Evaluation of the Portable THD® Anopress Device in Patients with Faecal Incontinence
Received: 15-Nov-2018 / Accepted Date: 25-Nov-2018 / Published Date: 05-Dec-2018 DOI: 10.4172/2472-1220.1000582
Abstract
Background: THD Anopress® has been promoted as a new portable anal manometer providing measurements of whole anal canal pressures. This study aims to report the anorectal function of patients suffering from Faecal Incontinence (FI) using this new device and the associated patient comfort.
Methods: We reviewed data from patients suffering from FI who had been evaluated with the Anopress. The St Mark’s score had been routinely used to evaluate the severity of FI. Manometric parameters and pressures were evaluated. Acceptability of this test was also assessed using the Visual Analogue Scale (VAS).
Results: THD Anopress® device was used to assess 60 patients with FI. The median baseline St Mark’s FI score was 14 (7-24). A mixed FI was seen in 34 (57%) patients. Resting pressures were 25 (0-60) in the female group and 30 (0-52) mmHg in the male group. Maximum squeeze increments were 27 (6-106) in the female and 42 (6-99) mmHg in male subject group. Maximum endurance squeeze in 10 seconds were 32 (5-89) in the female and 31 (10-65) mmHg in the male groups. Involuntary maximum squeeze was 33 (6-103) in the female group and 27 (7-90) mmHg in the male group. These values were significantly reduced when compared to previously report normative values. The median VAS is of 0 at insertion and during the procedure.
Conclusion: The THD Anopress® device appears to detect anal sphincter dysfunction in those with symptomatic FI and is well tolerated.
Keywords: Anorectal function; Anorectal manometry; Faecal incontinence; Anorectal physiology studies; THD Anopress®
Introduction
Faecal Incontinence (FI) is a socially debilitating condition with multiple aetiologies [1-5]. Its management includes Anorectal Physiological testing (ARP) which aims to define the function of the anorectum, providing diagnostic and prognostic information for a range of anorectal pathologies [6-9].
Manometric assessment of anal canal pressures is reflective of anal sphincter function. This includes the anal canal resting pressure, maximum voluntary squeeze increment, involuntary squeeze increment, and endurance squeeze increments [10-13]. Anal manometry is traditionally performed using a multi-channel water perfused catheter, which records along both longitudinal and radial axes [7,13-15].
These catheters are durable, require little maintenance and are reliable. More recently, high resolution solid state catheters and three dimensional High Resolution Manometry (HRM) have been introduced [10,12,16]. They have a greater number of sensors per surface area and a topographical display aiding easier interpretation of data.
THD Anopress® (THD SpA, Correggio (RE), Italy) is a new portable anal manometry device which uses high resolution air filled catheters to evaluate the sphincter pressures generated from the whole of the anal canal [17].
It is promoted as a small, portable, wireless device which can perform rapid manometric assessments away from the anorectal physiology laboratory [11,17,18]. It is CE (European Conformity) marked and has United States of America Food and Drug Administration approval.
The aim of this study was to determine whether this new device is able to detect anal sphincter dysfunction in patients with FI and compare these manometric parameters to previously reported normative values [17]. Acceptability of the device was also assessed.
Materials and Methods
We reviewed prospectively collected data on adult patients with symptomatic Faecal Incontinence (FI) who had been assessed in the outpatient clinic using the Anopress device. All patients were examined in a single tertiary care specialist centre. Research and Development office approval was gained prior to data review (R&D No. SE16/039 London North West NHS Trust).
A full clinical history had been taken, including the severity of FI estimation using the St Mark’s FI score [19], for all patients prior to THD Anopress® assessment. THD Anopress® assessment was performed by an experienced physician with a specialist interest in anorectal manometry and physiology (CAL).
A standard protocol was followed for all patients [17]: patients were instructed to defecate if required prior to the procedure and no bowel preparation was given. It was confirmed that all subjects understood the commands squeeze, cough and push prior to performing each procedure [20].
Resting pressure, voluntary squeezes, involuntary squeezes (cough manoeuvre) and endurance pressure increments were recorded in keeping with a previous published protocol [17].
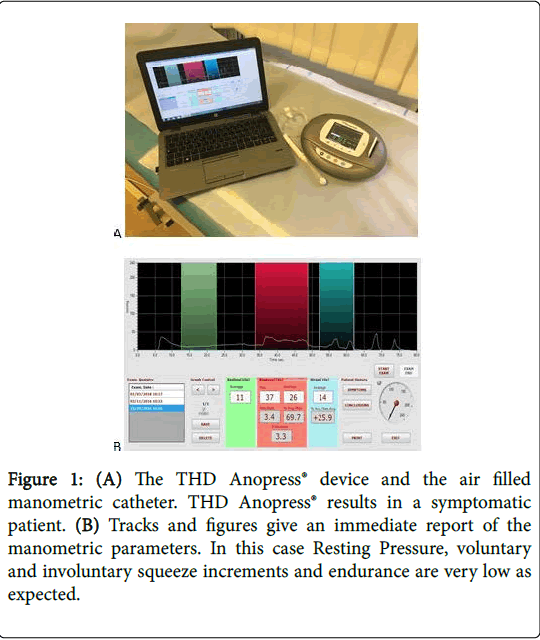
Endurance was defined as the progressive duration of contraction of up to 50% of the maximum squeeze pressure, and as a value expressed in mmHg at a maximum of ten seconds [11]. The data generated by this investigation had been recorded for each patient using the THD Anopress® device (Figure 1).
Figure 1:(A) The THD Anopress® device and the air filled manometric catheter. THD Anopress® results in a symptomatic patient. (B) Tracks and figures give an immediate report of the manometric parameters. In this case Resting Pressure, voluntary and involuntary squeeze increments and endurance are very low as expected.
A Visual Analogue Scale (VAS) pain score had been recorded for all patients during this test with the descriptor extremes “0=no pain at all” and “10=my pain is as bad as it could possibly be” to evaluate how comfortable patients found this new device [21].
Descriptive statistics are presented using median and range values. The Mann-Witney-U test was used analyse the study data using commercially available software (GraphPad Prism Software Version 6, La Jolla, CA, USA). A p value<0.05 was considered statistically significant.
Results
Between June 2016 and June 2017, 60 patients with symptomatic FI underwent assessment using the THD Anopress® device. Of these, 45 (75%) were female. The median (range) age was 51 (21-88) years for females and 61 (22-87) years for males respectively.
The predominant symptom was a combination of urge and passive FI in 34 (57%) patients, urge FI was found in 18 (30%) patients and the remaining 8 (13%) patients had pure passive FI. The median St Marks FI score was 15 (7-24) in females and 11 (9-24) in males respectively.
Resting pressures were 25 (0-60) mmHg in the female group and 30 (0-52) mmHg in the male group. Maximum squeeze increments were 27 (6-106) mmHg in the female and 42 (6-99) mmHg in male groups. Endurance squeeze increments were 3.9 (0.4-10) seconds in the female and 3.3 (0.3-4.3) seconds in the male groups.
Maximum endurance at 10 seconds was 32 (5-89) mmHg in the female group and 31 (10-65) mmHg in the male group. Involuntary maximum squeeze pressures were 33 (6-103) mmHg in the female group and 27 (7-90) mmHg in the male group.
Recorded measures are shown in Table 1. These results were compared to normal values previously published [17]. A statistically significant (p<0.05) reduction in all pressure measurements was seen (Table 1). Patients with higher incontinence scores had lower manometric results as expected.
| Parameters | Female symptomatic (No. 45) | Female Normal values | P-value | Male symptomatic (No. 15) | Male Normal values | P-value |
|---|---|---|---|---|---|---|
| Age | 51 (21-88) | 39.5 (19-79) | Not compared | 61 (22-87) | 41 (18-74) | Not compared |
| St Mark’s score | 15 (7-24) | 0 | <0.05 | 11 (9-24) | 0 | <0.05 |
| Resting Pressure | 25 (0-60) | 40.0-103.0 | <0.05 | 30 (0-52) | 38.3-99.6 | <0.05 |
| Max Squeeze Increment | 27 (6-106) | 35.0-140.6 | <0.05 | 42 (6-99) | 42.5-154.8 | <0.05 |
| Endurance in mmHg | 32 (5-89) | 44-98 | <0.05 | 31 (10-65) | 43-103 | <0.05 |
| Endurance in sec | 3.9 (0.4-10) | 1.3-9.0 | <0.05 | 3.3 (0.3-4.3) | 2.0-10.0 | <0.05 |
| Involuntary Squeeze | 33 (6-103) | 41.1-120.8 | <0.05 | 27 (7-90) | 40.0-123.6 | <0.05 |
Table 1: Demographic details and manometric parameters recorded in the cohort of 60 patients symptomatic for faecal incontinence. Parameters are expressed in Median and Range. Pressures are expressed in mmHg.
The procedure was well tolerated by all patients with a median VAS pain score of 0 (0-2) during insertion and 0 (0-1) during the procedure.
Discussion
Statistically significant reductions in all of the measured parameters in patients with symptomatic FI were demonstrated by this study, which indicates that the THD Anopress® device is able to detect impaired sphincter function in those with symptomatic FI.
The major advantage of this device includes the ease with which it can be calibrated taking only few seconds, the fact that water perfusion is not necessary and ease of catheter placement and positioning with minimal effort. Also this device appears to be well tolerated by the patients. This is suggested by the mean VAS scores of zero during both insertion and assessment.
Another significant advantage of the THD Anopress® device is that pressure measurements are taken from the entire anal canal: The catheters are air-filled and they provide measurements from the whole surface without the need for receptors or channels allowing the estimation of the average pressure from the whole anal canal at one time.
This may provide more clinically useful information also improving reproducibility of assessment when compared to existing anal manometry techniques which can take measurement from multiple points along the anal canal [16].
The device has some disadvantages. Firstly THD Anopress® is unable to measure anal canal length. Secondly a separate catheter with a balloon was needed to measure rectal sensitivity to distension and the recto-anal inhibitory reflex.
This is in contrast to existing catheters, in which the balloon is part of the manometry catheter. It is understood that newer generations of THD Anopress® catheters may combine these two additional parameters.
In conclusion further studies are required for comparison with manometric devices. This study demonstrates that THD Anopress® device appears able to detect anal sphincter dysfunction in those with symptomatic FI. It is also well tolerated in patients.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank THD SpA for providing the THD Anopress® device.
References
- Thekkinkattil DK, Lim M, Stojkovic SG, Finan PJ, Sagar PM, et al. (2008) Classification system for faecal incontinence based on anorectal investigations. Br J Surg 95: 222–228.
- Zorcolo L, Bartolo DCC, Leroi AM (2007) Pathophysiology of faecal incontinence. Fecal Incontinence 35–41.
- Kang HW, Jung HK, Kwon KJ, Song EM, Choi JU, et al. (2012) Prevalence and predictive factors of faecal incontinence. J Neurogastroenterol Motil 18: 86–93.
- Barrett JA, Akpan A (2004) Faecal incontinence and constipation. C J Geriatr Med 6: 99–108.
- Bharucha AE (2014) Epidemiology of Fecal Incontinence. In: GI Epidemiology: Diseases and Clinical Methodology: Second Edition. 285–295.
- Staller K (2015) Role of anorectal manometry in clinical practice. Curr Treat Options Gastroenterol 13: 418-431.
- Pucciani F (2010)Pelvic floor disorders: Imaging and multidisciplinary approach to management. Anorectal manometry.
- Buchanan GN, Nicholls T, Solanki D, Kamm M (2001). Investigation of faecal incontinence. Hosp Med 62: 533–537.
- Matzel KE, Bittorf B (2016) Management of fecal incontinence. Semin Colon Rectal Surg 27: 15-21.
- Carrington EV, Brokjær A, Craven H, Zarate N, Horrocks EJ, et al. (2014) Traditional measures of normal anal sphincter function using high-resolution anorectal manometry (HRAM) in 115 healthy volunteers. Neurogastroenterol Motil. 26: 625-635.
- Ihnat P, Vavra P, Gunkova P, Pelikan A, Zonca P (2014) 3D high resolution anorectal manometry in functional anorectal evaluation. Rozhl Chir 93: 524–529.
- Kim JH (2010) How to interpret conventional anorectal manometry. J Neurogastroenterol Motil 16: 437–439.
- Meunier PD, Gallavardin D (1993) Anorectal manometry: The state of the art. Dig Dis 11(4–5):252–64.
- Rao SSC, Azpiroz F, Diamant N, Enck P, Tougas G, et al. (2002) Minimum standards of anorectal manometry. Neurogastroenterol Motil 14:553-559).
- Lee TH, Bharucha AE (2016) How to perform and interpret a high-Resolution anorectal manometry test. J Neurogastroenterol Motil 22:46–59.
- Leo CA, Cavazzoni E, Thomas GP, Hodgkison J, Murphy J, et al. (2018) Evaluation of 153 asymptomatic subjects using the Anopress portable anal manometry device. J Neurogastroenterol Motil 24:431-436.
- Vaizey CJ, Carapeti E, Cahill JA, Kamm MA (1999) Prospective comparison of faecal incontinence grading systems. Gut 44:77-80.
- Heinrich H, Fox M, Kaufman E, Fried M, Fruehauf H (2009) The effect of instruction and motivation on the results of anorectal manometry. Neurogastroenterol Motil 139: 15S.
- Haefeli M, Elfering A (2006) Pain assessment. Eur Spine J 15: S17–S24.
Citation: Leo CA, Murphy J, Cavazzoni E, Thomas GP, Shaikh S, et al (2018) Evaluation of the Portable Anopress Device in Patients with Faecal Incontinence. J Gastrointest Dig Syst 8: 582. DOI: 10.4172/2472-1220.1000582
Copyright: © 2018 Murphy J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5863
- [From(publication date): 0-2018 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 4728
- PDF downloads: 1135