Environmental Impacts of Cage Culture in Lake Victoria, Kenya
Received: 01-Sep-2023 / Manuscript No. jmsrd-23-113986 / Editor assigned: 04-Sep-2023 / PreQC No. jmsrd-23-113986 / Reviewed: 18-Sep-2023 / QC No. jmsrd-23-113986 / Revised: 23-Sep-2023 / Manuscript No. jmsrd-23-113986 / Published Date: 30-Sep-2023 DOI: 10.4172/2155-9910.1000418
Abstract
Cage farming has become a common practice of rearing fish in marine and freshwater systems worldwide. Fish farming in cages in most East African countries seems to be a new technology, and this came about as a result of the decline in catches of fish around the Lake Victoria basin and the growing demand for protein from fish, which has ultimately resulted in strengthening strategies of boosting aquaculture productions to fill the growing gap of productions from capture fisheries. We conducted an experimental cage culture study at Anyanga, Dunga, Kiwa, and Mulukhoba beaches, Lake Victoria, Kenya, to investigate the impacts of cage aquaculture on the environment. We identified three locations at each beach for sampling. Physio-chemical parameters: Dissolved Oxygen (DO), Temperature, pH, and conductivity were taken. The water samples were then analyzed for nutrients: Total Nitrogen, Total phosphorus, and Ammonium. There were no significant changes in both physio-chemical characteristics of the water and nutrient concentrations. Even though nutrient concentrations were within the recommended ranges, close monitoring of the basin should be encouraged since it is a hyper-trophic lake.
Keywords: Cage aquaculture; Physio-chemical parameters; Nutrients; Hyper-trophic lake
Keywords
Cage aquaculture; Physio-chemical parameters; Nutrients; Hyper-trophic lake
Introduction
Lake Victoria which is one of the African Great Lakes, is the second-largest freshwater basin in the world by area after Lake Superior and the largest in Africa (Kayombo et al.,2006). Its surface area of 68,800 Km2 straddles three countries: Kenya (6%), Uganda (45%), and Tanzania (49%), while its catchment area extends to Rwanda and Burundi in East Africa (Kayombo et al.,2006). The Lake is a vital resource serving as a critical source of income, sustenance, transportation, and other ecological and socio-economic benefits to over 40 million of the rapidly increasing riparian population (Aura et al., 2022). The rich and unparalleled biodiversity host nature of the Lake has since changed as the Lake has undergone monumental shifts over the past years resulting from increased anthropogenic activities and climate-changing conditions (Nyamweya et al., 2016; Awange et al., 2019) [1]. Lake Victoria is presently dominated by the introduced Nile perch (Lates niloticus), tilapia (Oreochromis niloticus), and the native silver cyprinid Rastineobola argentae species contrary to the periods up until the 1970s when endemic tilapines and haplochromines were in abundance and constituted more significant percentage of the capture fisheries of Lake Victoria (Munyaho et al., 2014; Njiru et al., 2018). The changing ecology of the Lake is characterized by increasing human activities and increasing fishing pressure coupled with illegal, unreported, and unregulated (IUU) fishing practices that have led to the disappearance and extinction of crucial indigenous fish species, deteriorating water quality, and reduced wild capture fishery (Nyamweya et al., 2020) [2].
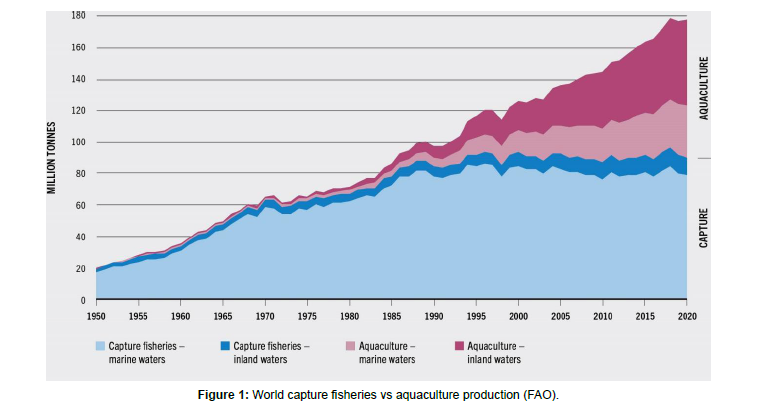
Lake Victoria’s commercial fishery has experienced monumental changes over the past decades. Before Nile perch was introduced into the Lake and became the dominant commercialized fish, the haplochromines accounted for 80% of the total catch (Nagl et al.,2000). There has been a decrease in the catch composition, hence the introduction of cage culture technology that has since developed into a critical component of the fish production system in the country (Obiero et al., 2021a). Cage culture is viewed as an alternative to reducing the widening gap between the demand and supply of fish within the basin (Aura et al.,2018) [3-5] (Figure 1).
Cage aquaculture, a form of intensive fish farming, has gained prominence to meet the region’s escalating demand for fish protein. Figure 1 elucidates how aquaculture production has tremendously increased compared to capture fisheries in both marine and freshwater systems in the past four decades worldwide.While it offers the promise of increased food security and economic growth, it also raises substantial concerns about its environmental consequences, particularly its potential impacts on the water quality of Lake Victoria (Aura et al.,2018). Emitting particulate and dissolved nutrients through uneaten residual feed, feces, and excretory secretions is one of the potential impacts of cage-based aquaculture (Masser,2008) [6].
This study seeks to understand the intricate interplay between cage farming and water quality by looking at the different scales of tilapia cage farming in Lake Victoria, evaluating the consequences for both the ecosystem and the human populations dependent on the lake’s resources. By examining the complex web of influences such as nutrient loading from excess fish feeds and fecal matter from the fish and the release of pollutants associated with cage farming, we aimed to provide a comprehensive analysis of the different impacts that both high and low-intensity cage aquaculture practice exerts on the delicate balance of Lake Victoria’s aquatic environment and consider potential mitigation strategies that can help strike a sustainable balance between the growing aquaculture industry and the preservation of this invaluable natural resource. Understanding these dynamics is essential for safeguarding Lake Victoria’s ecological integrity and ensuring the aquaculture sector’s long-term viability as a means to alleviate food security challenges in the East African region [7-9].
Materials and Methods
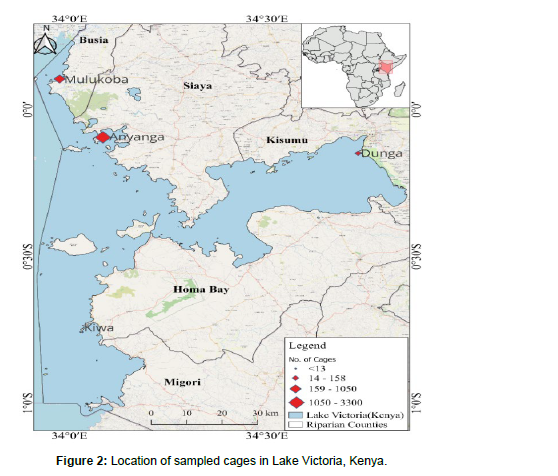
Study area: Sampling was done on the Kenyan parts of Lake Victoria, i.e., Dunga beach, Anyanga beach, Kiwa, and Mulukhoba beach. These sites were selected based on water pollution rates, the density of cages, the productivity of cages, and the impacts of human activities, respectively. The sampling was done quarterly for two years (Figure 2).
Sampling locations were selected randomly in each beach, along the cages, intermediate (located 100m from cages), and control site (located 800m away from cages). GPS (Global Positioning System) was used to mark the stations. The average depth of the cage, intermediate, and control sites was 4.5m, 5.2m, and 6.5m, respectively [10 ].
Water sampling and analysis: In situ, parameters such as Secchi, dissolved oxygen (DO), pH, conductivity, and temperature were measured using a YSI multi parameter. These physio-chemical parameters were measured along the water column,i.e., the bottom, middle, and surface waters. Thereafter, the values were averaged. Secchi depth(m) measurements were taken at each site to determine the water’s transparency. Total water depth was measured using a graduated rope. Triplicate water samples for nutrient analysis (Total Nitrogen, Total phosphorus, and Ammonium) were collected using a Vandorn sampler and stored in 250ml bottles [11]. The water samples were taken along the water column,i.e., surface, middle, and bottom, then mixed to form a composite sample. The samples were then stored in a cooler box filled with ice to prevent water degradation while transporting them to the laboratory. Nutrient concentrations were analyzed using standard methods,i.e., Total nitrogen (TN) determined by cadmium reduction and total phosphorus (TP) by persulfate digestion. A spectrophotometer was then utilized to measure the absorbance of the samples to evaluate the concentration of these nutrients [12 ].
Results and Discussions
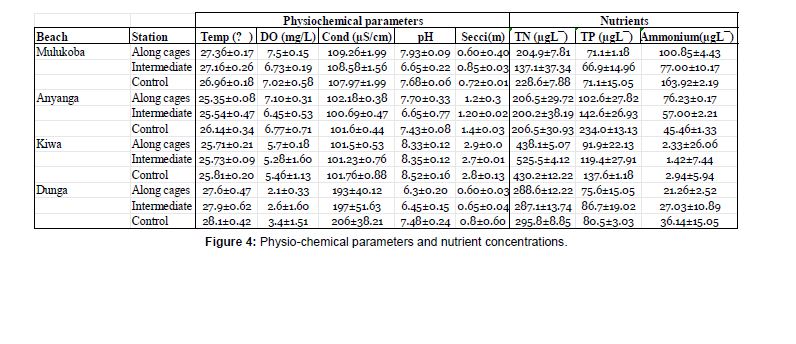
Physical-chemical parameters: The results of physical and chemical measurements at various selected cage sites in Busia (Mulukoba cages), Siaya (Anyanga cages), Kisumu (Dunga cages), and Homa Bay (Kiwa cages) counties have been summarized in Fig 3. above. The data points along the transects represent a combination of physical and chemical measurements of the water at different points: Around the cage area (along the cages), 100 meters past the cage area (Intermediate), open areas away from the cage activities reference point called Control (Ctrl). This sampling method applies to all cases (Figure 3).
There was visible water physio-chemical parameter variability regarding the beaches. Temperature is a critical factor affecting aquatic ecosystems as it influences metabolic rates and oxygen solubility (Coffill‐Rivera et al., 2023); Anyanga station showed no statistically significant differences between Control and along cages and Intermediate station. The average temperatures recorded were 25.35°C for Control, 25.5°C for Along cages, and 25.70°C for Intermediate point. The same trend was evident at the Dunga, Mulukoba, and Kiwa stations, as there were no remarkable variations with their respective control stations. The minor variations observed could be attributed to local environmental factors, such as sunlight exposure, water depth, and water flow dynamics (Sitoki et al.,2010) [13, 14].
Dissolved oxygen (DO) concentrations showed distinctive patterns in DO readings emerging across different stations, providing valuable information on potential shifts in water quality across stations from different regions. Notably, Stations Kiwa and Dunga consistently showed lower DO concentrations, around 2-5 mg/L, but generally, their averages are similar to the respective control stations. On the other hand, Mulukoba station maintained DO levels ranging from approximately 6.73±0.19 mg/L to 7.5±0.15 mg/L. These relatively stable values suggest that cage aquaculture practices in these areas have limited or no influence on dissolved oxygen concentrations [15].
Conductivity (Cond) and pH parameters primarily reflect on water’s ionic composition, chemical equilibrium, and alkalinity, respectively (Mwamburi et al., 2020.). The data showed relatively consistent conductivity values across most stations, except at Dunga beach, which exhibited a significantly higher value of 206±38.21. Compared to their respective controls, we did not experience significant differences as most had a difference range of (p>0.05), and pH values varied within a narrow range, indicating the water’s generally neutral to slightly alkaline nature. The deviations observed were not substantial enough to suggest significant pH-related impacts from cage aquaculture activities [16].
Secchi depth serves as an indicator of water transparency and light penetration. The data demonstrated varying levels of clarity across different beaches. Dunga and Mulukoba stations displayed lower Secchi depths of between 0.5±0.40m and 0.8±0.60m, implying potential influences on water clarity in their vicinity. Reduced Secchi depths may be attributed to suspended particles and sediment re-suspension (Herbeck et al., 2011; Henny et al., 2016) resulting in variable and often murkier water conditions. Kiwa had the most transparent waters, recording a Secchi depth of 2.9±0.01, supporting the understanding that deeper waters often lead to more transparent conditions. This pattern was attributed to reduced exposure to external influences, such as wind-induced turbulence or sediment deposition, that tend to be more prominent in shallower areas [17].
Nutrients: The study focused on key water quality parameters, including total nitrogen (TN), total phosphorus (TP), and ammonium concentration, across different cage locations. The highest TN concentration was recorded at the Kiwa station, reaching 525.47± 4.12μg/L, while the lowest concentration was observed at the Mulukoba station with a level of 137.05±37.34μg/L. In terms of total phosphorus concentration, the results indicated notable differences across the beaches but no significant changes in relation to control sites. The Anyanga and Kiwa stations exhibited the highest TP concentrations of 142.57±26.93μg/L and 137.57±1.18μg/, while the Dunga and Mulukoba stations had the lowest value of 80.53±μg/L and 66.86±14.96μg/L. Elevated phosphorus levels in Anyanga and Kiwa were attributed to eutrophication in the surrounding waters (Khan et al., 2014).
Kiwa station generally had the lowest ammonium concentration, 1.42±7.44μg/L, caused by the efficient vertical and horizontal mixing of water layers which help distribute nutrients evenly (Shang et al., 2021). In contrast, the Mulukoba and Anyanga stations recorded the highest concentration, 163.92±2.19μg/L. The limited water exchange in these areas leads to reduced dilution of ammonium, resulting in its elevated concentration (Constable et al., 2003).
Even though different beaches had different nutrient concentrations, the cage sampling stations per beach did not show any significant change in nutrient concentrations in relation to the control sites. Shows the concentrations of the studied nutrients, and the comparison after ANOVA test analysis shows there was no remarkable change in these nutrient concentrations in relation to the control sites at each beach [18]. (Table 1) (Figure 4).
| Comparison | t - value | p - value |
|---|---|---|
| Control vs Intermediate | -0.55 | >0.05 |
| Control vs Along cages | -0.12 | >0.05 |
Table 1: Comparison of intermediate sites and along cages in relation to control sites.
Conclusion
The observed values were within the desirable limits recommended by the World Health Organization. Physio-chemical parameters and nutrient concentrations along the cages and at control sites in the studied beaches did not differ significantly according to an ANOVA test using water quality measures (P >0.05).
Although the study demonstrates that cage culture has little to no environmental impact on Lake Victoria, careful water monitoring should be promoted to prevent water quality deterioration. Since Lake Victoria is already a hyper-eutrophic basin, fish cage farmers should also be urged to use best cage management practices.
References
- Aura C, Musa M, Yongo S, Okechi E, Njiru JK, et al. (2018) Integration of mapping and socio‐economic status of cage culture: Towards balancing lake‐use and culture fisheries in Lake Victoria, Kenya. Aquac Res 49: 532-545.
- Aura C, Roegner A, Owiti H, Birungi D, Fiorella KJ, et al. (2022) Mind the gaps for the best practices: Enhancing the management of Lake Victoria fisheries resources. Lakes Reserv: Res Manag 27: 124-131.
- Awange JL, Saleem A, Sukhadiya RM, Ouma YO, Kexiang H, et al. (2019) Physical dynamics of Lake Victoria over the past 34 years (1984–2018): is the lake dying? Sci Total Environ 658: 199-218.
- Coffill‐Rivera ME, Neal JW, Allen PJ (2023) Effects of temperature on metabolic rate and lower dissolved oxygen tolerance of juvenile speckled peacock bass Cichla temensis. J Fish Biol 102: 635-642.
- Constable M, Charlton M, Jensen F, McDonald K, Craig G, et al. (2003) An ecological risk assessment of ammonia in the aquatic environment. Hum Ecol Risk Assess 9: 527-548.
- Henny C, Nomosatryo S (2016) Changes in water quality and trophic status associated with cage aquaculture in Lake Maninjau, Indonesia. Environ Earth Sci 31: 20-27.
- Herbeck LS, Unger D, Krumme U, Liu SM, Jennerjahn TC, et al. (2011) Typhoon-induced precipitation impact on nutrient and suspended matter dynamics of a tropical estuary affected by human activities in Hainan, China. Estuar Coast Shelf Sci 93: 375-388.
- Kayombo S, Jorgensen SE (2006) Lake Victoria. Experience and lessons learned brief 8: 431-446.
- Khan MN, Mohammad F (2014) Eutrophication: challenges and solutions. Eutrophication: Causes, Consequences and Control 2: 1-15.
- Musa S, Aura CM, Tomasson T, Sigurgeirsson Ó, Thorarensen H, et al. (2022) Impacts of Nile tilapia cage culture on water and bottom sediment quality: The ability of a eutrophic lake to absorb and dilute perturbations. Lakes Reserv: Res Manag 27: 124-134.
- Mwamburi J, Basweti G, Owili M, Babu J, Wawiye P, et al. (2020) Spatio‐temporal trends of nutrients and physico‐chemical parameters on lake ecosystem and fisheries prior to onset of cage farming and re‐opening of the Mbita passage in the Nyanza Gulf of Lake Victoria. Res Manag 25: 292-313.
- Nagl S, Tichy H, Mayer WE, Takezaki N, Takahata N, et al. (2000) The origin and age of haplochromine fishes in Lake Victoria, East Africa. Proceedings of the Royal Society of London. Bio Sci 267: 1049-1061.
- Njiru J, van der Knaap M, Kundu R, Nyamweya C (2018) Lake Victoria fisheries: Outlook and management. Res Manag 23:152-162.
- Nyamweya CS, Natugonza V, Taabu-Munyaho A, Aura CM, Njiru JM, et al. (2020) A century of drastic change: Human-induced changes of Lake Victoria fisheries and ecology. Fish Res 230: 105-564.
- Nyamweya C, Sturludottir E, Tomasson T, Fulton EA, Taabu-Munyaho A, et al. (2016) Exploring Lake Victoria ecosystem functioning using the Atlantis modeling framework. Environ Model Softw 86: 158-167.
- Shang C, Liang C, Chen G, Gao Y (2021) The influence of turbulent mixing on the subsurface chlorophyll maximum layer in the northern South China Sea. J Oceanol Limnol 39: 2167-2180.
- Sitoki L, Gichuki J, Ezekiel C, Wanda F, Mkumbo OC, et al. (2010) The environment of Lake Victoria (East Africa): current status and historical changes. Int Rev Hydrobiol 95: 209-223.
- Taabu-Munyaho A, Nyamweya CS, Sitoki L, Kayanda R, Everson I, et al. (2014) Spatial and temporal variation in the distribution and density of pelagic fish species in Lake Victoria, East Africa. Aquat Ecosyst Health Manag 17: 52-61.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Omach Z (2023) Environmental Impacts of Cage Culture in Lake Victoria,Kenya. J Marine Sci Res Dev 13: 418. DOI: 10.4172/2155-9910.1000418
Copyright: © 2023 Omach Z. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3040
- [From(publication date): 0-2023 - Nov 20, 2025]
- Breakdown by view type
- HTML page views: 2629
- PDF downloads: 411