Effectiveness of Percutaneous Electrical Neurostimulation (PENS) for post-operative pain in Cesarean Section (C-section) patients using DyAnsys Primary Relief: A Case-Control Study
Received: 02-Jul-2022 / Manuscript No. JPAR-22-68597 / Editor assigned: 04-Jul-2022 / PreQC No. JPAR-22-68597 / Reviewed: 18-Jul-2022 / QC No. JPAR-22-68597 / Revised: 23-Jul-2022 / Manuscript No. JPAR-22-68597 / Published Date: 30-Jul-2022 DOI: 10.4172/2167-0846.1000448 QI No. / JPAR-22-68597
Abstract
Post-operative pain after Cesarean sections (C-sections) are frequently ignored and was found to affect the daily routine and quality of life and may even contribute to persistent post-operative pain in the mother. This study aims to find the effectiveness of the analgesic effect of the PENS device, DyAnsys Primary Relief, for post-operative pain management after C-Section.This interventional case-control study was conducted in Kalyani Hospital, Warangal,India, on 52 participants. After obtaining the IRB approval, the consent, and after considering the inclusion and exclusion criteria, the participants were randomized into case and control groups. The case group received the PENS device, DyAnsys Primary Relief, whereas the control group received a dummy device. The Numeric Rating Scale (NRS) was used to record the pain for 72 hours. The six variables, sleep, general activity, interpersonal relationship (IPR), anxiety, walking ability, and enjoyment of life, were a part of the Brief Pain Inventory (BPI) metric and were measured on a 0-10 scale in the BPI form. This minimally invasive nerve stimulation intervention using the PENS device was safe and effective in reducing pain and accelerating the mobilization of patients after a C-section.The PENS device could effectively reduce post-operative pain after a C-section without any pharmacological intervention. Considering personnel and time expenditures, this device can be recommended as routine for pain control in patients after an elective C-section.
Keywords: Cesarean Section; C-section; Dyansys; Pain management; PENS device; Primary Relief; Post operative pain
Keywords
Cesarean Section; C-section; Dyansys; Pain management; PENS device; Primary Relief; Post operative pain
Introduction
Cesarean Section (C-section) is one of the most common surgical procedures. An estimated one million women in the United States give birth by cesarean section [1]. The C-section rate increased from 5% in 1970 to 31.9%in 2016 [2]. Acute post-operative pain management after C-section remains a considerable clinical challenge. If not appropriately managed, the post-operative pain may result in persistent pain, delay in the functional recovery of the mother, more extended hospital stays, etc.
Nearly 20% of the women who underwent C-section experience acute post-operative pain of high severity, which would delay the functional recovery and increase post-partum depression, thus negatively impacting the mother’s ability to take care of her infant and breastfeeding [3]. Early maternal mobilization is recommended after C-section to reduce thromboembolic complications [4]. Posttraumatic stress disorder was also high in the case of women who underwent C-section surgery [5].
Post-c-section pain can be managed with oral, intravenous, or rectal analgesia, regional analgesia, transverse abdominis plane block, wound infiltration, or combinations of various interventions, and the adverse events due to these interventions include nausea, vomiting, sedation, constipation, diarrhea, drowsiness, sleepiness, or psychological impacts [6]. Therefore, non-pharmacological pain management after C-section holds much importance.
Minimally invasive percutaneous nerve stimulation (PENS) is a newer modality for post-operative pain management. The selective stimulation of the thick myelinated nerve fiber of the auricular branch of the vagus nerve helps achieve a selective modulation of afferent Aβ fibers that projects to the nucleus of the solitary tract of the brain stem. The study aims to assess the effectiveness of the PENS device, DyAnsys Primary Relief, for post-operative pain management after a C-section.
Methodology
This prospective, interventional, case-controlled clinical trial was conducted in Kalyani hospital, Warangal, India. After obtaining the approval from the Institutional Review Board, consent was obtained from the subjects for the participation. Those who met the inclusion criteria were recruited for the study. The study recruited 52 participants, out of which 30 were randomly selected using Research Randomizer. The case group received a test device called DyAnsys Primary Relief (FDA approved January 2022: K213188) to receive the auricular neurostimulation as the primary mode of analgesia postoperatively. The device was positioned on the auricular part of the ear to receive the stimulation [7]. The control group received a placebo (dummy device with no electrical stimulation) placed topically onto the backside of the ear. Among the 30 subjects, five participants refused to give consent for the study participation, and three had violated the protocol. Hence the study population in the interventional group dropped to 22. The duration of the study was 72 hours post-C-section. The study was registered in https://www.clinicaltrials.gov/- NCT03829774.
Inclusion Criteria:
● Age between 22 – 35 years
●Underwent C-section surgery
●History of maternal complication with surgery. Page 2 of 6
●Having pain one-hour post-C-Section surgery,
●Conscious and oriented after the surgery for device installation after the anesthetic effect
●Patients who completed required clinical and biochemical investigations as deemed necessary by the gynecologist after post C – section surgery.
●No previous poor obstetrical outcome
●No experience in Han’s Acupoint nerve stimulator and TENS for other reasons.
●Term pregnancy (> 37 weeks of gestation).
●Ready to give consent for participation in the study and can comply with study procedures.
●Patients with normal cognitive and communicative ability as judged by clinical assessment and ability to complete self-reported questionnaires.
Exclusion Criteria:
●Presence of maternal mental, neurological disease, affecting the evaluation of pains and disease condition, preoperatively
●Presence of gestational hypertension, gestational diabetes, gestational thyroid disease.
●History of intake of any analgesic drugs before C-Section surgery
●Use of diazepam, piperazine hydrochloride, or other sedative or analgesic drugs during the process of labor.
●Pre-pregnancy overweight or low pregnancy weight, Body mass index (< 18.5 or >25 kg/m2).
●Patients refused who refuse to consent to receive painless labor
●Neonatal issues require immediate separation from the mother for medical or NICU care.
●Severe placental abruption.
●Hydrops (fluid accumulation or edema in fetus body tissue and cavities) secondary to anemia or heart failure.
●Known twin to twin transfusion syndrome (TTS).
●Congenital anomalies hampering the procedure (gastroschisis, omphalocele, spina bifida).
●Homebirth.
●Hearing impairment.
●Legal abortion
●Twin pregnancy
●Instrumental birth
●Uterine anomalies with contraindication for a vaginal birth, e.g., previous opening of the uterine cavity, myomectomy, congenital abnormalities.
●Placental anomalies including placenta praevia, suspected acreta, increta, percreta, especially after the previous cesarean.
●Fetal abnormalities, growth restriction.
●Subjects on any investigational drug(s) or therapeutic device(s) within 30 days preceding screening; or subject or physician anticipate the use of any of these therapies by the subject during the study
●Previous participation in the Treatment Phase of the present Protocol
●Any malignant conditions not in remission for five years or more that has been medically or surgically treated without evidence of metastases
●Presence of one or more medical conditions, as determined by medical history, which seriously compromises the subject’s ability to complete the study, including a history of poor adherence with medical treatment, unstable pain intensity or pain medications six weeks before the study, renal, hepatic, hematologic, active autoimmune or immune diseases that, in the opinion of the investigator, would make the subject an inappropriate candidate for this study
●One or more abnormal blood biochemistry analyte result that is ≥ 3 times that of the upper limit of the normal range
●Known history of having Acquired Immunodeficiency Syndrome (AIDS) or with a history known to be infected with Human Immunodeficiency Virus (HIV)
●American Heart Association (AHA) Class III and IV congestive heart failure (CHF), as defined by the following criteria: a) Class III: Symptoms with moderate exertion b) Class IV: Symptoms at rest or c) Cardiac pacemakers.
●History of psychiatric disorders including but not limited to major depressive disorder, bipolar disorder, obsessive-compulsive disorder, generalized anxiety, dysthymia, or suicidality/suicide ideation
●Subjects not willing to take any treatment before discharge from the hospital.
The patients were maintained on the same medications throughout the study period, as medically feasible, with no introduction of new therapies. Standard therapy for C-Section patients was allowed, except for treatments noted in the exclusion criteria.
The auricular PENS device, DyAnsys Primary Relief, is a patented and FDA-approved neurostimulation product that provides analgesia by conducting cranial electrostimulation through the passage of tiny electric currents into the brain through the auricular cranial nerves [8]. It was placed by the investigator during the post-operative period about an hour after transfer to the post-anesthesia care unit, into the pinna of the study participants, into the locations recommended by the manufacturer. The device was set up to emit biphasic signals at frequencies that were swept from 1.14 to 2.28 to 4.56 to 9.12 and then 100 Hz and then back down again. The patients were informed that they could demand analgesia if they had any pain. The Numeric Rating Scale (NRS) for pain was recorded hourly for the next 72 hours. Any bleeding or rashes at the placement site or any other adverse side effects from the instrument were noted.
The variables sleep, general activity and normal work, interpersonal relationship (IPR), anxiety, walking ability, and enjoyment of life were assessed using a scale of 11 points. These six parameters were a part of the Brief Pain Inventory (BPI) metric and were measured on a 0-10 scale in the BPI form, which has been widely utilized in multiple studies [9,10]. This helped to compare the same set of metrics to gain insights and provide the changes between the two groups observed during the study period of 72 hours. For sleep, 0 signifies best sleep quality and 10 worst sleep quality. For the variable general activity, zero signifies the inability to work without support, and ten signifies the ability to work independently. For IPR, 0 signifies negative feelings toward others, and 10 signifies positive feelings toward others. For anxiety (called mood), 0 signifies extreme anxiety and pessimism, and 10 signifies lack of anxiety and a positive mood. A score of 3 or below would indicate pessimism, drastic mood swings, self-dissatisfaction, or a general sense of helplessness. For Walking ability, 0 signifies the inability to walk without support, and 10 signifies the ability to walk independently. Enjoyment of Life was assessed starting with 0 (signifying negative emotions) and ending with 10 (signifying strong positive emotions).
Statistical Analysis
The analysis summarized the baseline response data using mean and standard deviation. The longitudinal average values and the corresponding 95% were plotted using the scatter diagram and error bar joined with vertical lines. The effect of the treatment was evaluated using the linear mixed model, and the results were summarized as the difference in the least-square means obtained using the model. We model the individual responses separately, using treatment, time, and interaction as independent variables. The p-value and confidence limits were adjusted for multiple testing using the Tukey-Kramer method. The longitudinal time points considered for analysis varied according to the nature of the measurements. All hypotheses were tested for 5% two-sided significance unless specified. A P-value (adjusted P-value wherever applicable) less than 0.05 was considered as sufficient evidence to reject the null hypothesis under test.
Results
A total of 44 patients were randomized to either the intervention group (n=22) or the control group (n=22) for the study. Out of the total 52 recruited, eight patients dropped out by refusal of consent (5) and violation of protocol(3). The mean(SD) age for the study group and the control group were 24.7(2.6) and 26.6(3.9) years, respectively. The numerical rating scale assessed the pain score at 23 intervals spanning 72 hours.
Effect of treatment on pain score
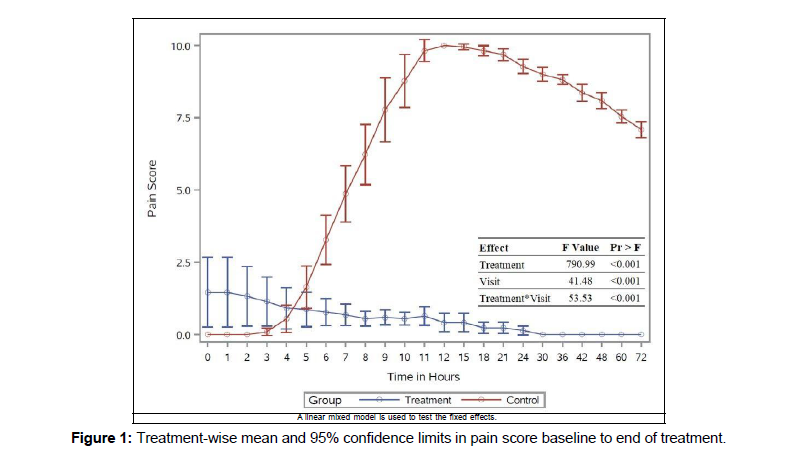
The pain score of the patients in the control group reported zero during the initial two hours, and in the study group, it was on one day of follow-up in Figure 1.
The control group showed an increase in mean score from 4-6 hours and the peak on 11–18 hours. There was a reduction in the score after 18 hours. In the intervention group, there was a gradual decrease in mean score from the beginning, and no pain was recorded after one day. There was a highly significant impact on the treatment, time, and interaction.
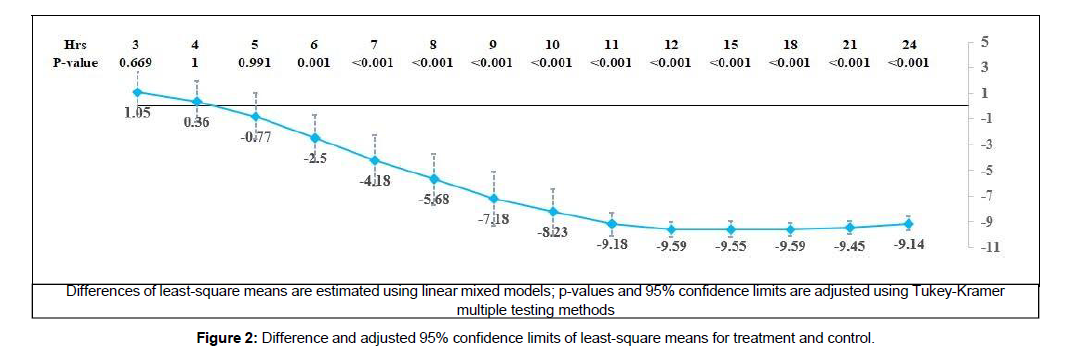
In Figure 2,
the treatment group showed an early significant 2.5- point reduction in least-square means from six hours. The difference in least-square means showed a rapid increase and a very high difference of more than 9 points from 11 to 24 hours. All these differences from six to 24 hours showed the effectiveness of the treatment, with a highly significant p-value of < 0.001.
Effect of treatment in general activity
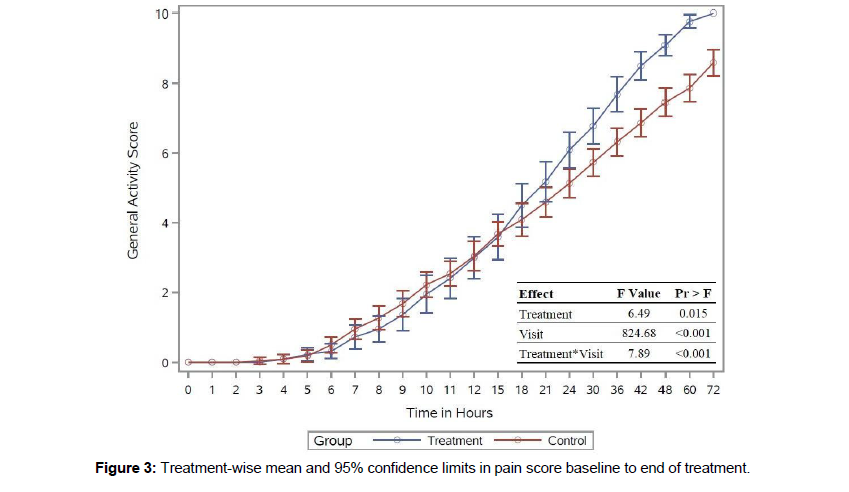
The control and intervention groups reported zero general activity scores during the initial two hours after C-section. Both groups showed increased mean activity scores from four hours in Figure 3.
The results significantly affected the treatment, time, and interaction. The average least-square means were slightly low in the intervention group at the beginning and increased after 18 hours. The average least-square means were slightly low in the intervention group at the beginning and started to show increased activity after 18 hours. There was no significant effect of treatment until 36 hours. The analysis showed an increase in the activity score in the intervention group from 18 hours to the end of the third day compared to the control group.
Effect of treatment on mood
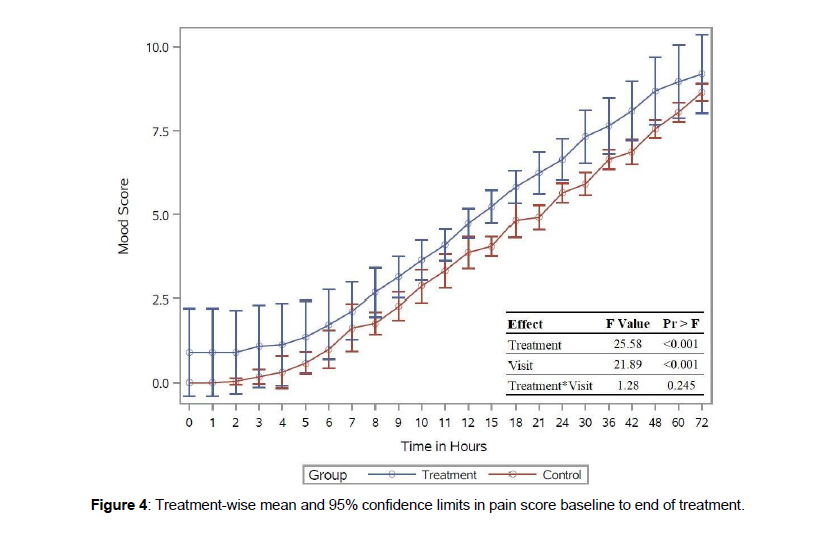
The control group reported a zero mood score during the initial two hours of measurements in Figure 4.
The average mood score in the intervention group was consistently higher from the beginning of the study. The difference in least-square mean mood score was one point higher in the intervention group than in the control group (p-value 0.03). There was evidence to prove the effect on treatment and time, but no evidence for the interaction.
Effect of treatment on walking ability
The results showed some parturients among the intervention group started having walking ability scores from seven hours, and a change in the score in both groups was noticed from 11 hours. There was a sharp increase in scores in both groups from 12 hours onwards. The test for fixed effects showed treatment, time, and interaction effects (p-value <0.001). The intervention group showed more than 1.5 points increase in the least-square means walking ability score from the end of the first day compared to the control group (p-value < 0.05).
Effect of treatment on relationship with other people
Parturients in the control group did not show any relationship with other people's scores for the first four hours. The intervention and the control groups showed a sharp increase in average relationship with other people scores from five hours onwards. The test for fixed effects showed evidence for treatment, time, and interaction effects (p-value <0.001). There was an increase in the least-square means among the intervention group from 30 hours onwards (p-value <0.002).
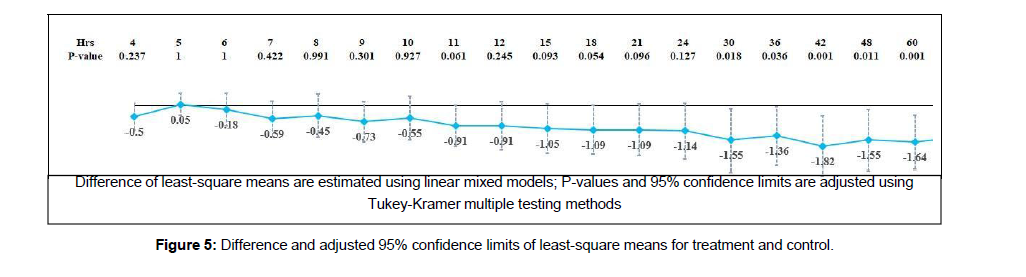
Effect of treatment on sleep
The control and intervention groups reported a ten sleep score during the initial hours. The control group showed a ten sleep score for up to three hours in Figure 5.
There was a gradual reduction in the score from the fourth hour onwards. The study showed evidence for treatment, time, and interaction effects. Analysis of the difference in the least-square means indicated an improved sleep score from 30- hour onwards, which means an improved score from 30-hour onwards (p-value <0.001).
The test showed an increase in the enjoying life score in both the groups from five-hour onwards. There was evidence for treatment, time, and interaction effects (p-value <0.001). Figure 5 showed evidence for an increase in the least-square means of enjoying life score from 30 hours onwards (p-value <0.02). The improvement in least-square mean values showed a more than a one-point difference in the score. Effect of treatment in normal work
There was a steady increase in normal work scores in both the groups from five-hour onwards. The average normal work score showed slightly higher growth in the intervention group than in the control group. The test shows treatment, time, and interaction effects. There was evidence for an increase in the normal work score in the intervention group after 42 hours (p-value <0.004).
Discussion
The study showed that the parturients in the intervention group reported a pain score of 0 after one day of follow-up compared to the control group. The control group reported 0 pain scores during the initial two hours of measurement. All the measurements were taken from three hours to twenty-four hours only. The pain score increased from four to six hours in the control group, and the peak pain score was reported at the peak of 11 to 18 hours which gradually declined. However, in the interventional group, the average pain score gradually decreased. No pain was reported after 24 hours. A similar study in another center using a PENS device in 2019 also showed similar results. Chakravarthy et al. used a PENS device that emits signals at 1 Hz and found that the analgesic effect was reduced by 36%. However, in this study, the frequencies swept from 1.14 to 2.28 to 4.56 to 9.12, then 100 Hz, and then back down again, and no parturients used analgesics. The auricular stimulation points used in both studies were the same. The least-square mean difference from 6 to 24 hours while comparing both the groups showed evidence for the effect of the intervention, which was highly statistically significant.
The study showed a significant improvement in the general activity score, mood, walking ability, interrelationship, or enjoyment in life in the interventional group compared to the control group. Excessive use of analgesics is not recommended after C-section due to increased risk of post-partum hemorrhage, gastrointestinal disturbances, and liver or renal impairment [11]. Moreover, the traces of the drug may be transferred to breast milk, and an association between pain and post-partum depression has already been reported [12]. The pain could interfere with the daily activities, delay the recovery, affect the mother-child bonding, and was also found to decrease the quality of sleep [13,14,15]. The sleep disturbances after C-section was found to associate with anxiety, wound pain, and breastfeeding. Persistent pain is more common after C-section than pain from the surgical site and other sources like musculoskeletal. Post-operative pain increases disability, anxiety, and depression [16,17]. Proper post-operative pain management could improve sleep and daily activity and reduce anxiety in mothers. The PENS device could reduce the post-operative pain significantly, and effective pain management would have improved the general activity score, mood, walking ability, and enjoyment of life in the interventional group.
This newer pain management method improved the quality of life of the intervention group, reducing their pain with no pain medications. The gate-control theory may account for the analgesic effect of the PENS device [18]. The low-frequency stimulation from the PENS device could activate the μ and δ opioid receptors via the release of enkephalin, β-endorphin, and endomorphin, and the highfrequency stimulation activates the κ-opioid receptors in the spinal cord, releasing the dynorphin(18). Chakravarthy et al. had cited that a naloxone opioid-receptor agonist could antagonize this analgesic effect(7). No adverse events were reported throughout the study. This is a single-center study, and a multi-center trial including a larger sample size would have provided a better picture.
Conclusion:
The auricular PENS device is an effective analgesic adjuvant that could reduce pain following the C-section with no pain medications. The pain score of the intervention group reached zero by the end of 24 hours. The least-square means analysis of the data indicates that the intervention treatment outperforms the standard treatment within the first six hours of the follow-up. A very high least-square means difference post six hours suggests that the intervention treatment is more effective in reducing the pain score than the standard treatment used in the control group.
Acknowledgement
We acknowledge the contributions from Mr. M. Robin for data collection, Dr. Abin Thomas and Mrs. Anithadevi T. S. for the statistical analysis, and Ms. Sulfia Jabbar P for the medical content writing
Conflict of Interest
None
References
- Berghella V, Baxter JK, Chauhan SP (2005) Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol EU 193:1607-1617.
- Grobman W (2019) ACOG Practice Bulletin No. 205: Vaginal Birth After Cesarean Delivery. Obstet Gynecol US 133:110-127.
- Karlstrom A, Engstrom OR, Norbergh KG, Sjoling M, Hildingsson I (2007) Postoperative pain after cesarean birth affects breastfeeding and infant care.JOGNN US 36:430-440.
- James AH, Jamison MG, Brancazio LR, Myers ER (2006) Venous thrombo embolism during pregnancy and the postpartum period: Incidence, risk factors, and mortality. Am J Obstet Gynecol EU194:1311-1315.
- Chen Y, Yang X, Guo C, Liao Y, Guo L, et al.(2020) Prevalence of Post-Traumatic Stress Disorder Following Caesarean Section: A Systematic Review and Meta-Analysis. J Womens Health US 29:200-209.
- Mkontwana N, Novikova N (2015) Oral analgesia for relieving post-caesarean pain. Cochrane database Syst Rev US 2015: 1-47.
- Chakravarthy M, Prashanth A, George A (2019) Evaluation of Percutaneous Electrical Nerve Stimulation of the Auricle for Relief of Postoperative Pain Following Cesarean Section. Med Acupunct.31: 281-288.
- Juncker RB, Gagnier JJ, Mirza FM (2019) Neurostimulation as an Efficacious Nonpharmacologic Analgesic following Arthroscopic Rotator Cuff Repair. Case Rep Anesthesiol 2022:1-5.
- Edgley C, Hogg M, De SA, Braat S, Bucknill A, et al.(2019) Severe acute pain and persistent post-surgical pain in orthopaedic trauma patients: a cohort study. Br J Anaesth US 123: 350-359.
- Hetmann F, Schou BI, Sandvik L, Kongsgaard UE (2022) Does chronic pre-operative pain predict severe post-operative pain after thoracotomy? A prospective longitudinal study. Acta Anaesthesiol Scand US 57:1065-1072.
- Sutton CD, Carvalho B (2021) Optimal Pain Management After Cesarean DeliveryAnesthesiol Clin EU 35: 107-124.
- Daly B, Young S, Marla R, Riddell L, Junkin R, et al.(2017) Persistent pain after caesarean section and its association with maternal anxiety and socioeconomic background. Int J Obstet Anesth EU 29: 57-63.
- Jasim HH, Sulaiman SABS, Khan AH, Rajah UAS (2017) Factors Affecting Post Caesarean Pain Intensity among Women in the Northern Peninsular of Malaysia. J Clin Diagn Res IND 11: 7-11.
- De Sousa L, Pitangui ACR, Gomes FA, Nakano AMS, Ferreira CHJ (2009) Measurement and characteristics of post-cesarean section pain and the relationship to limitation of physical activities. Acta Paul Enferm EU 22: 741-747.
- Aziz Ismail NIA, Elgzar WTI (2018) The Effect of Progressive Muscle Relaxation on Post Cesarean Section Pain, Quality of Sleep and Physical Activities Limitation. Int J Stud Nurs EU 3: 1-14.
- Baird A, Sheffield D (2016) The Relationship between Pain Beliefs and Physical and Mental Health Outcome Measures in Chronic Low Back Pain: Direct and Indirect Effects. Healthc 4: 1-58.
- Melzack R, Wall PD (2022) Pain mechanisms: a new theory. Science 150 (3699): 971-979.
- Han JS (2003) Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci EU 26: 17-22.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Guru Nath S (2022) Effectiveness of Percutaneous Electrical Neurostimulation (Pens) for Post-Operative Pain in Cesarean Section (C-section) Patients Using Dyansys Primary Relief: A Case-Control Study. J Pain Relief 11: 448. DOI: 10.4172/2167-0846.1000448
Copyright: © 2022 Guru Nath S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3316
- [From(publication date): 0-2022 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 2832
- PDF downloads: 484