Mallory trichrome stained sections of control group showed very thin collagen fibers in the lamina propria of the fundic mucosa (Figure 4). Increased collagen fibers in the lamina propria of fundic mucosa of LFN exposed rats as compared to control group (Figure 4). Thin collagen fibers were detected in the lamina propria of the fundic mucosa of LFN+vit.E group (Figure 4). The recovery group showed collagen fibers creeping in the lamina propria between the basal fundic glands (Figure 4).
Figure 4: 4.A) Mallory trichrome stained sections of the control group showing very thin collagen fibers (arrow) in the lamina propria of the basal part of fundic mucosa. 4.B) Increased collagen fibers (arrow) distributed in the lamina propria of fundic mucosa in LFN exposed group as compared to control group. 4.C) LFN+vit.E group showing thin collagen fibers (arrow) distributed in the lamina propria of the fundic mucosa. 4.D) Thin collagen fibers (arrow) creeping in the lamina propria between the basal fundic glands in the recovery group. (Mallory trichrome X 100. Bar, 50 μm).
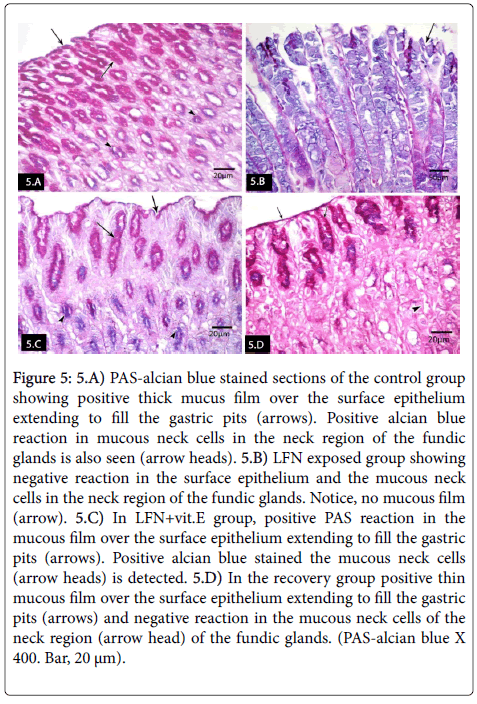
PAS-alcian blue stained sections of the control group revealed positive PAS reaction in the thick mucus film over the surface epithelium extending to fill the gastric pits. Positive alcian blue stained the mucous neck cells in the neck region of the fundic glands was also seen (Figure 5). LFN-exposed group revealed negative reaction in the surface epithelium and the mucous neck cells in the neck region of the fundic glands. No mucous film was noticed (Figure 5). In LFN+vit.E group, positive PAS reaction was seen in the mucous film over the surface epithelium extending to fill the gastric pits. Strong positive alcian blue stained the mucous neck cells in the neck region of the fundic glands was also seen (Figure 5). In the recovery group, positive reaction was detected in thin mucous film over the surface epithelium extending to fill the gastric pits and negative reaction in the mucous neck cells in the neck region of the fundic glands (Figure 5).
Figure 5: 5.A) PAS-alcian blue stained sections of the control group showing positive thick mucus film over the surface epithelium extending to fill the gastric pits (arrows). Positive alcian blue reaction in mucous neck cells in the neck region of the fundic glands is also seen (arrow heads). 5.B) LFN exposed group showing negative reaction in the surface epithelium and the mucous neck cells in the neck region of the fundic glands. Notice, no mucous film (arrow). 5.C) In LFN+vit.E group, positive PAS reaction in the mucous film over the surface epithelium extending to fill the gastric pits (arrows). Positive alcian blue stained the mucous neck cells (arrow heads) is detected. 5.D) In the recovery group positive thin mucous film over the surface epithelium extending to fill the gastric pits (arrows) and negative reaction in the mucous neck cells of the neck region (arrow head) of the fundic glands. (PAS-alcian blue X 400. Bar, 20 μm).
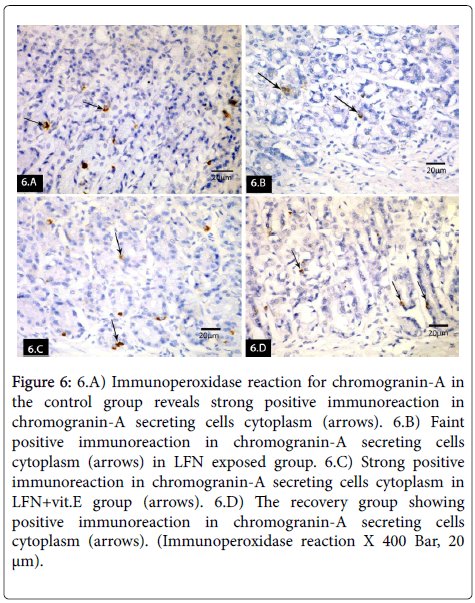
Immunoperoxidase reaction for chromogranin-A in control group revealed strong positive immuoreaction in chromogranin-A secreting cells cytoplasm (Figure 6). Faint positive immuoreaction was seen in LFN exposed group (Figure 6). Strong positive immuoreaction was detected in LFN+vit.E group (Figure 6). The recovery group revealed positive immunoreaction in chromogranin-A secreting cells cytoplasm (Figure 6).
Figure 6: 6.A) Immunoperoxidase reaction for chromogranin-A in the control group reveals strong positive immunoreaction in chromogranin-A secreting cells cytoplasm (arrows). 6.B) Faint positive immunoreaction in chromogranin-A secreting cells cytoplasm (arrows) in LFN exposed group. 6.C) Strong positive immunoreaction in chromogranin-A secreting cells cytoplasm in LFN+vit.E group (arrows). 6.D) The recovery group showing positive immunoreaction in chromogranin-A secreting cells cytoplasm (arrows). (Immunoperoxidase reaction X 400 Bar, 20 μm).
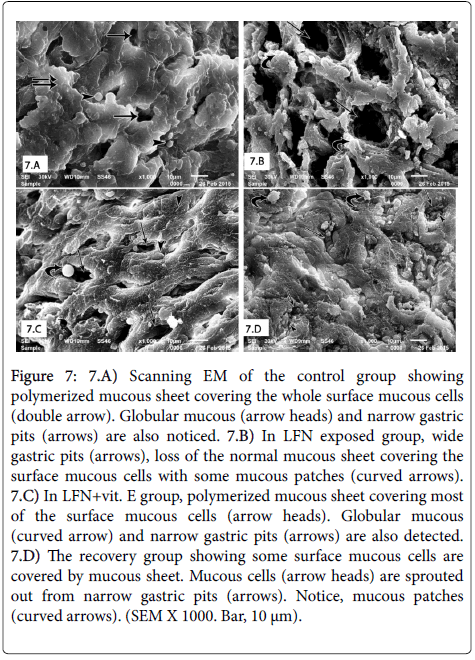
SEM of control group revealed polymerized mucous sheet covering the whole surface mucous cells. Globular mucous and narrow gastric pits were also seen (Figure 7). LFN-exposed group revealed dilated gastric pits, loss of the normal mucous sheet covering the surface mucous cells with some mucous patches and globular mucous form disappeared (Figure 7). LFN+vit.E group showed polymerized mucous sheet covering most of the surface mucous cells. Globular mucous and narrow gastric pits were also noticed (Figure 7). Finally, the recovery group revealed some surface mucous cells covered by mucous sheet. Mucous cells were sprouted out from the narrow gastric pits. Mucous patches were also noticed (Figure 7).
Figure 7: 7.A) Scanning EM of the control group showing polymerized mucous sheet covering the whole surface mucous cells (double arrow). Globular mucous (arrow heads) and narrow gastric pits (arrows) are also noticed. 7.B) In LFN exposed group, wide gastric pits (arrows), loss of the normal mucous sheet covering the surface mucous cells with some mucous patches (curved arrows). 7.C) In LFN+vit. E group, polymerized mucous sheet covering most of the surface mucous cells (arrow heads). Globular mucous (curved arrow) and narrow gastric pits (arrows) are also detected. 7.D) The recovery group showing some surface mucous cells are covered by mucous sheet. Mucous cells (arrow heads) are sprouted out from narrow gastric pits (arrows). Notice, mucous patches (curved arrows). (SEM X 1000. Bar, 10 μm).
2-Histomorphometrical and statistical results
Highly statistically significant increase in the mean area percent of collagen fibers of the fundic mucosa was detected in the LFN exposed group as compared to the control group and LFN+vit.E exposed group. Highly statistically significant increase in the mean area percent of collagen fibers of the fundic mucosa was detected in the recovery group as compared to the control group. No statistically significant difference between LFN+vit.E exposed group and the control group (Table 1).
| Mean ± SD | F | P-value | |
|---|---|---|---|
| G1 | 0.223± 0.02 | 1398.99 | <0.001** |
| G2a | 0.786± 0.03 | ||
| G2b | 0.232± 0.02 | ||
| G3 | 0.542± 0.02 | ||
| LSD (least significance difference for comparison between groups | |||
| G1 | G2a | G2b | |
| G2 a | <0.001** | <0.001** | |
| G2 b | 0.325 | <0.001** | |
| G3 | <0.001** | <0.001** | <0.001** |
*Significant (p<0.05)
**Highly Significant (p<0.001)
Table 1: Area percent of collagen fibers in different studied groups.
Highly statistically significant decrease in the mean thickness of mucous film in PAS-alcian blue stained sections of the fundic mucosa was detected in the LFN exposed group as compared to the control group and LFN+vit.E exposed group. Highly statistically significant decrease in the mean thickness of mucous film in PAS-alcian blue stained sections of the fundic mucosa was detected in the LFN exposed group as compared to the recovery group. Statistically significant difference in the mean thickness of mucous film in PAS-alcian blue of the fundic mucosa was detected in the recovery group as compared to the control group. No statistically significant difference between LFN +vit.E exposed group as compared to the control group and the recovery group (Table 2).
| Mean ±SD | F | P-value | |
|---|---|---|---|
| G1 | 65.3± 12.6 | 55.7 | <0.001* |
| G2a | 12.4± 3.1 | ||
| G2b | 55.2± 11.05 | ||
| G3 | 45.5± 11 | ||
| LSD for comparison between groups | |||
| G1 | G2 a | G2 b | |
| G2 a | <0.001** | <0.001** | |
| G2 b | ˃0.05 | <0.001** | |
| G3 | <0.01* | <0.001** | ˃0.05 |
*Significant (p<0.05)
**Highly Significant (p<0.001)
Table 2: Thickness of mucous film in in different studied groups.
Highly statistically significant decrease in the mean optical density of chromogranin-A immuoreaction the fundic mucosa was detected in the LFN exposed group as compared to the control group and LFN +vit.E exposed group. Statistically significant decrease in the mean optical density of chromogranin-A immuoreaction of the fundic mucosa was detected in the recovery group as compared to the control group. No statistically significant difference between LFN+vit.E exposed group and the control group (Table 3).
| Mean ± SD | F | P-value | |
|---|---|---|---|
| G1 | 45.2± 14.2 | 17.23 | <0.001** |
| G2a | 15.07± 6.1 | ||
| G2b | 37.3± 5.6 | ||
| G3 | 20.5± 10.8 | ||
| LSD for comparison between groups | |||
| G1 | G2 a | G2 b | |
| G2 a | <0.001** | <0.001** | |
| G2 b | 0.227 | <0.001** | |
| G3 | 0.029⃰ | 0.137 | 0.037* |
*Significant (p<0.05)
**Highly Significant (p<0.001)
Table 3: Optical density of chromogranin-A immunoreaction in different studied groups.
Discussion
Examination of fundic mucosa of LFN exposed rats in the present study showed shedding of the surface epithelial cells, glandular cells with pyknotic and karyolitic nuclei, parietal cells had pyknotic nuclei and vacuolated cytoplasm and chief cells appeared with pyknotic nuclei and separated basal lamina.
These findings were in agreement with Fonseca et al. [21] who proved that the proximal gut of LFN-exposed wistar rats suffered from diffuse gastric and duodenal erosions caused by considerable epithelial death. These epithelial cell lesions were similar to lesions previously found in other LFN-exposed tissues as rat pleura and bronchi. They were also similar to ulcerative lesions caused by whole body vibration; vibratory phenomenon comparable to noise Sukharevskaia et al. [22].
Our findings were also in agreement with Da-Fonseca et al. [7] who detected two striking features in LFN-exposed rats; increased frequency of cell death in the glandular epithelial layer of the distal stomach, by both LM and SEM and erosions observed both macroscopically and by histological examination reflecting cellular degeneration and death.
Moslehi et al. [6] explained gastritis seen in all groups of traffic noise exposed rats via activation of the vagus nerve in the medulla. Ising et al. [23] proved that, noise exposure activates hypothalamic pituitary adrenal axis. Therefore, hypothalamus has an important role in noise pathophysiological effects. They also added that, rats exposed to repeated restraint stress were found to have a higher level of plasma noradrenalin and corticosterone compared to the non-stressed rats.
During stress the underlying mechanisms involved are the activation of the hypothalamic-pituitary-adrenal axis and sympathoadrenal- medullary systems causing the release of corticosterone together with the release of noradrenalin and adrenalin [24,25].
Adrenal catecholamines play a physiological role in response to stressful situations. Rats exposed to stress were found to develop gastric lesions associated with reduced brain noradrenalin content and increased plasma catecholamines and corticosterone levels [26]. The elevation in catecholamine levels generates free radicals which are cytotoxic and mediate tissue damage by injuring cellular membranes and releasing intracellular components [27].
According to Shi and Nuttall [28], exposure to noise increased the activity of inducible nitric oxide synthase with excess nitric oxide production which caused oxidative stress, generating an excess of ROS leading to DNA damage.
Acoustic stressors can also impact genes in two ways; by setting off chemical cascades that can lead to DNA damage and ⁄or by altering gene expression. The neural activity required to process environmental noise leads to an increased number of free radicals, which were known to cause carcinogenic mutations [29]. ROS cause damage to DNA, as well as to proteins and lipids. ROS-induced damage was observed in the adrenal glands [30] and hearts [31] of noise-stressed rats.
In the current work, LFN-exposed group revealed also dilated fundic glands, creeping of collagen fibers between the basal parts of the fundic glands, mononuclear cell infiltration and thick muscularis mucosa.
These findings were in agreement with Hill et al. [32]. They stated that, the cellular components of organs particularly fibroblasts, endothelial cells and smooth muscle cells were subjected to a mechanical stress that goes beyond what happens under normal conditions. The transmission of such forces to cells in organs such as blood vessels caused production of growth factors, cytokines or hormones that led to hypertrophic, hyperproliferative or fibrotic responses. LFN increased collagen I and III in the extracellular matrix and induced ultrastructural alterations in the cardiomyocytes, interstitial collagen deposits and changes in mitochondria and intercalated discs of the cardiomyocytes in LFN-exposed animals [10].
In an experiment by Du et al. [33] rats exposed to noise, increased activation of microglial cells and macrophages in the brain and spinal cord that defend the central nervous system against immunological challenges.
In the present work, Mallory trichrome stained sections of LFN exposed rats showed increased collagen fibers distributed in the lamina propria of fundic mucosa. It was also proved statistically by highly significant increase in the mean area percent of collagen fibers of the fundic mucosa in the LFN exposed group as compared to the control group and LFN+vit.E exposed group. These results were in agreement with Ingber [34,35] who stated that mechanical forces which applied to individual cells could change cell reactions to biochemical stimuli or even induce entirely different cellular responses. Mechanical forces resulting from tissue vibration may be the initial stimulus for collagen production.
Unlike the fibrotic proliferation in response to an inflammatory stimulus, the tissue exposed to LFN seems to reflect a structural reinforcement in order to assimilate the abnormal vibration stress. This structural reinforcement would be achieved by massive production of collagen [36,37]. In a study done by Fonseca et al. [21], fibrotic changes started early in the first weeks of LFN-exposure. The thickness of the submucosa of 1 week exposed rats was significantly larger than that of control rats. In their study, LFN-exposure was continuous. Consequently, the fibrotic process was constantly increased with exposure. They found that, in rats exposed for longer periods (9 weeks or 13 weeks) fibrosis was due to collagen IV production and related to the neoangiogenesis process.
In the present study, PAS-alcian blue stained sections of the same group revealed negative reaction in the surface epithelium and the mucous neck cells in the neck region of the fundic glands. SEM revealed dilated gastric pits, loss of the normal mucous sheet covering the surface mucous cells with some mucous patches. Globular mucous form disappeared. These findings were in agreement with Mohamed [38], who attributed these changes to occurrence of damage in the gastric mucosal barriers. The first line of defense in the stomach; which is the mucus, was decreased due to suppressed prostaglandin production and damage of the surface epithelial cells and mucus neck cells.
Decreased mucus secretion allows hydrogen ions and pepsin to diffuse into the mucosa from the lumen. Back diffusion of acid and pepsin into the tissues stimulates further acid and pepsin secretion, decreases mucosal blood flow and decreases gastric motility. The acid also damages connective tissue and submucosal capillaries to cause focal mucosal hemorrhage [39].
In the current study, Faint positive immuoreaction in chromogranin-A secrerting cells cytoplasm with highly statistically significant decrease in the mean optical density of chromogranin-A immuoreaction the fundic mucosa of LFN exposed group as compared to the control group and LFN+vit.E exposed group. Chromogranins are acidic glycoproteins that play an active role in hormone and neuropeptide secretion in neuroendocrine cells. Chromogranin-A is the major member of the granin family. It plays multiple roles in the secretory process [40]. Such finding was in agreement with Biswas et al. [41]. They stated that, increased microvascular injury causes ischemia which leads to gastric mucosal cells necrosis. Low oxygen tension and the subsequent depletion of ATP generation, affect sodium-potassium pump leading to influx of sodium into the cell and osmotic gain of water. At the same time, the intracellular calcium increases through influx from the extracellular fluid and its release from intracellular stores. This activates phospholipases, protease and endonucleases which result in cellular damage. Sun et al. [42] also stated that, suppressed gastric mucosal cyclo oxygenase-1 and increased gastric mucosal TNF-α, Fas and Fas ligand level, increased death signal leading to activation of caspase-3 and caspase 8.
Examination of LFN+vitamine E group rats revealed an obvious improvement in the structure of fundic glands associated with strong positive PAS reaction in the mucous film over the surface epithelium extending to fill the gastric pits. Thin collagen fibers distributed in the lamina propria and strong positive immuoreaction in chromogranin-A secreting cells cytoplasm were also detected.
These findings were in agreement with the study reported by Ohta et al. [13]. Prostaglandin E2, the substance that maintains the gastric mucosal integrity, gastric acidity and gastrin level were found to be decreased in the stomach of rats exposed to water immersion restraint stress (WRS). Treatment with palm vitamin E was able to reverse the detrimental effects of WRS [11]. Vitamin E plays important roles in maintaining the integrity of the gastric mucosa. It had been shown to prevent the increase in stress-induced gastric contractions [43]. This may explain the protective effect of vitamin E in reducing the formation of gastric lesions.
A decrease in gastric mucosal vitamin E level and an increase in gastric mucosal lipid peroxidation were found in ischemiareperfusion- induced gastric mucosal injury. The severity of the injury was enhanced in vitamin E deficient rats Yoshikawa et al. [44]. It was also found that, stress can impair gastric blood flow and cause ischemic-like conditions leading to reperfusion-induced injury and finally development of gastric lesions [26].
In the presence of stress, vitamin E prevented the production of the enzyme xanthine oxidase in the stomach. In consequence, the free radicals formation was prevented. Xanthine oxidase promotes production of free radicals. Involvement of free radicals has been proposed as one of the mechanisms in the development of stress induced gastric ulcers. Free radicals promote lipid peroxidation and this process can be assessed by the production of its stable end product, malondialdehyde [45].
Nur-Azlina et al. [26,46] showed that gastric PGE2 content after 3.5 hours exposure to WRS was signifiantly suppressed compared to that of the control group. They recorded that, the increased gastric PGE2 content in their study after tocotrienol (TT) administration as a source of vitamine E, could possibly be due to the effect of vitamin E which was reported to stimulate prostaglandin synthesis by activating the calcium-dependent phospholipase enzyme A2 and inhibiting the lipoxygenase enzyme. They also stated that, gastroprotective effect of vitamin E was not only due to its antioxidant action but also its inhibitory action on neutrophil infiltration into the gastric mucosa.
Examination of recovery group showed partial improvement in fundic glands structure with positive PAS reaction in the mucous film over the surface epithelium extending to fill the gastric pits and negative reaction in the mucous neck cells in the neck region of the glands. Positive immuoreaction in chromogranin-A secrerting cells cytoplasm with significant decrease in the mean optical density of chromogranin-A immuoreaction of the fundic mucosa was detected in the recovery group as compared to the control group. Some authers [46,47] found that the exposure to stress led to ischemia reperfusion, which produced a significant fall in PGE2 generation in the gastric mucosa, but it was gradually restored during mucosal recovery from gastric lesions, suggesting that endogenous prostaglandin is involved in the spontaneous healing of these lesions. This is supported by the fact that PGE2 generation reached higher values during the course of healing of ulcerated gastric mucosa than it did in nonulcerated mucosa.
Konturek et al. [48] also showed that, the healing of stress lesions resulted in the restoration of mucosal prostaglandin generation, and this effect was accompanied by overexpression of epithelial growth factor (EGF) and tumor necrosis factor alpha (TNFα) as well as cyclooxygenases COX-1 and COX-2 mRNA and by the increased biosynthesis of gastroprotective prostaglandins.
Collagen fibers in the recovery group were creeping in the lamina propria between the basal fundic glands with highly significant increase in the mean area percent of collagen fibers as compared to the control group. This was explained by Verrecchia and Mauivel [49] who reported that, in fibrosis caused by chronic stimulation, the tissue injury and the attempt of regeneration process were implicated. Also, it was reported that despite the fibrogenesis is a repair mechanism; it tends to imbalance with extensive deposition of the matrix proteins and fibrosis with the chronicity of the stimulus on the tissue [50]. According to Kight and Swaddle [51], animals may habituate to stressors over time. Many neuroendocrine responses to noise are highly plastic; thus, ecological control of noise pollution could allow animals to achieve both structural and functional recovery.
Conclusion
Exposure to LFN has marked effects on fundic mucosa and its mucus barrier. So, it is recommended to meticulously follow up people exposed to LFN to avoid any possible complications. Moreover, marked improvement with vitamin E administration was approved. So, vitamin E is recommended to protect the fundic mucosa against these effects.
References
- Xie H, Kang J, Mills GH (2009) Clinical review: The impact of noise on patients' sleep and the effectiveness of noise reduction strategies in intensive care units. Crit Care 13: 208.
- Koc ER, Ersoy A, Ilhan A, Erken HA, Sahin S (2015) Is rosuvastatin protective against on noise-induced oxidative stress in rat serum? Noise Health 17: 11-16.
- Tamura H, Ohgami N, Yajima I, Iida M, Ohgami K, et al. (2012) Chronic exposure to low frequency noise at moderate levels causes impaired balance in mice. PLoS One 7: e39807.
- Reinhold K, Kalle S and Paju J (2014) Exposure to high or low frequency noise at workplaces: differences between assessment, health complaints and implementation of adequate personal protective equipment K. Agronomy Research. 12: 895-906.
- Mendes J, Santos J, Oliveira P and Branco CN (2007) Low frequency noise effects on the periodontium of the Wistar rat-a light microscopy study. Eur J Anat 11: 27-30.
- Moslehi A, Nabavizadeh-Rafsanjani F, Keshavarz M, Rouhbakhsh N, Sotudeh M, et al. (2010) Traffic noise exposure increases gastric acid secretion in rat. Acta Med Iran 48: 77-82.
- Da-Fonseca J, Dos Santos J, Branco N, Alves-Pereira M, Grande N, et al (2006) Noise Induced Gastric Lesions: A Light And Scanning Electron Microscopy Study Of The Alterations Of The Rat Gastric Mucosa Induced By Low Frequency Noise. Cent Eur J Public Health. 14: 35-38.
- Asar M, Kayisli UA, Izgut-Uysal VN, Oner G, Kaya M, et al. (2000) Cadmium-induced changes in epithelial cells of the rat stomach. Biol Trace Elem Res 77: 65-81.
- Bashandy M and Seleem H (2014) Effect of hunger and thirst stress on the fundic mucosa of the stomach of adult female albino rats (Histological, histochemical and immunohistochemical study). Journal of American Science 10: 264-273.
- Antunes E, Borrecho G, Oliveira P, De Matos APA, Brito J, et al. (2013) Effects of low-frequency noise on cardiac collagen and cardiomyocyte ultrastructure: an immunohistochemical and electron microscopy study. Int J ClinExpPathol 6: 2333-2341.
- Ibrahim A, Kamisah Y, Ismail N, Fahami N (2008) Protective effect of palm vitamin E and a-tocopherol against gastric lesions induced by water immersion restraint stress in Sprague-Dawley rats. Indian J Pharmacol 42:73-77.
- Baldwin AL, Primeau RL, Johnson WE (2006) Effect of noise on the morphology of the intestinal mucosa in laboratory rats. J Am Assoc Lab AnimSci 45: 74-82.
- Ohta Y, Kobayashi T, Imai Y, Inui K, Yoshino J, et al. (2006) Effect of oral vitamin E administration on acute gastric mucosal lesion progression in rats treated with compound 48/80, a mast cell degranulator. Biol Pharm Bull 29: 675-683.
- Aziz Ibrahim IA, Kamisah Y, Nafeeza MI, NurAzlina MF (2012) The effects of palm vitamin E on stress hormone levels and gastric lesions in stress-induced rats. Arch Med Sci 8: 22-29.
- Ozmen J, Bobryshev YV, Lord RS, Ashwell KW (2002) Identification of dendritic cells in aortic atherosclerotic lesions in rats with diet-induced hypercholesterolaemia. Histol Histopathol 17:223-237.
- Bancroft J, Layton C (2013) The Hematoxylin and eosin. In: Suvarna SK, Layton C and Bancroft JD editors. Theory and Practice of histological techniques (7thed.), Churchill Livingstone of El Sevier Philadelphia 172-214.
- Drury RAB, Walington EA (1980) Carleton’s histological techniques (5th ed.), Oxford: Oxford University Press, pp. 250-266.
- Kiernan JA (2000) Histological and Histochemical Methods, Theory and Practice (3rded.), Oxford, Boston, Johannesburg and New Delhi, pp. 129-139.
- Amer M, Mohamed D and Karam R (2013) Protective role of curcumin against 2,3,7,8 tetrachlorodibenzodioxin-induced histological and biochemical changes in fundic mucosa of the adult rat stomach. Egyptian J Histol 36:13-27.
- Dykstra MJ, Reuss LE (2003) Biological electron microscopy: theory, techniques, and troubleshooting. (2nded.), New York: Library of congress, pp. 74-100
- Fonseca J, Santos M, Oliveira P, Laranjeira N, Aguas A, et al. (2012) Noise-induced gastric lesions: a light and electron microscopy study of the rat gastric wall exposed to low frequency noise. Arq Gastroenterol 49: 82-88.
- Sukharevskaia TM, Nepomniashchikh GI, Bobrova SV, BelovIIu, AÄÂdagulova SV, et al. (1999) [Clinical endoscopic and pathomorphologic study of the stomach in vibration disease]. Med Tr Prom Ekol : 16-19.
- Ising H, Lange-Asschenfeldt H, Moriske H, Born J, Eilts M (2004) Low frequency noise and stress: bronchitis and cortisol in children exposed chronically to traffic noise and exhaust fumes. Noise Health. 6: 21-28.
- Ainsah O, Nabishah B, Osman C, Khalid B (2000) Naloxone but not Glycyrrhizic acid modifies stress induced changes in brain serotonin levels. Asia Pac J Pharmacol 14:1-7
- NurAzlina MF, Nafeeza MI (2008) Tocotrienol and alpha-tocopherol reduce corticosterone and noradrenalin levels in rats exposed to restraint stress. Pharmazie 63: 890-892.
- Nur- Azlina M, Ibrahim I, Kamisah Y, Ismail N (2012) Palm vitamin E reduces catecholamines, xanthine oxidase activity and gastric lesions in rats exposed to water-immersion restraint stress. BMC Gastroenterology 12: 54-58.
- Huang CJ, Webb HE, Evans RK, McCleod KA, Tangsilsat SE, et al. (2010) Psychological stress during exercise: immunoendocrine and oxidative responses. Exp Biol Med (Maywood) 235: 1498-1504.
- Shi X, Nuttall AL (2003) UpregulatediNOS and oxidative damage to the cochlear striavascularis due to noise stress. Brain Res 967: 1-10.
- Samson J, Sheela Devi R, Ravindran R, Senthilvelan M (2005) Effect of noise stress on free radical scavenging enzymes in brain. Environ Toxicol Pharmacol 20: 142-148.
- Frenzilli G, Lenzi P, Scarcelli V, Fornai F, Pellegrini A, et al. (2004) Effects of loud noise exposure on DNA integrity in rat adrenal gland. Environ Health Perspect 112: 1671-1672.
- Lenzi P, Frenzilli G, Gesi M, Ferrucci M, Lazzeri G, et al. (2003) DNA damage associated with ultrastructural alterations in rat myocardium after loud noise exposure. Environ Health Perspect 111: 467-471.
- Hill M, Davis M, Meininger G, Potocnik S, Murphy T (2006) Arteriolar myogenic signalling mechanisms: implications for local vascular function. Clin Hemorheol Microcirc 34: 67-79.
- Du F, Yin L, Shi M, Cheng H, Xu X, et al. (2010) Involvement of microglial cells in infrasonic noise-induced stress via upregulated expression of corticotrophin releasing hormone type 1 receptor. Neuroscience 167: 909-919.
- Ingber DE (2003) Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 116: 1397-1408.
- Ingber DE (2004) The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst 1: 53-68.
- Ferreira JR, Albuquerque e Sousa J, Foreld P, Antunes M, Cardoso S, et al. (2006) Abnormal respiratory drive in vibroacoustic disease. Rev Port Pneumol 12: 369-374.
- Oliveira P, Brito J, Mendes J, DA- Fonseca J, Ãguas A, et al. (2013) Effects of Large Pressure Amplitude Low Frequency Noise in the Parotid Gland Perivasculo-Ductal Connective Tissue. Acta Med Port 26: 237-242.
- Mohamed A (2010) Postulated Protective Role of Curcumin on Indomethacin-induced Acute Gastric Mucosal Damage in Adult Albino Rats (Histological and Immunohistochemical Study). Egypt J Histol 33: 583-593.
- Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, et al. (2002) Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med 33: 311-322.
- Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh Y P, et al. (2011) The Extended Granin Family: Structure, Function, and Biomedical Implications. Endocr Rev 32: 755-797.
- Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, et al. (2003) A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem 278: 10993-11001.
- Sun Y, Chen X, Liu J, Cheng X, Wang X, et al. (2006) Differential caspase-3 expression in non-cancerous, pre-malignant and cancer tissues of stomach and its clinical implication. Cancer Detect Prev 30: 168- 173.
- Ibrahim A, Kamisah Y, Ismail N, Fahami N (2011) Modulation of gastric motility and gastric lesion formation in stressed rats given enteral supplementation of palm vitamin E and a-tocopherol. Int Med J 18: 47-52.
- Yoshikawa T, Yasuda M, Ueda S, Naito Y, Tanigawa T, et al. (1991) Vitamin E in gastric mucosal injury induced by ischemia-reperfusion. Am J ClinNutr 53: 210S-214S.
- Kamisah Y, Ibrahim I, Nafeeza M, Nur-Azlina M (2011) Palm tocotrienol-rich fraction supplementation suppressed stress-induced gastric oxidative stress in rats. J Applied Pharmaceutical Science. 1: 118-122.
- NurAzlina MF1, Kamisah Y, Chua KH, Qodriyah HM (2013) Tocotrienol Attenuates Stress-Induced Gastric Lesions via Activation of Prostaglandin and Upregulation of COX-1 mRNA. Evid Based Complement Alternat Med 2013: 804796.
- Brzozowski T, Konturek P and Sliwowski Z, Drozdowicz D, Burnat G, et al, (2008) “Gastroprotective action of orexin-A against stress-induced gastric damage is mediated by endogenous prostaglandins, sensory affrent neuropeptides and nitric oxide,†Regulatory Peptides. 148: 6-20.
- Konturek PC, Brzozowski T, Duda A, Kwiecien S, Löber S, et al. (2001) Epidermal growth factor and prostaglandin E(2) accelerate mucosal recovery from stress-induced gastric lesions via inhibition of apoptosis. Journal Physiology Paris 95: 361-367.
- Verrecchia F, Mauviel A (2004) TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal 16: 873-880.
- Kisseleva T, Brenner DA (2008) Mechanisms of fibrogenesis. ExpBiol Med (Maywood) 233: 109-122.
- Kight C, Swaddle J (2011) How and why environmental noise impacts animals: an integrative, mechanistic review. Ecology Letters 14: 1052-1061.
Citation: Ahmed SM, Abdelrahman SA, Hassan EZ (2015) Effect of Low Frequency Noise on Fundic Mucosa of Adult Male Albino Rats and the Role of Vitamin E Supplementation (Histological and Immunohistochemical Study). J Clin Exp Pathol 5:256. DOI: 10.4172/2161-0681.1000256
Copyright: © 2015 Ahmed SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12568
- [From(publication date): 12-2015 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 11537
- PDF downloads: 1031