Exploring 30 day Mortality Variability in the Context of Radiological Gastrostomy: How Important Are Month to Month Differences?
Received: 25-Feb-2022 / Manuscript No. JGDS-22-55577 / Editor assigned: 28-Feb-2022 / PreQC No. JGDS-22-55577(PQ) / Reviewed: 14-Mar-2022 / QC No. JGDS-22-55577 / Revised: 18-Mar-2022 / Manuscript No. JGDS-22- 55577(R) / Published Date: 25-Mar-2022 DOI: 10.4172/2161-069X.1000678
Abstract
Purpose: Radiological gastrostomy catheter insertion is a well-established procedure to provide enteral nutrition to patients unable to do so themselves. It carries risks and an associated 30 day mortality rate. After referring clinicians perceived increase in early mortality after gastrostomy insertion, we aimed both to determine whether this was a true difference or random variation and how best to communicate this.
Material and Methods: Data for 696 consecutive gastrostomy insertions in 661 patients performed between 2014 and 2020 were collected retrospectively. A literature search was performed for gastrostomy series with 30 day mortality data. Mortality variation was compared to random variation and visualised with a funnel plot. Mortality rate was compared to existing literature. Survival analyses were performed to compare survival among indications.
Results: We found 30 day mortality variability to be indistinguishable from random variation and visualised it to demonstrate no significant outlier and also showed that it compared favourably to published literature. Patients with motor neurone disease had a significantly worse survival than those with ear, nose and throat cancers at 30 days and one year (p<0.05).
Conclusion: Mortality after gastrostomy catheter insertion varies over time but statistical methods can be used to help assess whether the variability is random. A funnel plot is an ideal visualisation for such data and should not be limited to comparing institutions or operators. Some patient groups have significantly worse outcomes after gastrostomy than others.
Keywords: Gastrostomy; Mortality rate; Statistics; Radiological
Introduction
Radiologically inserted gastrostomy (RIG) is an interventional radiology (IR) procedure with a history four decades long [1]. Broadly speaking, there are two insertion mechanisms, one by which the gastrostomy catheter passes into the stomach through the skin (Percutaneous Radiological Gastrostomy-PRG), and the other by which the gastrostomy catheter passes out of the skin through the stomach (Per-Orum Inserted Gastrostomy- PIG); the techniques are well described elsewhere [2-4]. The procedure is widely accepted, with technical success in the literature between 93% and 100% and procedure related mortality between 0% and 3.9%, comparing reasonably with endoscopic and surgical insertion methods [5-8]. Guidelines from the Society for Interventional Radiology (SIR) and Cardiovascular and Interventional Radiology Society of Europe (CIRSE) suggest procedure related mortality should be 0% to 2% and 0.3%, respectively, but do not specifically define procedure related mortality [9,10].
Thirty day mortality after gastrostomy varies between 0% and 40% in the literature with SIR guidelines suggesting 6.7% to 26% and CIRSE guidelines commenting on the variability in the literature without specifying a recommended range [9-12].
With wide variability in the literature, it is unsurprising that RIG 30 day mortality also varies with time, from low and reassuring to higher and potentially worrying. It is important therefore to be able to confidently and correctly differentiate random variability in 30 day mortality from practices causing that variability. Visualisation of 30 day mortality variability has been key in assessing high profile situations such as paediatric cardiac surgery [13].
In December 2016 and January 2017, referring clinicians perceived an unacceptable rise in RIG 30 day mortality at our institution and after consideration, changes in process and practice were implemented in the department. At no point did we seek to determine if the mortality increase was real and the changes were justified.
Today, only one of the changes made is still in place and there have never been further concerns raised. With that in mind, the primary aim of this paper is to explore and compare statistical methods by which variability in mortality rates can be quickly assessed and visualised. The first method will be to perform a post-hoc sample size calculation to conceive a clinical trial comparing mortality rates between groups. The second method is to test whether RIG 30 day mortality has a Poisson distribution. A Poisson distribution expresses the statistical probability of an event (in this case RIG 30 day mortality) occurring in a fixed time interval based on a known mean event rate and assuming new events are independent from previous events [14]. Thirdly, we visualise our RIG 30 day mortality using a funnel plot. Secondary aims are to succinctly present our large volume gastrostomy data and to compare our RIG 30 day mortality to the published literature, again using a funnel plot.
Materials and Methods
Procedural data were collected from the Radiology Information System.
Patient data were collected from the electronic patient record. Mortality data were collected from a national patient database. We included procedures from 1st January 2014 to 30th June 2020 performed at three local hospitals. For mortality calculations, each patient was entered into the dataset once. Gastrostomy catheter insertion was performed on some patients more than one time. Re-insertion procedures were only considered as a data point for survival analysis if a new gastric puncture was required for re-insertion.
The UK National Research Ethics Service (NRES) guidelines define the methods we used as service evaluation and state that ethical approval is not required for service evaluation [15]. Data were analysed using the language and environment for statistical computing, R (R Foundation for Statistical Computing, Aut.) in the RStudio indegrated development environment using packages Tidyverse, survival, lubridate, bibtex, pander, ggpubr, pwr, plyr and survminer [16,17]. Shapiro-Wilk normality test was used to assess normality of distributions. Chi-squared test was used to compare the number of patients who died each month with a number predicted by a Poisson distribution. For abnormally distributed data, the non-parametric Kruskal-Wallis test was used to compare the age of patients in each diagnosis group and Wilcoxon multi-pairwise comparison was used to determine which groups differed. Kaplan-Meier method was used for all survival analyses, with Log-Rank test being used to compare survival between groups. Bonferroni correction of p-values was performed when multiple comparisons were made.
Results
Patients
Between January 1st 2014 and June 30th 2020, 696 consecutive gastrostomies were performed in 661 patients. Some patients who had gastrostomy in late 2019 and all patients who had gastrostomy in 2020 did not have full one year follow up and so one year mortality may increase from the values presented. 630 patients had one gastrostomy insertion, 27 patients had two gastrostomy insertions and 4 patients had three gastrostomy insertions.
The patients’ underlying condition was grouped into seven categories: ear, nose and throat cancers (ENT) and their management, motor neurone disease (MND), stroke (CVA), oesophageal disease (OES), other neurodegenerative diseases (NEU), traumatic brain and spine injuries (INJ) and the remaining miscellaneous or un-diagnosed conditions (UNK). Due to the low number of cases, OES, NEU, INJ and UNK groups were excluded from direct statistical comparisons between underlying diagnoses.
A summary of patient diagnoses, age, number and method of procedures, survival and mortality rates is provided in (Table 1). CVA patients were older than both ENT and MND patients (p<0.05). There was no significant difference between the ages of ENT and MND patients (p=1).
Table 1: Number of patients, their gender and age, 30 day and one year mortality, number of procedures, gastrostomy type and median survival broken down by diagnosis.
| Diagnosis | Patients | Men | Women | Mean age (years) | Median age (years) | 30 day mortality | 1 year mortality | Procedures | PRG | PIG | Median survival (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 661 | 421 | 240 | 64 | 66 | 38 (6) | 299 (45) | 696 | 523 (75) | 173 (25) | 463 |
| ENT | 325 | 226 | 99 | 64 | 65 | 11 (3) | 140 (43) | 345 | 329 (95) | 16 (5) | 514 |
| MND | 107 | 63 | 44 | 64 | 65 | 12 (11) | 63 (59) | 110 | 64 (58) | 46 (42) | 299 |
| CVA | 82 | 42 | 40 | 74 | 77 | 5 (6) | 37 (45) | 83 | 29 (35) | 54 (65) | 562 |
| INJ | 37 | 29 | 8 | 57 | 54 | 3 (8) | 14 (38) | 41 | 25 (61) | 16 (39) | 612 |
| NEU | 40 | 27 | 13 | 57 | 60 | 0 (0) | 12 (30) | 43 | 26 (60) | 17 (40) | 941 |
| UNK | 45 | 23 | 22 | 56 | 62 | 5 (11) | 18 (40) | 46 | 25 (54) | 21 (46) | 762 |
| OES | 25 | 11 | 14 | 69 | 73 | 2 (8) | 15 (60) | 28 | 25 (89) | 3 (11) | 227 |
| All=all patients, ENT=Ear, nose and throat cancers and their management, MND=motor neurone disease, CVA=stroke, OES=oesophageal disease, NEU=other neurodegenerative diseases, INJ=traumatic brain and spine injuries and UNK=miscellaneous or un-diagnosed conditions. Unspecified values are numbers. Values in parentheses are percentages. The location of age data in the table is emboldened to reflect the distribution of age in the diagnosis group, ie. groups with patients of normally distributed age have mean age emboldened and those abnormally distributed have median age emboldened. 1 year mortality values are a minimum, as not all patients had one year follow up at the time of data analysis. | |||||||||||
Post-Hoc sample size calculation for a conceptual clinical trial
The observed 30 day mortality rate was 6% (661 patients, 38 deaths). Choosing a minimum clinically significant 30 day mortality increase as 10%, power of 0.8 and significance level of 0.05, two equal groups of 113 patients would be required for a comparison of 30 day mortality. Alternatively, using the 661 patients in these data as one experimental group, 62 patients would be required in a second group. To increasing the power to 0.95, 116 patients would be required in a second group. For reference, 21 patients had gastrostomy insertion in December 2016 and January 2017 (the two months which caused concern in our situation) combined.
Monthly variation in gastrostomy procedures and 30 day mortality
Between 1 and 18 patients had a gastrostomy insertion each month; these were abnormally distributed with a median of 8 and interquartile range (IQR) of 6-11. Between 0 and 3 patients died within 30 days of gastrostomy insertion each month; abnormally distributed with a median of 0 and IQR 0-1. RIG 30 day mortality each month ranged from 0 to 33% (median 0%, IQR 0%-9%).
The mean number of patients who died within 30 days each month was 0.49 (38 patients in 78 months). If RIG 30 day mortality is a Poisson random variable, the expected frequency that zero, one, two or three patients would die within 30 days each month can be calculated by Poisson probability. These are displayed, alongside the actual observed frequency, in (Table 2). Chi-squared test of these observed and expected numbers is not significant (p=1). In other words, the observed RIG 30 day mortality is statistically indistinct from predicted random variation. A Poisson probability distribution assumption predicts a probability that more than two patients die within 30 days of gastrostomy in any month is 1.3%.
Table 2: Observed and expected (based on assumed Poisson probability distribution) frequency of number of 30 day mortality patients each month.
| Patients dead at 30 days | Observed frequency | Expected frequency |
|---|---|---|
| 0 | 50 | 48 |
| 1 | 20 | 23 |
| 2 | 6 | 6 |
| 3 | 2 | 1 |
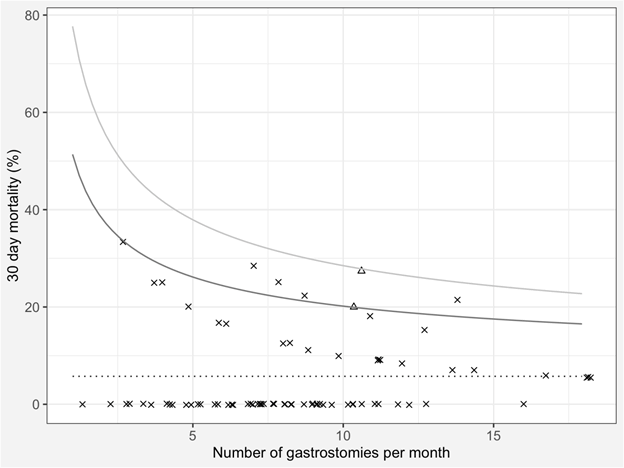
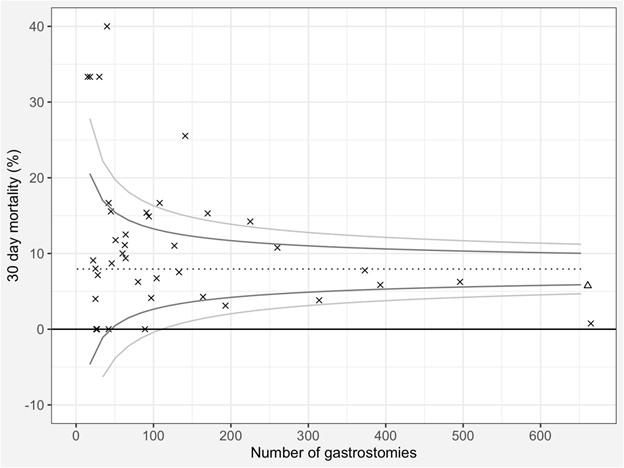
The RIG 30 day mortality for each month is shown as a funnel plot in (Figure 1). 39 published series with 30 day mortality data were found in the literature. Added to our data, they comprise 5711 patients with 7.9% 30 day mortality (454 deaths) and are shown in (Figure 2).
Figure 1: Funnel plot of monthly 30 day mortality after radiologically inserted gastrostomy. The dotted line represents the benchmark/mean, the dark grey line represents two and the pale grey line three standard deviations from the benchmark/mean. The two months of concern are plotted as triangles, the remainder are an ‘x’. Random jitter has been applied along the x axis to reduce the overlap of individual points.
Figure 2: Funnel plot of 30 day mortality after radiologically inserted gastrostomy from 39 published series and the current series. The dotted line represents the benchmark/mean, the dark grey line represents two and the pale grey line three standard deviations from the benchmark/mean. The current series is plotted as a triangle, the remainder are an ‘x’.
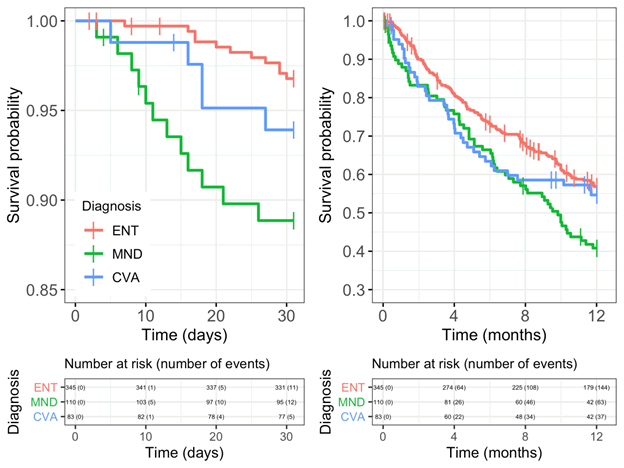
Survival analysis
30 day and one year Kaplan Meier survival curves for ENT, MND and CVA are shown in (Figure 3). At 30 days and one year, pairwise log-rank test shows the difference between ENT and MND is statistically significant (p=0.0023 and p=0.0082 respectively) while the differences between ENT and CVA and between MND and CVA are not statistically significant.
Discussion
In this paper we have explored methods to objectively assess changes in a common and important outcome: 30 day mortality. We believe that funnel plots are a simple and, importantly, easily understood method for assessing, visualising and communicating these data. Their use is perhaps limited by a perception that their primary purpose is to compare institutional or even operator performance [18]. However, their methodology allows for their use in many other comparisons, in this case a change in outcome over a period of time.
To compare an outcome between groups, clinical trials are the gold standard in medical research. However, prospective analysis, blinding and randomisation would be impossible in the context of RIG 30 day mortality. Moreover, we calculated that a group of 116 patients would be needed to have a 95% chance of detecting a 10% rise in 30 day mortality compared to the control group of our current dataset of 661 patients. The maximum number of patients who had gastrostomy in any given month was 18; too small for a valid clinical trial.
Assuming Poisson distribution, we calculated the expected frequency of zero to three RIG 30 day mortality cases in a month. When compared to our observed monthly RIG 30 day mortality, the values were statistically indistinguishable. In other words, our 30 day mortality is indistinguishable from random distribution.
The simplicity of this calculation is its main benefit, but there are drawbacks to Poisson probability statistics. As discussed, it takes no variable into account other than the mean frequency of the outcome being assessed. In these data, our denominator (number of patients undergoing gastrostomy each month) was also highly variable, including two months in which fewer than three gastrostomies took place, invalidating any prediction of greater than two deaths occurring those months. For this reason, Poisson probability is perhaps better reserved for rare outcomes in large, stable populations (for example daily number of homicides in England and Wales) [14]. Additionally, while a Chi-squared table is familiar, Poisson probability may require explanation which is counter to our aim of quick and simple communication of information.
When each month’s number of gastrostomies and 30 day mortality rate are displayed on a funnel plot, the results are immediately visible. There is no significant outlier (above three standard deviations) in terms of high mortality. While both of the months which concerned our clinicians were on or above two standard deviations from the mean, there was a further five months when this was also true, and no concerns were ever raised about them. The fact that the months of concern were consecutive is a likely reason they caught the attention of the clinicians when the others did not. “We are far too willing to reject the belief that much of what we see in life is random [19].
For a wider perspective, we compared our data to 39 published data sets [7,11,12,20-55]. Ours is the second largest, and 30 day mortality compares favourably at around two standard deviations below the mean 30 day mortality of 7.9%. There were eight outliers above three standard deviations, half of which were small series below 50 patients, but the most striking outlier is the publication with the largest number of patients which reports well below three standard deviations of the mean 30 day mortality [50]. This seems to violate the Law of Large Numbers [14]. An explanation may be the fact that of all the publications, this is the only one in which its patients were voluntarily submitted to the researchers (the other publications largely being consecutive series) and so it was subject to selection bias.
A simple criticism would be that both funnel plots presented have made no attempt to correct for case mix. Our local survival data would suggest that if MND patients were over represented, 30 day mortality may appear higher. This was a deliberate decision. One of ours aims was simple data analysis and quick visualisation. Leaving the data ‘raw’ satisfies this aim. Moreover, in Figure 2, the case series with the fourth highest number of patients on the plot had a case mix with close to 90% neurological disease and yet their reported mortality remains close to the mean, perhaps showing that the effect of large numbers can balance a skewed case mix. There is an even simpler and more reason why we have not corrected our data before visualising it. Our funnel plots contain no judgement, blame or conclusions. They are not being used as tools in a meta-analysis or systematic review. They are smiply visualising data and allowing the observer to quickly assess if there is something worthy of investigation. Seeing a significant outlier would be the start of an investigation, not the end.
Another limitation is that we presented no root cause analysis into the cause of death of the patients in the months which raised concern with our clinicians. One of the responses to the concerns raised was to present all RIG 30 day mortality at the monthly departmental morbidity and mortality meeting for one year. In that year, no direct procedure related 30 day mortality occurred. While this does not mean it had not happened in the past, it is at least somewhat reassuring.
There may also be a perception that this exercise has little applicability outside interventional radiology or even outside the setting of gastrostomy practice. However, the methods presented can be applied to myriad outcomes rate of positive CTPA against source of referral, radiation dose during barium swallow by operator or monthly rate of pneumothorax after lung biopsy, for example. With these methods at your disposal, true, impartial feedback is available which is essential for meaningful self-reflection and institutional progress.
Conclusion
Apophenia is an all too human tendency to see patterns or correlations when there are none. When increased mortality is what you see, it is understandable that you want to act now for the good of your patients. It is therefore vital that we have methods to quickly and objectively analyse such observations and decide whether action is really required. Funnel plots are easy to produce and immediately understandable, with simple axes and readily explainable annotations (ie. the mean and curves). They also avoid the stigma of rankings or league tables. Their use should not be limited to institutional or operator comparisons, nor to gastrostomy, but offer a straightforward tool to visualise any outcome.
References
- Preshaw RM (1981) A percutaneous method for inserting a feeding gastrostomy tube. Surg Gynecol Obstet 152:658-660.
[CrossRef] [Google Scholar] [Pubmed]
- Simons M, Yeung E, Ho CS (1996) Percutaneous Fluoroscopic Gastrostomy and Transgastric Jejunostomy. Semin intervent Radiol 13:159-167.
- Rosenzweig TB, Palestrant AM, Esplin CA, Gilsdorf RB (1994) A method for radiologic-assisted gastrostomy when percutaneous endoscopic gastrostomy is contraindicated. Am J Surg 168:587-591.
[CrossRef] [Google Scholar] [Pubmed]
- Ho SGF, Marchinkow LO, Legiehn GM, Munk PL, Lee MJ (2001) Radiological Percutaneous Gastrostomy. Clin Radiol 56:902-910.
[CrossRef] [Google Scholar] [Pubmed]
- Thornton FJ, Fotheringham T, Haslam PJ, McGrath FP, Keeling F, et al (2002) Percutaneous Radiologic Gastrostomy With and Without T-Fastener Gastropexy: A Randomized Comparison Study. CardioVasc Intervent Radiol 25(6):467-471.
[CrossRef] [Google Scholar] [Pubmed]
- Perona F, Castellazzi G, De Iuliis A, Rizzo L (2010) Percutaneous Radiologic Gastrostomy: A 12-Year Series. Gut Liver 4:44-49.
[CrossRef] [Google Scholar] [Pubmed]
- Yip D, Vanasco M, Funaki B (2004) Complication Rates and Patency of Radiologically Guided Mushroom Gastrostomy, Balloon Gastrostomy, and Gastrojejunostomy: A Review of 250 Procedures. CardioVascu Intervent Radiol 27:3-8.
[CrossRef] [Google Scholar] [Pubmed]
- Grant DG, Bradley PT, Pothier DD (2009) Complications following gastrostomy tube insertion in patients with head and neck cancer: A prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol 34(2):103-112.
[CrossRef] [Google Scholar] [Pubmed]
- Itkin M, DeLegge MH, Fang JC (2011) Multidisciplinary Practical Guidelines for Gastrointestinal Access for Enteral Nutrition and Decompression From the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, With Endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology 141(2):742-65.
[CrossRef] [Google Scholar] [Pubmed]
- Sutcliffe J, Wigham A, Mceniff N, Dvorak P, Crocetti L, et al (2016) CIRSE Standards of Practice Guidelines on Gastrostomy. CardioVascu Intervent Radiol 39(7):973-987.
[CrossRef] [Google Scholar] [Pubmed]
- McAllister P, MacIver C, Wales C (2013) Gastrostomy insertion in head and neck cancer patients: A 3 year review of insertion method and complication rates. Br J Oral and Maxfac Surg. 51(8):714-718.
[Cross Ref] [Google Scholar] [Pubmed]
- Laskaratos FM, Walker M, Walker M (2013) Predictive Factors for Early Mortality after Percutaneous Endoscopic and Radiologically-Inserted Gastrostomy. Dig Dis Sci 58(12):3558-3565.
[CrossRef] [Google Scholar] [Pubmed]
- Spiegelhalter DJ (2002) Mortality and volume of cases in paediatric cardiac surgery: Retrospective study based on routinely collected data. BMJ 323:1-5.
[CrossRef] [Google Scholar] [Pubmed]
- Spiegelhalter D (2019) Statistics: The Art of Learning from Data. PELICAN.
- A percutaneous method for inserting a feeding gastrostomy tube
- R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
- RStudio Team (2020) RStudio: Integrated Development Environment for R. RStudio, Inc.
- Spiegelhalter DJ (2005) Funnel plots for comparing institutional performance. Stat Med 24:1185-1202.
[CrossRef] [Google Scholar] [Pubmed]
- Kahneman D (2011) Thinking, Fast and Slow. 1st ed. Farrar, Straus; Giroux.
- Ho CS, Yee ACN, McPherson R (1988) Complications of surgical and percutaneous nonendoscopic gastrostomy: Review of 233 patients. Gastroenterology 95(5):1206-1210.
[CrossRef] [Google Scholar] [Pubmed]
- Halkier BK, Ho CS, Yee AC (1989) Percutaneous feeding gastrostomy with the Seldinger technique: Review of 252 patients. Radiology 171:359-362.
[CrossRef] [Google Scholar] [Pubmed]
- O’Keeffe F, Carrasco CH, Charnsangavej C, Richli WR, Wallace S, et al. (1989) Percutaneous drainage and feeding gastrostomies in 100 patients. Radiology. 1989;172(2):341-343.
[CrossRef] [Google Scholar] [Pubmed]
- Saini S, Mueller PR, Gaa J (1990) Percutaneous gastrostomy with gastropexy: Experience in 125 patients. Am J Roentgenol 54(5):1003-1006.
[CrossRef] [Google Scholar] [Pubmed]
- Hicks ME, Surratt RS, Picus D, Marx MV, Lang EV (1990) Fluoroscopically guided percutaneous gastrostomy and gastroenterostomy: Analysis of 158 consecutive cases. Amr J Roentgen 154(4):725-728.
[Cross Ref] [Google Scholar] [Pubmed]
- Deutsch LS, Kannegieter L, Vanson DT, Miller DP, Brandon JC (1992) Simplified percutaneous gastrostomy. Radiology 184(1):181-183.
- Wollman B, D’Agostino HB, Walus-Wigle JR, Easter DW, Beale A (1995) Radiologic, endoscopic, and surgical gastrostomy: An institutional evaluation and meta-analysis of the literature. Radiology 197(3):699-704.
[CrossRef] [Google Scholar] [Pubmed]
- Elliott LA, Sheridan MB, Denyer M, Chapman AH (1996) PEG Is the E necessary? A comparison of percutaneous and endoscopic gastrostomy. Clin Radiol. 1996;51(5):341-344.
[CrossRef] [Google Scholar] [Pubmed]
- Economou G, Lee SH (1996) Radiologically-guided percutaneous gastrostomy: Three-year follow up and literature review. Min Inva Ther Alied Tech 5(4):342-345.
- Ryan JM, Hahn PF, Boland GW, McDowell RK, Saini S (1997) Percutaneous gastrostomy with T-fastener gastropexy: Results of 316 consecutive procedures. Radiology 203(2):496-500.
[CrossRef] [Google Scholar] [Pubmed]
- Wollman B, D’Agostino HB (1997) Percutaneous radiologic and endoscopic gastrostomy: A 3-year institutional analysis of procedure performance. Amr J Roent 169(6):1551-1553.
[CrossRef] [Google Scholar] [Pubmed]
- Szymski GX, Albazzaz AN, Funaki B (1997) Radiologically guided placement of pull-type gastrostomy tubes. Radiology 205(3):669-673.
[CrossRef] [Google Scholar] [Pubmed]
- Dewald CL, Hiette PO, Sewall LE, Fredenberg PG, Palestrant AM (1999) Percutaneous Gastrostomy and Gastrojejunostomy with Gastropexy: Experience in 701 Procedures. Radiology 211(3):651-656.
[Cross Ref] [Google Scholar] [Pubmed]
- Baere T de, Chapot R, Kuoch V (1999) Percutaneous Gastrostomy with Fluoroscopic Guidance: Single-Center Experience in 500 Consecutive Cancer Patients. Radiology 210(3):651-654.
[CrossRef] [Google Scholar] [Pubmed]
- Möller P, Lindberg CG, T Zilling (1999) Gastrostomy by Various Techniques: Evaluation of Indications, Outcome, and Complications. Scand J Gastroenterol 34(10):1050-1054.
[CrossRef] [Google Scholar] [Pubmed]
- Funaki B, Zaleski GX, Lorenz J (2000) Radiologic Gastrostomy Placement: Pigtail- Versus Mushroom-Retained Catheters. Amr J Roentgenol 175(2):375-379.
[CrossRef] [Google Scholar] [Pubmed]
- Funaki B, Peirce R, Lorenz J (2001) Comparison of Balloon- and Mushroom-Retained Large-Bore Gastrostomy Catheters. Amr J Roentg 177(2):359-362.
[CrossRef] [Google Scholar] [Pubmed]
- Deurloo EE, Schultze Kool LJ, Kröger R, Coevorden F, Balm AJM (2001) Percutaneous radiological gastrostomy in patients with head and neck cancer. Europ J Surg Onco 27(1):94-97.
- Dinkel HP, Beer KT, Zbären P, Triller J (2002) Establishing radiological percutaneous gastrostomy with balloon-retained tubes as an alternative to endoscopic and surgical gastrostomy in patients with tumours of the head and neck or oesophagus. Br J Radiol 75(892):371-377.
[CrossRef] [Google Scholar] [Pubmed]
- Bazarah SM, Al-Rawas M, Akbar H, Qari Y (2002) Percutaneous Gastrostomy and Gastrojejunostomy: Radiological and Endoscopic Approach. Annals of Saudi Medicine 22:38-42.
[CrossRef] [Google Scholar] [Pubmed]
- Neeff M, Crowder VL, McIvor NP, Chaplin JM, Morton RP (2003) Comparison of the Use of Endoscopic and Radiologic Gastrostomy in a Single Head and Neck Cancer Unit: Gastrostomy in Head and Neck Cancer Unit. ANZ J Surg 73(8):590-593.
[CrossRef] [Google Scholar] [Pubmed]
- Chio A (2004) Percutaneous radiological gastrostomy: A safe and effective method of nutritional tube placement in advanced ALS. J Neuro, Neurosur Psych 75(4):645-647.
[CrossRef] [Google Scholar] [Pubmed]
- Silas AM, Pearce LF, Lestina LS (2005) Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: A comparison of indications, complications and outcomes in 370 patients. Eur J Radiol 56(1):84-90.
[CrossRef] [Google Scholar] [Pubmed]
- Rio A, Ann Ampong M, Turner MR (2005) Comparison of two percutaneous radiological gastrostomy tubes in the nutritional management of ALS patients. Amyotr Later Scler 6(3):177-181.
[CrossRef] [Google Scholar] [Pubmed]
- Rustom IK, Jebreel A, Tayyab M, England RJA, Stafford ND (2006) Percutaneous endoscopic, radiological and surgical gastrostomy tubes: A comparison study in head and neck cancer patients. J Laryngol Otol 120(6):463-466.
[CrossRef] [Google Scholar] [Pubmed]
- Eze N, Jefford JM, Wolf D, Williamson P, Neild P (2007) PEG and RIG tube feeding in Head and Neck patients: A retrospective review of complications and outcome. J Eval Clin Pract. 13(5):817-819.
[CrossRef] [Google Scholar] [Pubmed]
- Lewis D, Ampong MA, Rio A (2009) Mushroom-cage gastrostomy tube placement in patients with amyotrophic lateral sclerosis: A 5-year experience in 104 patients in a single institution. European Radiology 19(7):1763-1771.
[CrossRef] [Google Scholar] [Pubmed]
- Blondet A, Lebigot J, Nicolas G (2010) Radiologic versus Endoscopic Placement of Percutaneous Gastrostomy in Amyotrophic Lateral Sclerosis: Multivariate Analysis of Tolerance, Efficacy, and Survival. J Vasc Interv Radiol 21(4):527-533.
[Cross Ref] [Google Scholar] [Pubmed]
- Leeds JS, McAlindon ME, Grant J, Robson HE, Lee FKT, et al (2010) Survival analysis after gastrostomy: A single-centre, observational study comparing radiological and endoscopic insertion. Eur J Gastroenterol Hepatol 22(5):591-596.
[Cross Ref] [Google Scholar] [Pubmed]
- Pruthi D, Duerksen DR, Singh H (2010) The Practice of Gastrostomy Tube Placement Across a Canadian Regional Health Authority. Am J Gastroenterol 105(7):1541-1550.
[Cross Ref] [Google Scholar] [Pubmed]
- Lowe AS, Laasch HU, Stephenson S (2012) Multicentre survey of radiologically inserted gastrostomy feeding tube (RIG) in the UK. Clin Radiol 67(9):843-854.
[CrossRef] [Google Scholar] [Pubmed]
- La Nauze RJ, Collins K, Lyon S (2012) Outcomes of percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy tube insertion at a tertiary hospital. Clin nutr 7(4):e144-e148.
- Power S, Kavanagh LN, Shields MC (2013) Insertion of Balloon Retained Gastrostomy Buttons: A 5-Year Retrospective Review of 260 Patients. Cardiovasc Intervent Radiol 36(2):484-491.
[CrossRef] [Google Scholar] [Pubmed]
- Allen JA, Chen R, Ajroud-Driss S (2013) Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: A retrospective study of complications and outcome. Amyotrophic Lateral Sclerosis and Frontotemporal Degenerationm 14(4):308-314.
[CrossRef] [Google Scholar] [Pubmed]
- ProGas Study Group (2015) Gastrostomy in patients with amyotrophic lateral sclerosis (ProGas): A prospective cohort study. Lancet Neuro 14(7):702-709.
- Delf J, Jepson S, Ramachandran S (2020) Predictors for 30-day mortality and complications following radiologically inserted gastrostomies: A single centre, large cohort review. Clin Radiol 75:375-382.
Citation: Hennessy M (2022) Exploring 30 day Mortality Variability in the Context of Radiological Gastrostomy: How Important Are Month to Month Differences? J Gastrointest Dig Syst.12:678. DOI: 10.4172/2161-069X.1000678
Copyright: © 2022 Hennessy M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3523
- [From(publication date): 0-2022 - Dec 15, 2025]
- Breakdown by view type
- HTML page views: 2857
- PDF downloads: 666