Effect of Tianeptine on Spared Nerve Injury-Induced Allodynia and Prefrontal Cortex Vascular Endothelial Growth Factor
Received: 21-Oct-2021 / Accepted Date: 08-Nov-2021 / Published Date: 15-Nov-2021
Abstract
Objectives: Antidepressant drugs are widely used in the management of neuropathic pain. Emerging studies have shown that tianeptine (TNT), an atypical antidepressant with distinct neurochemical properties, is effective in reducing neuropathic pain symptoms. However, the neural mechanisms underlying the analgesic action of TNT are not fully understood. Alteration in vascular endothelial growth factor (VEGF) expression appears to play an important role in both pain and antidepressant mechanisms. Although the involvement of VEGF in inflammatory or neuropathic pain at the spinal level has been reported, the effect of neuropathic pain on cortex VEGF is unknown. Additionally, the TNT effect on cortex VEGF is not reported. The present study examined changes in cortex VEGF levels following TNT treatment in the neuropathic pain state.
Methods: The experiments were performed in a rat model of spared nerve injury (SNI)-induced neuropathic pain. TNT (75 mg/60 kg/day/orally) or saline was administered to SNI rats on days 14-18 post-injury. The effects of TNT on SNI-induced mechanical and cold allodynia were assessed by von Frey and acetone drop tests, respectively. The changes in the prefrontal cortex (PFC) VEGF protein expression following SNI and TNT treatments were measured by a Simple Western automated system.
Results: Rats that underwent the SNI protocol displayed both mechanical and cold allodynia, as expected. Single and repeated administration of TNT significantly reduced mechanical allodynia but had no effect on cold allodynia. Additionally, SNI rats showed increased VEGF protein expression in the PFC and this was reversed by TNT treatments, suggesting a link between the TNT-mediated antinociceptive effect and PFC VEGF expression.
Conclusion: Repeated oral administration of TNT reduces SNI-induced mechanical allodynia, and this effect appears to be associated with the regulation of prefrontal cortex PFC VEGF expression.
Keywords: Neuropathic Pain; Tianeptine; Vascular Endothelial Growth Factor; Mechanical Allodynia; Cold Allodynia
Abbrevations
PWT: Paw Withdrawal Threshold; PFC: Prefrontal Cortex; SAL: Saline; SNI: Spared Nerve Injury; TNT: Tianeptine; VEGF: Vascular Endothelial Growth Factor.
Introduction
Persistent neuropathic pain is a complex neurological disorder that results from a disease or direct damage to the somatosensory system. High prevalence of neuropathic pain in the general population has been reported worldwide. For example, in the United States around 1.6% of the population experiences chronic neuropathic pain [1]. Neuropathic pain impairs quality of life and causes a high economic burden to health care systems worldwide [2-5].
Allodynia, hyperalgesia, and hyperpathia are the hallmark symptoms of chronic neuropathic pain. The underlying mechanisms of chronic neuropathic pain are not fully understood. Nerve injury- induced activation of immune cells, alteration of neurochemicals, neuropeptides, neurotransmitters, ion channels, and release of pro- inflammatory mediators are some of the mechanisms that have been implicated in the etiology of chronic neuropathic pain [6-8]. Existing drug therapies are effective in reducing neuropathic pain symptoms only in a small population of patients and often they produce significant side effects when used repeatedly [7, 9, 10]. Therefore, there is an urgent need for novel analgesics with minimal side effects and for a better understanding of neuropathic pain mechanisms.
At present, tricyclic antidepressants (e.g., amitriptyline), serotonin and noradrenaline re-uptake inhibitors (e.g., duloxetine) are used as first-line drugs to reduce neuropathic pain symptoms [8]. TNT is an atypical antidepressant that possesses distinct and diverse neurochemical properties compared to classical antidepressants.
Studies indicate the anti-allodynic effect of TNT in nerve injury models [11-13]. Further, TNT has shown full agonist activity at the mu opioid receptor and mimics opioid-like behavioral effects [14]. Additionally, a recent study demonstrates that TNT prevents morphine-induced respiratory depression without altering antinociceptive activity in conscious rats [15].
Mammalian Vascular Endothelial Growth Factor (VEGF) gene family members include VEGF-A, VEGF-B, VEGF-C, VEGF-D, and Placental Growth Factor (PlGF). Members of the VEGF family signal by binding to three types of receptor tyrosine kinases (RTKs), VEGFR-1 (Flt-1), VEGFR- 2 (KDR/Flk-1), and VEGFR-3 (Flt-4) [16]. VEGF-A (hereafter referred to as VEGF) signals by binding and activating VEGFR-1 and VEGFR-2. VEGF activity has been extensively investigated in different types of pain mechanisms [16-21]. For example, in neuropathic pain states increased expression of VEGF at the site of nerve injury, dorsal root ganglion, trigeminal ganglion, spinal cord and plasma have been reported [19, 22-24]. Similarly, upregulation of VEGF protein levels in inflammatory pain conditions such as interstitial cystitis and osteoarthritis have been reported [16, 25, 26].
Previous reports have shown that VEGF-mediated signaling pathways in the cortex plays an important role in the mechanisms of action of antidepressants [27, 28]. Furthermore, in chronic pain states, the prefrontal cortex (PFC) area of the brain undergoes structural and functional changes in both human patients and animal models of pain [29-31]. However, the effect of antidepressants on PFC VEGF protein expression in neuropathic pain is unknown.
Thus, the objectives of the present study were: (1) to determine whether peripheral nerve injury alters PFC VEGF expression levels; and (2) to examine the association between the antinociceptive effect of TNT and PFC VEGF levels in a neuropathic pain state. We utilized a rat model of Spared Nerve Injury (SNI) and western blot methods to meet these objectives.
Methods
Animals
Experiments were performed on male Sprague-Dawley rats of body weight 200-220g. The rats were purchased from Charles River Laboratories, USA. Rats were housed 2/cage on a 12 h light/dark cycle (6 am – 6 pm) with ad libitum access to food and water. Rats spent one week in the vivarium prior to the start of experiments. This study was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. The facility’s Institutional Animal Care and Use Committee approved all research conducted in this study. The facility where this research was conducted is fully accredited by the AAALAC.
Spared Nerve Injury (SNI)
Neuropathic pain was induced by performing SNI surgery as previously described [32]. Briefly, a rat was deeply anesthetized and fur over left hind limb trimmed. Following limb immobilization, an incision was made at mid-high level. The three peripheral branches (sural, common peroneal and tibial) of the sciatic nerve were exposed. Forceps were placed below the tibial and common peroneal nerves to slide thread (sterile 6.0 silk Ethicon) around these nerves and then tightly ligated. About 3-4 mm of the nerve stump that is distal to the ligation was sectioned and removed. The muscle and skin were closed with 5.0 absorbable sterile sutures. The sham procedure involved the same surgery under anesthesia without ligation. Silver sulfoxide was applied on the sutured area one time to minimize infection. In addition, the animals’ general appearance and wound assessment were monitored throughout the experimental period.
Experiment Design and Drug Treatment
(Figure 1) shows the experimental procedures and treatments. We used 3 groups of rats (n=5-6/group) for this study. Group 1 was sham surgery and groups 2 and 3 underwent SNI procedure. Rats from groups 1 and 2 were given saline, whereas group 3 rats received TNT (75 mg/60 kg), orally one time/day on day’s 14-18 post-SNI. Oral administration was performed using a 16-guage gavage needle in a volume of 5 mL/kg. The TNT oral dose was calculated as shown in previous study [13]. Additionally, 75 mg/60 kg of TNT was found ineffective in producing antinociceptive activity in uninjured rats (data not shown). Mechanical and cold allodynia tests were performed before and one hour after administration of TNT on day 14 and 18 post-SNI.
Mechanical Allodynia Test
To test for mechanical allodynia, rats were subjected to non- noxious stimuli as described previously [33]. Rats were placed in clear Plexiglas chambers (non-restrictive) on an elevated grid platform. Using an electric anesthesiometer (Ugo Basile), a blunt mechanical stimulus was applied to the plantar surface of the hind paw with slowly increasing force until the rat voluntarily withdraws the paw. The force of the mechanical stimulus was set to produce a ramp of 3 grams/sec over 10 sec with a cutoff of 30 grams. The threshold (in grams) for the rat to remove the paw from the stimulus was recorded automatically as the paw withdrawal threshold (PWT). Each hind paw was tested in triplicate at each time point and an average was taken for analysis.
Cold Allodynia Test
Rats were placed in a Plexiglas box resting on an elevated grid platform and a stream of either 100 μl acetone or saline was applied to the plantar surface of the injured and uninjured hind paws. The number of shakes and/or licking episodes evoked due to cooling effect produced by acetone was measured over a 2 min observation period. The mean of 2 consecutive trials with a 5 min inter- trial interval was calculated as a measure of nocifensive behavior to a cold stimulus [34, 35].
Tissue Isolation
Following the final behavioral tests, rats were humanely euthanized by decapitation in accordance with USAISR IACUC Policy: Use and Maintenance of Guillotine’s for Rodents. Briefly, rats without analgesics/anesthetics were restrained in a plastic Decapicone and were decapitated using a guillotine (Harvard Apparatus) by a trained technician. This method allows intact brain tissue to be obtained without chemical contamination. The brains were immediately removed, flash-frozen in liquid nitrogen and stored at − 80 °C until fixation in a pre-chilled rat brain slicing matrix (Zivic instruments) at 4°C. The brain was maintained in a semi-frozen state and all dissections were completed prior to full thawing. The dissection of the prefrontal frontal cortex was performed as described previously [36, 37]. Briefly, the frontal cortex was separated from the whole brain by cutting at the first appearance of the corpus callosum at bregma 0.70 mm. The ventral area containing the olfactory nuclei was removed; leaving the dorsal prefrontal cortex intact, which was further separated into left and right hemispheric regions.
Total Protein Isolation and Simple Western Protein Analysis
To isolate protein, 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES) (20 mM HEPES; 1 mM EDTA; 40 units/mL RNAse inhibitor; mini complete protease inhibitor tablet) buffer was added to the dissected right (ipsilateral) and left (contralateral) dorsal prefrontal cortex samples. Tissue was homogenized 2 times for 20 s each, split into two separate tubes, and centrifuged at 13,000×g for 20 min at 4°C. The pellet for the protein isolate was solubilized in RIPA buffer for 20 min on ice. Following another centrifugation step, the supernatant was subjected to the bicinchoninic acid assay (BCA; Pierce) to determine protein concentration.
Simple Western Protein Analysis
VEGF antibody (LSBio, Cat#LS-C48549, at 45 to 55 kD) and GAPDH (39-41 kD) were assayed for protein expression by the ProteinSimple Wes System, utilizing kit items SM-W004 and DM- 001. Briefly, as per the kit protocol, 400 mM dithiothreitol (DTT) and 10X sample buffer were mixed to prepare 5X fluorescent master mix. A final concentration of 0.2 mg/mL was prepared by combing 5X fluorescent master mix and the protein lysate. A biotinylated protein standard ladder was prepared with 10X sample buffer, 400 mM DTT, and deionized water. Ladder was denatured for 5 min at 95 °C and loaded onto lane 1 of the pre-filled plate provided in the assay kit. Samples then followed at 5uL per lane observing lane design. The VEGF primary antibody (1:50 dilution in kit Antibody 2 buffer), the kit secondary antibody, and luminol-S/peroxide combined substrate were loaded onto the plate following the pre-set assay application design. The assay was run using pre-set conditions for a 25 lane 25-230kD electrophoresis resolution, and the resulting data assessed by Wes Systems analysis software, as well as ImageJ software [38].
Statistical Analysis
GraphPad Prism 5 statistical software (GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the experimental data. All data were expressed as mean ± standard error of mean (SEM). Repeated measures two-way analysis of variance (ANOVA) for behavioral data and one-way ANOVA (western blot data) were used to find interaction, time, and treatment effects. This was followed by Bonferroni’s post-hoc test to compare the differences among individual groups. The statistical significance was set at a level of P-values of < 0.05.
Results
Effect of TNT on SNI-induced mechanical and cold allodynia
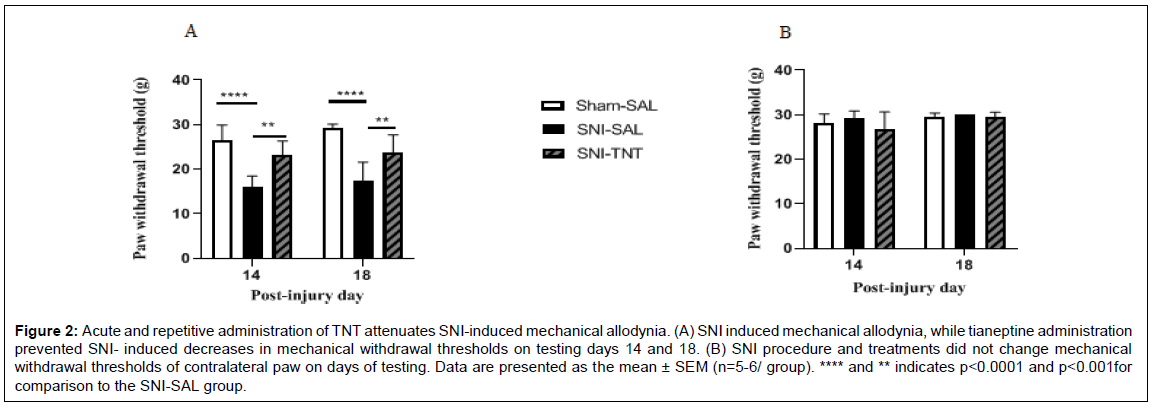
Consistent with the results shown previously [39], the SNI procedure produced mechanical and cold allodynia that lasted through the day 18 post-injury period (Figure 2A & 3A). Two-way RM ANOVA analysis of the mechanical allodynia data showed F(1,14) = 2.545, P=0.1330; F(2,14)=29.53, P< 0.0001; F(14,14)=1.340, P = 0.3131 for the time, TNT treatment, and for the interaction of TNT treatment × time, respectively, (Figure 2A). Significant decreases in PWTs in response to mechanical stimulation between sham-saline and SNI-saline were observed on day 14 and 18 post-injury (Post hoc test, P < 0.001 for both day 14 and 18). TNT treatment significantly increased withdrawal threshold on day 14 (Post hoc test, P < 0.0029) and on day 18 (Post hoc test, P<0.0092) post-injury. Repeated administration of saline did not alter PWT in sham and SNI rats (P>0.05). Additionally, withdrawal thresholds after the first and before the last injections of TNT were not different. No change in contralateral withdrawal threshold among experimental groups was observed on day 14 and day 18 post-injury/ treatment (All P’s>0.05), (Figure 2B). Ipsilateral paws of SNI rats treated with saline and TNT showed comparable increase in response to acetone application on both testing days 14 and 18. Two-way RM ANOVA showed F(1,14)=0.7921, P=0.3885; F(2,14) =12.68, P<0.0001; F(14, 14) = 2.017, P=0.1008 for the time, TNT treatment, and for the interaction TNT treatment × time, respectively, (Figure 3A). Post hoc test showed a significant difference between Sham- Saline and SNI-Saline (P<0.0001) and Sham- Saline verses SNI- TNT (P<0.0001) but the SNI-Saline verses SNI-TNT groups were not different (P>0.05). Analysis of contralateral paw measurements showed no significant change in nocifensive behaviors among experimental groups on day 14 and day 18 post-injury/treatment (All P values >0.05) (Figure 3B).
Figure 2: Acute and repetitive administration of TNT attenuates SNI-induced mechanical allodynia. (A) SNI induced mechanical allodynia, while tianeptine administration prevented SNI- induced decreases in mechanical withdrawal thresholds on testing days 14 and 18. (B) SNI procedure and treatments did not change mechanical withdrawal thresholds of contralateral paw on days of testing. Data are presented as the mean ± SEM (n=5-6/ group). **** and ** indicates p<0.0001 and p<0.001for comparison to the SNI-SAL group.
Figure 3: Effect of TNT on SNI-induced cold allodynia. (A) Compared to sham rats receiving saline treatment, the mean cold response duration was significantly high in SNI rats receiving saline and tianeptine treatments on days 14 and 18. Cold response was similar between SNI-SAL and SNI-TNT rats on days 14 and 18. (B) Cold stimulus did not evoke significant response on contralateral paw among experimental groups. Data are presented as the mean ± SEM (n=5-6/ group). *** and * indicates p<0.0001 and p<0.05 for comparison to the Sham-SAL group.
Effect of TNT on VEGF protein expression level in the PFC
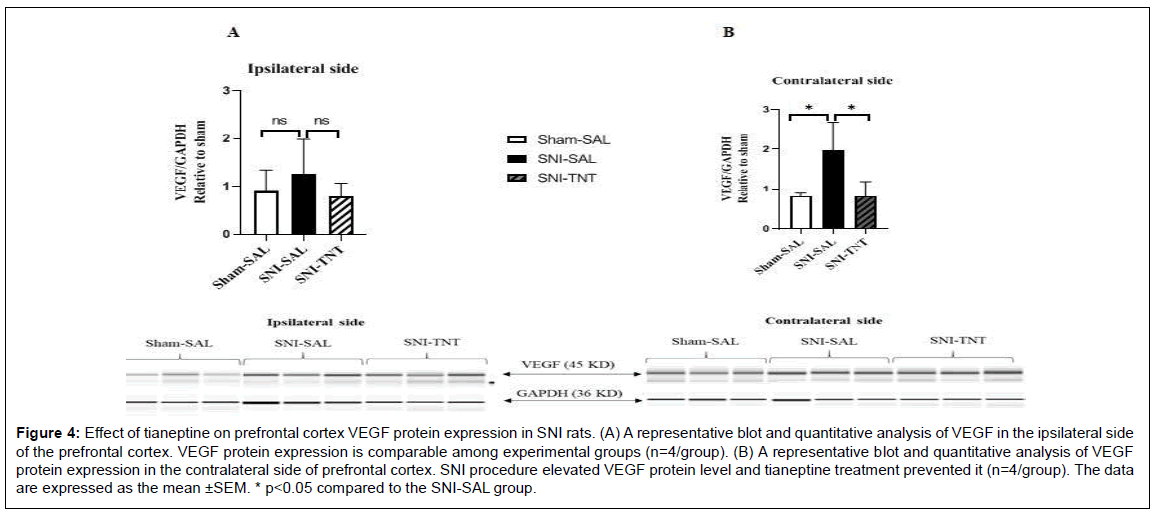
Alteration of VEGF protein levels in the PFC of both ipsilateral and contralateral sides to the nerve injury at the termination of the experiment (day 18 post-injury), was assessed using the Simple Wes method. SNI increased VEGF protein levels in the contralateral side of PFC (Sham+Saline vs. SNI+Saline, P<0.05). VEGF protein level was reduced in the PFC contralateral side in rats that received TNT treatments (SNI+Sal vs. SNI+TNT, P<0.05) (Figure 4B). Among experimental groups, no significant changes in the VEGF protein levels were observed in the ipsilateral side of the PFC (P>0.05) (Figure 4A).
Figure 4: Effect of tianeptine on prefrontal cortex VEGF protein expression in SNI rats. (A) A representative blot and quantitative analysis of VEGF in the ipsilateral side of the prefrontal cortex. VEGF protein expression is comparable among experimental groups (n=4/group). (B) A representative blot and quantitative analysis of VEGF protein expression in the contralateral side of prefrontal cortex. SNI procedure elevated VEGF protein level and tianeptine treatment prevented it (n=4/group). The data are expressed as the mean ±SEM. * p<0.05 compared to the SNI-SAL group.
Discussion
These results demonstrate two important points. First, a peripheral nerve injury (SNI)-induced mechanical and cold allodynia response is accompanied by increased PFC VEGF protein expression. Second, the TNT-mediated mechanical anti-allodynic effect is associated with reduced PFC VEGF protein levels in the SNI animals. Together, these data provide the first evidence of a possible mechanistic link between TNT-mediated antinociceptive activity and PFC VEGF dysregulation in a neuropathic pain condition.
In a L5 spinal nerve ligation (SNL) neuropathic pain model, it has been reported that intrathecal and oral administration of TNT dose- dependently reduced mechanical allodynia [11,12]. Our findings on the antinociceptive effect of TNT on SNI-induced mechanical allodynia is compatible with these previous reports. Additionally, we show that both a single and repeated administration of TNT produces a comparable increase in PWT in the mechanical allodynia test. This suggests that the TNT-mediated anti-allodynic effect is transient and does not produce antinociceptive tolerance following repeated administration. We also examined the effect of TNT on SNI-induced cold allodynia and found it to be ineffective. The precise reason for this difference in TNT effect between mechanical and cold allodynia is unknown. It is likely due to different neural mechanisms involved in mechanical and cold allodynia. For instance, activation of TRPM8 but not TRPA1, is required to mediate behavioral and neuronal responses to noxious cold [40]. Further studies examining the antinociceptive effect of TNT in TRPM8 knockout animals would allow us to better understand the specificity of TNT in reducing mechanical allodynia but not cold allodynia.
Antidepressants are widely used in painful disorders. However, the analgesic mechanisms of antidepressants are unclear. Several studies suggest that clinically used tricyclic antidepressants and selective serotonin reuptake inhibitors produce analgesia by inhibiting reuptake of monoamines and serotonin (5-hydroxytryptamine, 5-HT). However, TNT neurochemical properties and antidepressant action differs from classical tricyclic and non-tricyclic antidepressants [41,42]. A previous report shows that intravenous administration of TNT reduces visceral pain through 5HT and 5-HT3 receptor signaling [43]. Kim et al have shown that antagonists of 5-HT, and α-1 and α-2 adrenoreceptor antagonists, partially attenuate TNT-induced antinociceptive behaviors in an inflammatory pain model [44]. Another report showed that spinal 5-HT7 receptors of the GABAergic interneurons play a role in the antinociceptive activity of TNT [45]. Furthermore, one study showed involvement of both peripheral and central opioid receptors in TNT produced antinociceptive activity [46]. These reports clearly indicate involvement of multiple neurotransmitters and neuroreceptors in the antinociceptive mechanisms of TNT.
Previous studies have implicated VEGF as a potential target for the action of antidepressants. For example, VEGF expression in the PFC and hippocampus is reduced in animals exposed to chronic stress and in cerebrospinal fluid of patients who have attempted suicide [28,47-49]. Chronic but not acute treatment with antidepressants reduced stress-induced depression by increasing VEGF expression [28,47,48]. However, in animal models of pain, VEGF protein expression is increased (please see introduction section). These reports suggest that alteration in VEGF protein expression depends on the pathological state being examined. Our data showed increased VEGF protein expression in the contralateral side of the PFC to peripheral injury. Five days of treatment with TNT reduced SNI-induced changes in VEGF expression to control levels. It would be informative to determine if reducing VEGF levels by other means, such as anti-VEGF antibodies or siRNA, will replicate the TNT effect. If such approaches reduced PFC VEGF levels in SNI rats but did not replicate the anti-allodynic effect of TNT, it would suggest that TNT reduces allodynia through other signaling pathways.
The present study provides evidence that SNI alters PFC VEGF levels, and TNT reduces SNI- induced mechanical allodynia but not cold allodynia. Repeated administration of TNT does not produce antinociceptive tolerance, and TNT treatments regulate SNI-induced changes in PFC VEGF protein expression levels. In conclusion, the VEGF signaling system in the cortex could be a novel target for treating neuropathic pain with antidepressant drugs like TNT.
Declaration
Ethics approval and consent to participate
All procedures performed on rats were approved by the US Army Institute of Surgical Research (USAISR) Institutional Animal Care and Use Committee (IACUC). The facility where this research was conducted is fully accredited by the AAALAC. This study was conducted in compliance with the Animal Welfare Act, by implementing Animal Welfare Regulations and the Principles of the Guide for the Care and Use of Laboratory Animals, National Research Council.
Consent for publication
Not applicable.
Availability of data and materials
All data are included in the manuscript. However, the raw data used and/ or analyzed in the present study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests. Additionally, the opinions or assertions contained herein are those of the author(s) and do not reflect are not to the official policy or position of the U.S. Army Medical Department, Department of the Army, DoD, or the U.S. Government.
Authors’ Contributions
B.C. conceived the project supervised all experiments, performed data analysis, and wrote manuscript draft. A.T. and B.C. performed in vitro and in vivo experiments. C.D., J.C., K.W., and T.S. proofread the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to acknowledge Roger J Chavez and Pain & Sensory Trauma Care Combat Research Team for assistance in animal work.
References
- Carter GT, Galer BS (2001) Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am 2001, 12: 447-459.
- Kerstman E, Ahn S, Battu S, Tariq S, Grabois M (2013) Neuropathic pain. Handb Clin Neurol 110:175-87.
- Liedgens H, Obradovic M, De Courcy J, Holbrook T, Jakubanis R (2016) A burden of illness study for neuropathic pain in Europe. Clinicoecon Outcomes Res 8:113-26.
- Caruso R, Ostuzzi G, Turrini G, Ballette F, Recla E, et al. (2019) Beyond pain: can antidepressants improve depressive symptoms and quality of life in patients with neuropathic pain? A systematic review and meta- analysis. Pain 160: 2186-98.
- Hiyama A, Watanabe M, Katoh H, Sato M, Sakai D, et al. (2015) Evaluation of quality of life and neuropathic pain in patients with low back pain using the Japanese Orthopedic Association Back Pain Evaluation Questionnaire. Eur Spine J 24: 503-12.
- Leung L, Cahill CM (2010) TNF-alpha and neuropathic pain--a review. J Neuroinflammation 7:27.
- Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, et al. (2017) Neuropathic pain. Nat Rev Dis Primers 3: 17002.
- Kremer M, Salvat E, Muller A, Yalcin I, Barrot M (2016) Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience 338: 183-206.
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, et al. (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14: 162-73.
- Attal N (2019) Pharmacological treatments of neuropathic pain: The latest recommendations. Rev Neurol (Paris) 175: 46-50.
- Lee HG, Choi JI, Yoon MH, Obata H, Saito S, et al. (2014)The antiallodynic effect of intrathecal tianeptine is exerted by increased serotonin and norepinephrine in the spinal dorsal horn. Neurosci Lett  583: 103-07.
- Han SM, Kim YH, Jo HU, Kwak JA, Park HJ (2017) Tianeptine Reduces Mechanical Allodynia in Spinal Nerve-ligated and Chemotherapy-induced Neuropathic Mice. Pain Physician 20: E593-E600.
- Heo BH, Shin JY, Park KS, Lee HG, Choi JI, et al. (2016) Effects of tianeptine on the development and maintenance of mechanical allodynia in a rat model of neuropathic pain. Neurosci Lett 633: 82-6.
- Samuels BA, Nautiyal KM, Kruegel AC, Levinstein MR, Magalong VM, et al. (2017) The Behavioral Effects of the Antidepressant Tianeptine Require the Mu-Opioid Receptor. Neuropsychopharmacology 42: 2052-63.
- Cavalla D, Chianelli F, Korsak A, Hosford PS, Gourine AV, et al. (2015) Tianeptine prevents respiratory depression without affecting analgesic effect of opiates in conscious rats. Eur J Pharmacol  761: 268-72.
- Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, et al. (2016) Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J Bone Miner Res 31: 911-24.
- Hulse RP (2017) Role of VEGF-A in chronic pain. Oncotarget  8: 10775-76.
- Lee GW, Son JY, Lee AR, Ju JS, Bae YC, et al. (2019) Central VEGF-A pathway plays a key role in the development of trigeminal neuropathic pain in rats. Mol Pain 15: 1744806919872602.
- Di Cesare Mannelli L, Tenci B, Micheli L, Vona A, Corti F, et al. (2018) Adipose-derived stem cells decrease pain in a rat model of oxaliplatin-induced neuropathy: Role of VEGF-A modulation. Neuropharmacology 131: 166-75.
- Jere M, Cassidy RM (2018) VEGF-A165 b to the rescue: vascular integrity and pain sensitization. J Physiol 596: 5077-78.
- Tooke K, Girard B, Vizzard MA (2019) Â Functional effects of blocking VEGF/VEGFR2 signaling in the rat urinary bladder in acute and chronic CYP-induced cystitis. Am J Physiol Renal Physiol 317: Â F43-F51.
- Lin J, Li G, Den X, Xu C, Liu S, et al. (2010)VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X(2)(/)(3) receptor of primary sensory neurons. Brain Res Bull 83: 284-91.
- Jeric M, Vukojevic K, Vuica A, Filipovic N (2017) Diabetes mellitus influences the expression of NPY and VEGF in neurons of rat trigeminal ganglion. Neuropeptides 62: 57- 64.
- Nesic O, Sundberg LM, Herrera JJ, Mokkapati VU, Lee J, (2010) Vascular endothelial growth factor and spinal cord injury pain. J Neurotrauma 27: 1793-803.
- Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, et al. (2008) Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F826-F836.
- Kiuchi H, Tsujimura A, Takao T, Yamamoto K, Nakayama J, et al. (2009) Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: its association with pain severity and glomerulations. BJU Int 104: 826-831.
- Warner-Schmidt JL, Duman RS (2007) VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A 104: 4647- 4652.
- Deyama S, Bang E, Wohleb ES, Li XY, Kato T, et al. (2019) Role of Neuronal VEGF Signaling in the Prefrontal Cortex in the Rapid Antidepressant Effects of Ketamine. Am J Psychiatry 176: 388-400.
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, et al. (2004) Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24: 10410-10415.
- Wu XB, Liang B, Gao YJ (2016) The increase of intrinsic excitability of layer V pyramidal cells in the prelimbic medial prefrontal cortex of adult mice after peripheral inflammation. Neurosci Lett 61: 40-45.
- Cardoso-Cruz H, Lima D, Galhardo V (2013) Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J Neurosci  33: 2465-2480.
- Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149-158.
- Sosanya NM, Trevino AV, Chavez RL, Christy RJ, Cheppudira BP (2017) Sound-stress- induced altered nociceptive behaviors are associated with increased spinal CRFR2 gene expression in a rat model of burn injury. J Pain Res 10: 2135-2145.
- Werner MF, Kassuya CA, Ferreira J, Zampronio AR, Calixto JB, et al. (2007) Peripheral kinin B(1) and B(2) receptor-operated mechanisms are implicated in neuropathic nociception induced by spinal nerve ligation in rats. Neuropharmacology 53: 48-57.
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM (1994) Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59: 369-376.
- Schulte-Herbruggen O, Chourbaji S, Ridder S, Brandwein C, Gass P, Hortnagl H, et al. (2006) Stress-resistant mice overexpressing glucocorticoid receptors display enhanced BDNF in the amygdala and hippocampus with unchanged NGF and serotonergic function. Psychoneuroendocrinology 31:1266-1277.
- Sosanya NM, Garza TH, Stacey W, Crimmins SL, Christy RJ, et al. (2019) Involvement of brain-derived neurotrophic factor (BDNF) in chronic intermittent stress-induced enhanced mechanical allodynia in a rat model of burn pain. BMC Neurosci  20: 17.
- Hall FM (1984) Post-fracture pubic osteolysis simulating malignancy. J Bone Joint Surg Am 66: 975.
- Taylor BK, Abhyankar SS, Vo NT, Kriedt CL, Churi SB, et al. (2007) Neuropeptide Y acts at Y1 receptors in the rostral ventral medulla to inhibit neuropathic pain. Pain 131: 83-95.
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD (2010) TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150: 340-350.
- Uzbekov MG (2009) Antidepressant action of tianeptine is connected with acceleration of serotonin turnover in the synapse: a hypothesis. Neuropsychopharmacol Hung 11: 83-87.
- Wagstaff AJ, Ormrod D, Spencer CM (2001) Tianeptine: a review of its use in depressive disorders. CNS Drugs 15: 231-259.
- Bilge SS, Bozkurt A, Ilkaya F, Ciftcioglu E, Kesim Y, et al. (2012)The antinociceptive effects of intravenous tianeptine in colorectal distension-induced visceral pain in rats: the role of 5-HT(3) receptors. Eur J Pharmacol 681: 44-49.
- Kim WM, Lee SH, Jeong HJ, Lee HG, Choi JI, (2012) The analgesic activity of intrathecal tianeptine, an atypical antidepressant, in a rat model of inflammatory pain. Anesth Analg 114:683-689.
- Lin H, Heo BH, Kim WM, Kim YC, Yoon MH (2015) Antiallodynic effect of tianeptine via modulation of the 5-HT7 receptor of GABAergic interneurons in the spinal cord of neuropathic rats. Neurosci Lett 598: 91-95.
- Bilge SS, Ilkaya F, Darakci O, Ciftcioglu E, Bozkurt A (2018) Opioid Receptors Contribute to Antinociceptive Effect of Tianeptine on Colorectal Distension-Induced Visceral Pain in Rats. Pharmacology 101: 96-103.
- Warner-Schmidt JL, Duman RS (2008) VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol 8:14-19.
- Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116-1127.
- Isung J, Mobarrez F, Nordstrom P, Asberg M, Jokinen J (2012) Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J Biol Psychiatry 13: 468-473.
Citation: Cheppudira BP, Trevino MAV, Castillo SA, Daniels CC, Clifford JL, et al (2021) Effect of Tianeptine on Spared Nerve Injury-Induced Allodynia and Prefrontal Cortex Vascular Endothelial Growth Factor. J Pain Relief 10: 408.
Copyright: © 2021 Cheppudira BP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2428
- [From(publication date): 0-2021 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 1756
- PDF downloads: 672