Effects of Resistance Training Accompanied by Sustanon Abuse on Hepatotoxicity in Rats with and without Training Experience
Received: 03-Aug-2017 / Accepted Date: 21-Aug-2017 / Published Date: 31-Aug-2017 DOI: 10.4172/2473-6449.1000126
Abstract
Objectives: Abuse of anabolic androgenic steroids (AAS) has increased. Previous studies have shown that resistance training (RT) is the main exercise modality practiced by AAS abusers and people often use them in different training situations. Thus propose of this study was to evaluate the hepatotoxic effects of sustanon (Su) as an example of anabolic androgenic steroids in male rats with and without RT experience.
Methods: Rats were divided into seven groups: control; Su - untreated sedentary rats (non-Su/ Sed); Su - treated sedentary rats (Su/Sed); Su - untreated trained rats (non-Su/Tr); Su - treated trained rats (Su/Tr); Su - untreated experience trained rats (non-Su/XTr); Su - treated experience trained rats (Su/XTr). Su - treated groups received sustanon 10 mg/kg intramuscularly once a week for 8 weeks. In the Tr and XTr groups, the animals climbed a 1.1 m vertical ladder, 3 days per week for 8 and 12 week, respectively.
Results: After Su - treatment, the mean values of serum parameters related to hepatic function were within normal ranges. Superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GR) activities were higher (P<0.05) in the liver of Su treatment groups. Tr and XTr did not change any of the above parameters.
Conclusion: The present findings suggest that the injection of Su during 8 weeks, either with or without RT up-regulation of enzymatic antioxidant activities. Moreover, these data demonstrated that liver function tests do not always reflect liver abnormalities particularly at the initial stages.
Keywords: Anabolic androgenic steroids; Antioxidant; Liver
27869Introduction
The prevalence of anabolic androgenic steroids (AAS) use by athletes, bodybuilders, and youths in order to increase muscle mass or enhance physical endurance has risen dramatically over the last two decades [1]. AAS are a group of synthetic compounds similar in chemical structure to the natural testosterone used in medical clinics for treatment of hypogonadism, impotence, delayed puberty, muscle wasting, etc. [2]. In addition to their therapeutic uses, nontherapeutic abuse also occurs [3].Chronic and unregulated use of AAS may lead to serious side effects such as testicular dysfunction, gynecomastia, hepatotoxicity, and psychological disorders [2,3]. Reactive oxygen species (ROS) may also be classified as free radicals such as superoxide anion (O2-), hydroxyl radical (OH), and non-radical species like hydrogen peroxide (H2O2), singlet oxygen (1O2), and so forth [4]. These are produced by living organisms as a result of normal cellular metabolism and are necessary for certain normal biological processes, but, at high concentrations, they can damage cell structures such as carbohydrates, DNA, lipids, and proteins and alter their functions [5]. Cumulatively, this is known as oxidative stress. A variety of pathological conditions are induced by oxidative stress such as cancer, neurological disorders, atherosclerosis, hypertension, ischemia/perfusion, diabetes, acute respiratory distress syndrome, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, and asthma [4].
Living organisms have evolved highly complex antioxidant systems, which include enzymatic and non-enzymatic antioxidants that are usually effective in blocking harmful effects of ROS [5,6]. Available data have shown that AAS abusing is accompanied by increased of oxidative stress condition in the liver, brain, and heart [7-10].
Exercise training is a natural way to improve health and decrease the incidence of a number of diseases. During physical exercise, depending on the intensity and duration, there is an increased generation of ROS and the activity of antioxidant/repair systems [5,6], thus altering the cellular redox milieu. Hence, exercise training can enhance the antioxidant capacity and lessens oxidative stress [9,10]. In addition, available data have shown that regular exercise can reduce drugs toxic effects. Camiletti-Moirón et al. reported that high intensity exercise training can reduce the AAS-mediated negative effect on brain redox [8]. Mallikarjuna et al. and, Lew and Quintanilha demonstrate that regular exercise can modify the hepatotoxic effects of the abuse of ethanol and acetaminophen in rats [11,12]. Thus, the role of exercise in reducing drugs side effects is noticeable. Based on aforementioned facts, and since it is very common to illicit the use of AAS among athletes, especially in bodybuilding, and it is often used along with resistance training, as the data on the effects of combining this exercise type with AAS abuse are scanty, we decided to test this combination in our studies. On the other hand, the athletes often use them in different training situations (starting at the beginning of exercise program or after a period of exercise training sessions). Thus the aim of the present study was to investigate the interaction effects of resistance training and sustanon (Su) abuse on hepatotoxicity in rats with and without training experience, by analyzing the responses of the rat liver antioxidant defense enzymes and liver function tests.
Materials and Methods
Animals and experimental treatments
Fifty six healthy male Wistar rats (age: 60-days old, weight: approximately 250 g) provided by Anistito Pastor (Karaj, Iran) were housed under environmentally controlled conditions (at 22-24°C on a 12 h light-and-dark cycle) and permitted free access to food and water throughout the experiment. Rats care, handling, and all of the experimental procedures employed were in accordance with the Ethics Committee on Animal Experimentation of University of Guilan. The Su ampoules (manufactured by N.V. Organon Oss Inc. Holland) have been obtained from the local pharmacy in Guilan - Iran. Each ampoule contains 1 mL of oily solution of Su (250 mg Su per 1 mL). Su was diluted with olive oil as needed to ensure equal injection volume for dosage, and was given intramuscularly once a week; Su - untreated rats received the same volume of the olive oil by this schedule. The dose of Su was based on the cases of Su abuse in athlete [13]. After a 7 day adaptation period, rats were divided randomly into 7 groups (first group served as control and the other groups as the treated groups). Each group consisted of eight rats per cage. Group (A) Control (sedentary): without any injection or exercise protocol; group (B) Su - untreated sedentary rats (non-Su/Sed): each rat injected once a week with 1 mL of olive oil intramuscularly (IM) as a vehicle for 8 weeks; group (C) Su - treated sedentary rats (Su/Sed): rats received 10 mg/kg body weight of Su suspension once a week by IM injection for 8 weeks; group (D) Su - untreated trained rats (non-Su/Tr): rats injected once a week with 1 mL of olive oil IM and the resistance training (RT) protocol was employed 3 days a week for 8 weeks; group (E) Su - treated trained rats (Su/Tr): rats received 10 mg/kg body weight of Su suspension once a week by IM injection and the RT protocol were employed 3 days a week for 8 weeks; group (F) Su - untreated experience trained rats (non-Su/ XTr): RT protocol for 4 weeks and then RT protocol along with 1 ml of olive oil IM injection once a week for 8 weeks; group (G) Su - treated experience trained rats (Su/XTr): RT protocol for 4 weeks and then RT protocol along with 10 mg/kg body weight of Su suspension once a week by IM injection for 8 weeks. The rats scheduled for RT were climbed on the vertical ladder (110 cm high, 18 cm wide, with 2 cm grid steps) with weights attached to their tails, 3 sessions per week for 8 and 12 week in the Tr and XTr groups respectively. RT program in first day was initiated with 75% of the animal’s body weight and, after that, the load was progressively increased (30 g weight was added) until reaching a load with which the rat could not climb the entire length of the ladder. The highest load successfully carried along the entire length of the ladder was considered to be the rat’s maximal carrying capacity for that training session. During subsequent training sessions, the rats carried 50%, 75%, 90%, and 100% of their pre-training maximal carrying capacity, and subsequent, 30 g of weight was progressively added to the prior weight until the rat’s new maximal carrying capacity was achieved. The rest interval between the repetitions was 120 s. The RT protocol was adapted from Hornerberger and Farrar [14].
Tissue collection and preparation
At the end of the experiments, the animals were anesthetized with ketamine-xylazine and sacrificed by cannulation of the abdominal aorta. Blood samples were then collected via cardiac puncture according to the method of Hoff and Rlatg [15], and centrifuged, and serum was frozen at -20°C for later analysis. Livers were rapidly excised and washed with cold saline and frozen in liquid nitrogen and stored at −80°C for further analysis.
Serum analyses
The activities of the serum enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were assayed using routine enzymatic methods (Pars Azmoon, Tehran, Iran) on an automated chemistry analyzer (Mindray Bs-380, China).
Liver homogenate preparation for antioxidant activity
One ml of homogenization buffer (0.1 M phosphate buffer, pH 7.4 containing 1 mM EDTA and 0.005% BHT) per 100 mg of tissue was added. Then liver tissue samples were homogenized. After homogenization, samples centrifuged at 8000 rpm for 10 minutes and the resulting supernatant was used for the measuring activity of antioxidant enzymes such as SOD [16], GPx [17] and GR [18].
Statistical analysis
All data were expressed as mean ± standard deviation (M ± SD) and statistical analyses were carried out using statistically available software of statistical package for social science (SPSS) version 19.0. All results were evaluated by one-way analysis of variance (ANOVA) test followed by Tukey test, if necessary. Significance was set at P <0.05.
Results
Results of the liver serum parameters (ALT, AST and ALP) are listed in Table 1. Neither the RT nor the treatment of Su modified significantly mean values of AST, ALT, and ALP in male rats with and without experience resistance training in compared to Su - untreated groups(P>0.05).
| Parameters | ALT (IU/L) | AST (IU/L) | ALP (IU/L) |
|---|---|---|---|
| Control | 41.5 ± 10/54 | 114.75 ± 15/32 | 97.10 ± 14/12 |
| non-Su/Sed | 42 ± 11/51 | 114.62 ± 17.98 | 96.87 ± 25.17 |
| Su/Sed | 47.62 ± 13/14 | 119.37 ± 18.76 | 99 ± 16/57 |
| non-Su/Tr | 42.75 ± 13/20 | 116.35 ± 18/81 | 95.12 ± 15/68 |
| Su/Tr | 46.25 ± 10.23 | 120.38 ± 21/56 | 98.75 ± 17/11 |
| non-Su/XTr | 42.75 ± 12/37 | 112.38 ± 18/32 | 94.5 ± 14/45 |
| Su/XTr | 48.5 ± 12/10 | 123.12 ± 16/53 | 100.06 ± 15/95 |
Values are expressed as mean± S.D. (n=8).
Sed:Sedentary; Tr:Trained Rats; XTr:Experience Trained Rats;ALT:Alanine Aminotransferase; AST:Aspartate Aminotransferase; ALP:Alkaline Phosphatase.
There were no statistically significant differences between groups.
Table 1: Effects of resistance training along with sustanon (Su) abuse on serum parameters in rats with and without training experience.
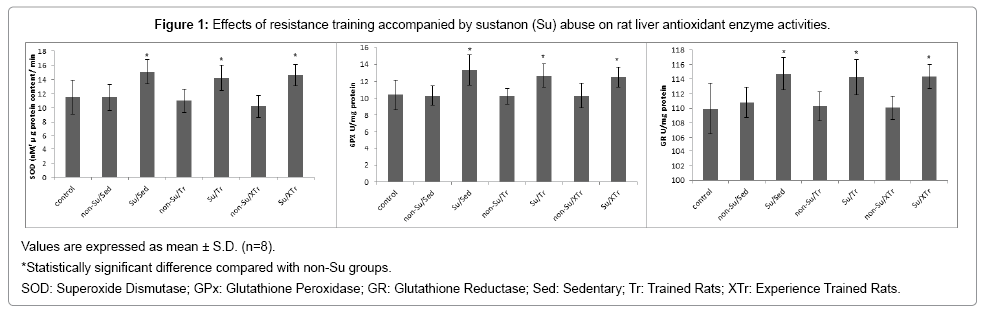
Measurements of enzymatic antioxidants (SOD, GPx and GR) activity in the liver homogenate are reported in Figure 1. The activity of enzymatic antioxidants in the Su - treatment groups significantly increased compared with the Su - untreated groups (P <0.05). There were no differences in antioxidants activity in both sedentary and trained animals (P> 0.05).
Discussion
Among the various anabolic steroids available, Su is presented as one of the most popular AAS that is used extensively throughout the bodybuilding community and in medicine alike [2,19]. Evidence from the current study indicated that IM injection of Su at supraphysiological dose (10 mg/Kg BW) for 8 weeks induced a significant increase (P<0.05) in the activities of the hepatic antioxidant enzymes SOD, GPx, GR, while resistance training (RT) had no effect on antioxidants levels in male rat with and without training experience. This significant increase in hepatic antioxidants activity in the Su - treated groups is in harmony with Pey et al. who observed that abuse of AAS for 8 weeks, either with or without concurrent exercise training, causes an increase the activities of the antioxidant enzymes in hepatic tissue as compared with control group [7]. On the contrary, Frankenfeld et al. reported that the abuse of AAS decreases the liver antioxidants activity [20].
The elevation of SOD, GPx and GR levels may be attributed to hepatic dysfunction of mitochondrial respiratory chain complexes and mono-oxygenase systems in response to Su administration [7,21], it would be possible that these alterations were accompanied by an increased ROS production. Excessive ROS formation could lead to oxidative stress and cell damage [22].
SOD is the first enzyme to deal with ROS [16]. SOD protects cells from oxidant injury by catalysing the conversion of superoxide anion to molecular oxygen (O2) and hydrogen peroxide (H2O2), which is then catalyzed either by CAT (catalase) or GPx. Induction of SOD activity occurs during high production of superoxide anion radical [16,23]. The significant increase of SOD activity in the present study might have occurred as a direct response to Su induced superoxide anion production. The increase in GPx activities in the liver as observed in the present study might be in response to H2O2 produced by SOD activity since GPx play a significant role in the elimination of hydrogen peroxide [22]. Our results were in accordance with the previous reports by Camiletti-Moirón et al. and Pey et al. where brain and hepatic SOD and GPx activities were elevated in rat after chronic exposure to AAS [7,8]. GR activity significantly increased in the Su - treated groups. Enhanced GR activity is indicative of a compensatory mechanism to maintain the endogenous GSH (glutathione) level or a response to an insufficient level of NADPH (nicotinamide adenine dinucleotide phosphate) [22].
In this study, the activity of liver antioxidant enzymes were not affected by RT. Results from previous studies analyzing the response of liver antioxidants activity to exercise training are disparate. Increases [23-25], and decreases in this parameter have been described [26]. Nevertheless, most authors have reported absence of variations [27,28]. These discrepancies can be related to the differences in the intensity and/ or duration of the exercise sessions employed in the aforementioned studies. It is known a single bout of High intensity or prolonged exercise can elevate ROS production in the liver [5,6,23] and therefore exercise training could activate the synthesis of antioxidant enzymes as a long-term strategy to cope with the encountered increased generation of ROS during exercise sessions. The resistance training session used in this experiment likely poses minimal oxidative stress to the liver, probably owing to its high intrinsic antioxidant capacity.
The liver serum parameters (ALT, AST and ALP) were unchanged by Su - treatment. This observation is consistent with previously reported results [7,21]. On the contrary, some studies reported that the supraphysiologic doses of AAS are associated with an increase in the plasma levels of these parameters [29-31]. The reason for the discrepancy observed in the effect on ALT, AST and ALP after AAS administration may be the different study designs used, sampling time, type of AAS used, administration route, etc.
The AAS biochemical structure could be related to hepatotoxicity, in addition to previous studies demonstrated that 17α-alkyl steroids are mainly taken orally (introduced to retard hepatic degradation) compared to 17β-hydroxyl steroids (injectable testosterone) in high dosages, which potentially damage liver cells due to the high steroid load [3,13,19].
The Su is an oil-based injectable testosterone blend containing 4 different types of testosterone (testosterone propionate, testosterone be phenylpropionate, testosterone isocaproate and testosterone decanoate), and it has a slow absorption rate into the blood stream, so that the liver experiences a low concentration of the drug compared to the substance taken orally [2]. In addition, the conventional liver function tests do not always reflect liver abnormalities particularly at the initial stages. In this regard, previous studies indicate that prolonged AAS administration causes inflammatory or degenerative lesions in centrilobular hepatocytes, ultrastructural alterations in the canaliculi and degenerative changes in mitochondria and lysosomes without modifying classical serum indicators of hepatic function [6,7,21].
A limitation of this study was that we did not evaluate oxidative stress markers and histopathological parameters. With regard to the observed undesirable effects of Su, future human studies on people who take Su are greatly recommended to investigate the side effects of Su and its optimal dose.
Conclusions
Our result indicates that chronic treatment with high doses of Su increases hepatic antioxidant enzymes activity and the activity of these parameters were not affected by RT and experience of RT. Furthermore, Su abuse in this dose did not make any significant variations in the liver function tests. These data support the finding that liver function tests do not always reflect liver abnormalities particularly at the initial stages.
Acknowledgements
The authors would like to thank director of animal laboratory in faculty of sport sciences, University of Guilan for friendly cooperation and facilitating condition of this study.
Conflict of Interests
The authors declare that they have no contradiction regarding this study.
References
- Harmer PA (2010) Anabolic-androgenic steroid use among young male and female athletes: is the game to blame. Br J Sports Med 44: 26-31.
- Hartgens F, Kuipers H (2004) Effects of androgenic-anabolic steroids in athletes. Sports Med 34: 513-554.
- Kicman AT (2008) Pharmacology of anabolic steroids. Br J Pharmacol 154: 502-521.
- Birben E, Sahiner UM, Sackesen C,Erzurum S,Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9-19.
- Cooper CE, Vollaard NBJ, Choueiri T, Wilson MT (2002) Exercise free radicals and oxidative stress. Biochemical Society Transactions 30: 280-285.
- Bejma J, Ramires P, Ji LL (2000) Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand 169: 343-351.
- Pey A, Saborido A, Blazquez I, Delgado J, Megias A (2003) Effects of prolonged stanozolol treatment on antioxidant enzyme activities oxidative stress markers and heat shock protein HSP72 levels in rat liver. J Steroid Biochem Mol Biol 87: 269-277.
- Camiletti-Moirón D, Aparicio AV, Nebot E, Medina G, MartÃnez R, et al.(2015) High-intensity exercise modifies the effects of Stanozolol on brain oxidative stress in rats. Int J Sports Med 36: 984-991.
- Chaves EA, Fortunato RS, Carvalho DP, Nascimento JH, Oliveira MF (2013) Exercise-induced cardioprotection is impaired by anabolic steroid treatment through a redox-dependent mechanism. J Steroid Biochem Mol Biol 138: 267-272.
- Chaves EA, Pereira-Junior PP, Fortunato RS, Masuda MO, de Carvalho AC,et al.(2006) Nandrolone decanoate impairs exercise-induced cardioprotection: role of antioxidant enzymes. J Steroid Biochem Mol Biol 99: 223-230.
- Mallikarjuna K, Nishanth K, Kuo CH, Reddy KS (2009) Effect of exercise training on ethanol-induced oxidative damage in aged rats. Alcohol 43: 59-64.
- Lew H, Quintanilha A (1991) Effects of endurance training and exercise on tissue antioxidative capacity and acetaminophen detoxification. Eur J Drug Metab Pharmaco Kinet 16: 59-68.
- Hassan NA, Slem MF, Sayed MA (2009) Doping and effects of anabolic androgenic effects on the heart: histological Utrastructural and echocardiographic assessment in strength athletes. Hum Exp Toxicol 28: 273-283.
- Hornberger TA, Farrar RP (2004) Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol 29: 16-31.
- Hoff J, Rlatg L (2000) Methods of blood collection in the mouse. J Lab Anim 29: 47-45.
- Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cells Superoxide dismutase activity. J Lab Clin Med 85: 337-41.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, et al. (1973) Selenium: Biochemical role as a component of glutathione peroxidase. Science 179: 588-590.
- Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res 44: 5086-91.
- Monaghan L (2001) Bodybuilding Drugs and Risk. Taylor and Francis New York USA.
- Frankenfeld SP, Oliveira LP, Ortenzi VH, Rego-Monteiro ICC, Chaves EA, et al. (2014) The Anabolic Androgenic Steroid NandroloneDecanoate Disrupts Redox Homeostasis in Liver Heart and Kidney of Male Wistar Rats. PLoS ONE 9: e102699.
- Molano F,Saborido A, Delgado J, Morán M,Meg´ıas A (1999) Rat liver lysosomal and mitochondrial activities are modified by anabolic-androgenic steroids. Med Sci Sports Exerc 3: 243-250.
- Ji LL (1995) Exercise and oxidative stress: role of the cellular antioxidant systems. Exerc Sports Sci Rev 23: 135-166.
- Venditti P, Di Meo S (1996) Antioxidantstissue damage and endurance in trained and untrained young male rats. Arch Biochem Biophys 23: 63-68.
- Kanter MM, Hamlin RL, Unverferth DV, Davies HW, Merola AJ (1985) Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol 59: 1298-1303.
- Wilson DO, Johnson P (2000) Exercise modulates antioxidant enzyme gene expression in rat myocardium and liver. J Appl Physiol 88: 1791-1796.
- Hong H, Johnson P (1995) Antioxidant enzyme activities and lipid peroxidation levels in exercised and hypertensive rat tissues. Int J Biochem Cell Biol 27:923-931.
- Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, et al. (1997) Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol 272: R363-R369.
- Duncan K, Harris S, Ardies M (1997) Running exercise may reduce risk for lung and liver cancer by inducing activity of antioxidant and phase II enzymes. Cancer Lett 116:151-158.
- Vieira RP, Franca RF, Damaceno-Rodrigues NR, Dolhnikoff M, Caldini EG, et al.(2008) Dose-dependent hepatic response to subchronic administration of nandrolone decanoate. Med Sci Sports Exerc 40: 842-847.
- TasginE, Lok S, Demir N, Ozdemi M (2010) The Effect of Testosterone Used in Sportsmen on Routine Biochemical Parameters. JAnim Vet Adv 9: 2038-2040.
- LokS, Erdal T, Nagehan D, Mehmet O (2010) Long term used testosterone may cause heart and liver damage.Journal of Animal and Veterinary Advances 9: 2343-2345.
Citation: Rahmati S, Arazi H, Ghafoori H (2017) Effects of Resistance Training Accompanied by Sustanon Abuse on Hepatotoxicity in Rats with and without Training Experience. Sports Nutr Ther 2: 126. DOI: 10.4172/2473-6449.1000126
Copyright: © 2017 Rahmati S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 7155
- [From(publication date): 0-2017 - Aug 25, 2025]
- Breakdown by view type
- HTML page views: 6250
- PDF downloads: 905