Exploring Lifetime Experiences of People with Breast Cancer: A Cross-Sectional Study
Received: 03-Mar-2021 / Accepted Date: 17-Mar-2021 / Published Date: 24-Mar-2021 DOI: 10.4172/aot.1000160
Abstract
This cross-sectional study explored the relationship between childhood and adult life events, emotional and psychological experiences and breast cancer status. Participants were 2041 women between the ages of 35-90 in the United States-1041 breast cancer patients (cases) and 1000 women who had not had breast cancer (controls). Participants completed a survey on life events and physical and emotional trauma in childhood and adulthood. The data were analyzed with inferential components using primary logistic regression, forward and backward stepwise regression, lasso, conditional inference tree and a random forest. For all models, the association between age at first live birth, major health problems, diethylstilbestrol use, hormone therapy, education, income and race and breast cancer status was consistently significant and they were selected as important predictors for all regression models. Emotional neglect (age 0-7), physical neglect (age 8-18), sexual abuse (age 0-7), experiencing a fire or explosion (age 8-18), exposure to a toxic substance as an adult, assault with a weapon as an adult, severe human suffering as a child (age 8-18 and 19-90) and a competitive environment in childhood and adulthood were associated with increased breast cancer odds. Two life events--a competitive environment and severe human suffering-demonstrated a relationship of first occurring as a child, then again as an adult, with a subsequent breast cancer diagnosis. Overall, the results suggest that adverse events in childhood that are then experienced again in adulthood may increase breast cancer risk. While the study is exploratory and correlative and results should be viewed and interpreted as such, the results warrant further research. These results suggest that emotional and psychological factors should be considered when developing preventative breast cancer strategies.
Keywords: Breast cancer trauma; Adverse events; Psychology competition; Emotional stress; Cancer prediction
Introduction
Breast cancer is the most common cancer in women globally. The World Health Organization’s International Agency for Research on Cancer reports that almost 2.1 million new breast cancer cases were diagnosed in 2018, with over 626,000 deaths in the same year. As survival rates improve, increasing numbers of breast cancer survivors struggle with the lingering physical and psychological side effects from the disease and its invasive treatments [1].
While the individual, social and global burden of disease looms large, research has made advances in treatment and identifying risk factors. Extensive research has been conducted which establishes demographic, lifestyle, and high-risk biological factors for breast cancer. These factors include race [2], Body Mass Index (BMI) [3], hormonal factors and hormonal replacement therapies [4], earlier menarche or later menopause [5], nulliparity, increasing age at first pregnancy [6], personal and family history of breast cancer [7], and genetic factors, among others.
Researchers have also investigated what psychological factors might be correlated with breast cancer incidence and results have been mixed. An early study looking at “cancer-prone” personality traits found no difference between breast carcinoma subjects and controls based on measures of mature, immature, and neurotic defense style; locus of control of behavior; emotional expression-in, emotional expression-out, and emotional control; self-esteem; anxiety; or depression [8]. More recently, a larger and longer prospective study in Japan (n=29,098) found that having “ikigai” (a Japanese word meaning something that makes one’s life worth living), decisiveness, ease of anger arousal, and perceived stress were not associated with developing breast cancer [9]. A second study followed 15,107 Japanese women over 17 years and again found no significant association between personality (extraversion, neuroticism, psychoticism and honesty) and cancer incidence [10]. Both studies concluded that personality is not significantly correlated with breast cancer development and progression, further supported by the lack of association found between extraversion or neuroticism and cancer in a large Swedish and Finnish study [11]. However another large study did find an association between personality traits like “suppressed emotional expression” and “rational/anti-emotional” hostility and cancers [12].
Links between early life traumas and later cancer development have also been explored. Extensive research has explored links between Adverse Childhood Experiences (ACEs) and many harmful outcomes [13]. ACEs represent a child’s exposure to adverse events, including abuse (physical, emotional, and sexual), parental absence, domestic violence, mental or substance abuse illness in the home [14]. Many of the harmful outcomes of ACEs are interrelated rather than independent [15] and include psychological problems such as depression [16] and physical manifestations of illness such as cardiovascular disease and diabetes [17] and, most germane to this discussion, cancer [13-17]. How these various illnesses manifest in ACE survivors’ lives is also multifactorial, such as the intersecting issues of poverty, environmental exposures, and lack of access to healthcare. ACEs are also associated with lower odds of cancer screening later in life [18], resulting in delayed detection of cancers, which are then harder to treat and lead to higher mortality rates. In general, stress alters neurobiological processes during development [19] and can lead to an adult phenotype characterized by heightened stress responses, which in turn activate pro-inflammatory tumor-promoting activities in the body [20].
While many studies have investigated the association between these adverse childhood events and cancer, few have investigated a potential relationship between adult traumatic events and cancer incidence. One recent study found a strong association between adult trauma and the risk of bilateral oophorectomy [21] and another found an association with sexual assault and gynecological symptoms leading to hysterectomy [22]. However, no study has evaluated relationships between childhood traumatic events, adult traumatic events, and breast cancer as far as we know.
This project builds on the breast cancer predictor literature. This study’s primary objective was to evaluate, in an exploratory fashion, the relationship between childhood and adult emotional and psychological experiences and breast cancer through a cross -sectional survey in women who have or have not had breast cancer. We hypothesized that childhood trauma re-experienced as an adult might predict breast cancer. We also examined the relationship between cancer incidence and childhood and adult traumas independently.
Materials and Methods
Study design
An anonymous cross-sectional survey of 2041 women in the United States who have (1041 cases) and have not (1000 controls) had breast cancer.
Setting
The survey was administered online through the Survey Monkey platform, which allows for HIPAA compliant data collection and provides detailed tracking of invitations and survey completion. Case surveys were collected from November 8, 2018, to December 10, 2018, and control surveys were collected from December 10, 2018 to January 25, 2018.
Eligibility criteria determined by self-report
Participants in both groups had to be between 35 and 90 and willing to complete a 30-minute anonymous survey. Cases had to have current or past breast cancer diagnosis but no other cancer types. Controls had no breast cancer history or family history and no other cancer history. Controls were recruited to match age and race with 5% variance to breast cancer cohort to reduce selection bias. These criteria were assessed through items inthe survey.
Recruitment
We contracted with Lucid, LLC (New Orleans, Louisiana) to obtain completed surveys. LUCID collects data directly from targeted audiences through surveys and cross-media measurement. LUCID marketed to potential participants who completed an online screening questionnaire with inclusion/exclusion criteria. Eligible participants were then routed through the LUCID platform to the Survey Monkey survey. The participants were compensated directly through the affiliate marketing contractor whom LUCID has partnered with to obtain survey participants. Breast cancer participants were paid approximately $18, and controls were paid approximately $5 to complete the survey. These payment amounts are approximate and differ between cases and controls because the exact amounts paid are different according to the supplier LUCID partners with to obtain participants. Additionally, cases were compensated at a higher level as they are more difficult to recruit than controls. Lucid, LLC (New Orleans, Louisiana) was contracted to obtain completed surveys directly from targeted potential participants.
Consenting procedures
The informed consent was the first survey page where participants had to acknowledge: 1) “I have read and understand the information about this study” and 2) “I would like to participate in this study” to continue. The Institute of Noetic Sciences Institutional Review Board (IRB ID#: WAHH_2018_04) approved all activities.
Measures
The survey was developed to include questions about lifetime trauma, including physical, emotional, and energy trauma experiences in early and adult life. It also included questions about breast cancer risk, family history, lifestyle factors, and breast cancer diagnosis and treatment. The questions/measures included in the survey were as follows:
Demographics: Age, race, relationship status, years of formal education, annual household income, state of residence, setting.
General health and lifestyle: Basal metabolic index, reproductive health (age of first menses, age of first live birth); first-degree relatives with breast cancer, hormone therapy history, diethylstilbestrol (DES) history, and other chronic diseases and exercise.
Breast cancer history: Time since the first diagnosis, age at diagnosis, method of breast cancer discovery, state of cancer when diagnosed, involved breast(s), lymph node involvement, cancer treatment, current treatment (if applicable), remission status, recurrence (if applicable), last mammogram, and BRCA1 or BRCA2 status were asked of the cases only.
Childhood Trauma Questionnaire (CTQ) short form: A 28-item scale evaluating maltreatment histories in both clinical and non-referred groups [23]. It results in five subscales of emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. An additional component was added to the questionnaire to ascertain time frames for when the trauma occurred. For each question, participants were asked to “Please choose HOW OFTEN you experienced these growing up as a child and a teenager. If you experienced the item, please choose Yes or No if you were 0-7 years old and/or 8-18 years old. Then, please choose Yes or No if you had a similar experience as an adult from 19-90 years old”.
Adverse Childhood Experience (ACE) questionnaire item #7: The ACE Questionnaire is a 10-item self-report measure developed for the ACE study to identify childhood experiences of abuse and neglect [17]. Only item #7 was included to reduce duplication from items included in other scales. “Your mother or stepmother was often pushed, grabbed, slapped, or had something thrown at her? Or sometimes or often kicked, bitten, hit with a fist, or hit with something hard? Or ever repeatedly hit over at least a few minutes or threatened with a gun or knife?” If applicable, the participant was then asked what age the experience occurred in childhood and if a similar experience happened in adulthood.
Life Events Checklist (LEC): The LEC is a brief, 17-item, selfreport measure designed to screen for potentially traumatic events in a respondent’s lifetime [24]. The LEC assesses exposure to 16 events known to potentially result in PTSD or distress and includes one item assessing any other extraordinarily stressful event not captured in the first 16 items. For each item, the respondent checks whether the event (a) happened to them personally, (b) they witnessed the event, (c) they learned about the event, (d) they are not sure if the item applies to them, and (e) the item does not apply to them. The LEC was developed concurrently with the Clinician-Administered PTSD Scale but has demonstrated good psychometric properties as a stand-alone assessment of traumatic exposure. Two life events were added to the Life Events Checklist due to feedback from volunteers during the testing phase of the survey: Death of a loved one; Divorce and/or major break in relationships. If applicable, the participant was then asked at what age the experience occurred in childhood and if a similar experience happened in adulthood.
Post Traumatic Growth Inventory Short-Form (PTGI-SF): The PTGI-SF is a 10-item questionnaire asking about transformative growth from traumatic life experiences [25]. Respondents are asked to rate whether the questions apply to them on a Likert Scale (no change, very small degree, moderate degree, great degree, very great degree). Items are summed for a total score, with higher scores reflecting greater positive change due to the experience. The scale results in sub scores for I. Relating to others, II. New Possibilities, III. Personal Strength, IV. Spiritual Change and V. Appreciation of Life and a total score. Unfortunately, due to a coding error, the last three items of this scale were not included in the survey. Thus, only the subscales of new possibilities (items 1 and 2) and Appreciation of Life (items 3 and 6) are included in the analysis.
Institute of Noetic Science Items (INS): Additionally, the survey included questions developed by the study team addressing emotional, psychological, and energetic trauma (of a controlling, neglectful or abusive nature), which were not covered in the other questionnaires.
1. I experienced bullying in school or my community when I was growing up.
2. I experienced religious, spiritual or political persecution while growing up.
3. I felt a sense of isolation, loneliness and being undervalued.
4. I felt that people around me were impulsive and domineering.
5. I didn’t feel like people had sympathy or concern for my suffering.
6. I felt that people were always focused on what I did or my performance rather than who I really was.
7. I had a very tortured relationship with my mother or mother figure.
8. I was very competitive and felt that I had to win in order to survive.
9. My environment was very competitive and performance and appearance meant everything.
10. Women and girls were not as important as and less valuable than men.
11. My thoughts and opinions were not honored or acknowledged.
12. I felt like I was supported and could work with others.
13. I had a very tortured relationship with my father or father figure.
If applicable, the participant was then asked at what age the experience occurred in childhood and if a similar experience happened in adulthood.
Statistical methods
All computations and statistical analyses were performed using the statistical programming language R v. 3.6.1 (R Core Team, 2019).
Sample size: Sample size was determined by evaluating the estimated prevalence of child abuse and traumatic events for US general population adults, examining previous cross-sectional and case-control studies on this general topic [26,27]. Two-thousand women as a feasible participant number was chosen based on time, budget, feasibility, and the research question.
Data processing: CTQ subscales were generated by summing how often items were experienced in childhood across subscale components (Emotional Abuse, Emotional Neglect, Physical Abuse, Physical Neglect, and Sexual Abuse). Age-based subscales (0-7, 8-18, 19-90 years) were generated by summing how often items were experienced in each age range across subscale items. INS and LEC items asking about occurrence were re-defined into a single variable with the following levels: Happened as a child (0-7 years) only, Happened as a child (8- 18 years) only, Happened as an adult (19-90 years) only, Happened as a child (0-7 years) and again as an adult (19-90 years), Happened as a child (8-18 years) and again as an adult (19-90 years), Happened as a child (both 0-7 and 8-18 years) and again as an adult (19-90 years), Happened as a child (both 0-7 and 8-18 years) but not as an adult (19- 90 years) and did not happen. Did not happen was set as the baseline for interpretive purposes. The LEC items included questions with binary responses, specifically whether the event “Happened to me”, “Witnessed it”, “Learned about it”, “Part of my job”, “Not sure”, “Doesn’t apply” and “Decline to Answer/Disclose”. “Part of my Job” responses were included as provided in model 6. The remaining questions were assigned a value and summed to create a scale that reflects the impact the event had on the subject. Specifically, “Happened to me” was assigned 3, “Witnessed it” was assigned 2, “Learned about it” was assigned 1 and the remaining three were assigned 0. Therefore, if a subject only witnessed an event, the score was 2. If a subject experienced an event (“Happened to me”) and learned about it, the score was 4. This resulted in an ordinal variable taking on values from 0 to 6. These independent ordinal variables were included in the logistic regression as polynomial contrasts.
Demographic, lifestyle and breast cancer history: First, demographic variables, general health and lifestyle and breast cancer history measures were described qualitatively (percentages endorsed for categorical variables and means and standard deviations for continuous variables). A primary logistic regression was then fit, including only demographic variables as explanatory variables and breast cancer status as the response.
Statistical analysis plan: The analysis objective was to examine the relationship between childhood and subsequent adult trauma and its relationship to breast cancer status. This was accomplished with two types of analysis. 1) Fit models that allow for inference about independent variables and their strength as regressors using logistic regression models. 2) Identify the question-specific and overall (fullsurvey) variables that best predict breast cancer status using forward and backward stepwise selection and fitting a lasso for each logistic regression model, conditional inference trees, and a random forest fitted on the full data set. It was not a goal to quantify predictive models’ capability, only to explore important predictors in the context of the scientific questions and the complete survey data set.
1. Fit models that allow for inference about independent variables and their strength as regressors. Trauma variables that explain or predict breast cancer status in adulthood: To evaluate the effect of various childhood trauma on the status of breast cancer and if childhood events subsequently experienced in adulthood influenced the status of breast cancer in adulthood, logistic regression models were performed. Because of the number of trauma variables, 15 separate logistic regression models were conducted:
Model 1: Effect of childhood trauma (ctq) on status of breast cancer: Does the frequency of childhood trauma (CTQ) explain breast cancer status, controlling for demographic variables? Which of these are important predictors?
Model 2: Effect of childhood trauma (ins/acs07) on status of breast cancer: Does the frequency of certain life experiences (INS and ACS07) explain breast cancer status, controlling for demographic variables? Which of these are important predictors?
Model 3: Effect of trauma (ctq age-based) at specific ages on status of breast cancer: Does the occurrence of childhood trauma (CTQ age - based) and subsequently in adulthood explain breast cancer status, controlling for demographic variables? Which of these are important predictors? Does the repetition of similar trauma in adulthood predict breast cancer status?
Model 4: Effect of trauma (ins/acs07 age- based) at specific ages on status of breast cancer: Does the occurrence of childhood trauma related to family relationships (INS7, 13; ACS7, limiting environment (INS 1, 2, 6, 8 ,9) and psychological well-being (INS3-5, 10-12) and subsequently experiencing similar trauma in adulthood explain breast cancer status, controlling for demographic variables? Which of these are important predictors? Does the repetition of similar trauma in adulthood predict breast cancer status?
Model 5: Effect of trauma (lec age-based) at specific ages on status of breast cancer: Does the occurrence of natural disaster and environmental accidents (LEC 1-5), violence (LEC 6-9), combat, imprisonment, severe suffering (LEC 10-13) or indirect suffering (LE C 14- 17) in childhood and subsequently experiencing similar trauma in adulthood explain breast cancer status, controlling for demographic variables? Which of these are important predictors? Does the repetition of similar trauma in adulthood predict breast cancer status?
Model 6: Effect of job experience (lec part of job) on status of breast cancer: Do the occurrences of certain life experiences as part of a subject’s job explain the status of breast cancer, controlling for demographic variables? Which are important predictors?
Model 7: Effect of type of trauma exposure (lec impact) at specific ages on status of breast cancer: Does the impact of certain life experiences (“Happened to Me”, “Witnessed It”, etc. expressed as an impact on a scale from 0-6) explain the status of breast cancer, controlling for demographic variables? Which are important predictors?
For each model, variables were examined visually for separability. The variance inflation factors (VIFs) were then generated using the R package car [28], and regressors with VIF exceeding five were removed one at a time until all regressors had VIFs below 6. The logistic regression was then fit with the remaining regressors. Logistic regression diagnostics and model assumptions were evaluated using the R package tools DHARMA [29]. The fitted model results were exported as a table of estimated coefficients, standard errors, and z and p values.
2a. Identify the question-specific variables that best predict breast cancer status.
Forward and backward stepwise regression was performed on each model using the step AIC procedure in the R package MASS [30]. Models selected by the forward/backward stepwise procedure were denoted in the fitted model results table. The lasso was performed on each model using the R package glmnet [31]. The lasso is a linear model regularization method that has the effect of variable selection by ‘shrinking’ coefficients down to 0, effectively eliminating them from the model, by minimizing the residuals sum of squares subject to the L1 penalty [31,32]. The complexity parameter (λ) determines the amount of shrinkage selected by cross-validation by the function cv.glmnet. Since the folds for cross-validation are selected randomly every time the procedure is employed, the ‘best’ value of λ may change from iteration to iteration. Friedman et al. [31] suggest averaging over error curves to select a final ‘best’ λ. This procedure was employed using 100 iterations to select λ and fit a final lasso. Variables selected by this lasso were denoted in the fitted model results table. Updated p-values for the stepwise regression and lasso-selected variables are not included, as (1) These steps specifically address the goal of prediction, not inference and (2) this would necessitate post-selective inference [33]. Coefficient estimates are presented, as they define the predictive model.
2b) Identify the overall (full survey) variables that best predict breast cancer status. A conditional inference tree and a randomForest were fitted to the full data set using the R packages party kit [34,35] and random Forest [36] to evaluate the importance of the trauma and life experience variables when examined together. Building a conditional inference tree is done by binary recursive partitioning. Regressors with the strongest relationship to the response variables are selected until no further improvement is demonstrated by testing the global null of independence between the response and predictors [34,35]. The conditional inference tree was generated using α=0.10, the significance level for variable selection. Random forests are decision trees built so that variance is reduced [37]. The random forest of classification trees was grown using the default m=√p variables at each split and 500 trees. Conditional inference tree results were visualized using a plot of the tree and most important random forest variables were visualized in an importance plot.
Results And Discussion
Recruitment resulted in 1,041 cases and 1,000 controls (Table 1).
| Cases | Controls | ||
|---|---|---|---|
| Began Survey | 1490 | Began Survey | 2541 |
| No to consent | 114 | No to consent | 227 |
| Male | 3 | Male | 7 |
| No BrCA | 31 | Yes BrCA/No resp | 170 |
| Other CA | 218 | ||
| Family Hx CA | 630 | ||
| 11 | |||
| Did not continue | 21 | Did not continue | 71 |
| In complete Survey | 284 | In complete Survey | 217 |
| 1041 | 1000 | ||
Table 1: Recruitment numbers for cases and controls.
Demographics, lifestyle variables and breast cancer history
Percentages, means, and standard deviations of demographic variables and lifestyle factors are reported in Table 2. The cases had significantly higher PTGI New Possibilities scores (Controls 6.4 ± 3.1; Case 7.1 ± 2.6, F(1,2038)=32.1, p<0.00005). There was no difference on the PTGI Appreciation of Life score (Control 5.7 ± 3.3; Case 5.9 ± 2.9, F(1,2038)=1.2, p=0.27) (Table 2).
| Factor | Level | Controls | Cases |
|---|---|---|---|
| Age, mean(SD) | 55.5(11.3) | 57.0(11.0) | |
| Race | White or Caucasian | 76.6 | 84.3 |
| African American/black | 10.2 | 8.3 | |
| Hispanic or Latina/Latino | 4.3 | 3.9 | |
| American Indian/Alaskan Native | 1.9 | 1.1 | |
| Asian/Pacific Islander | 4.2 | 1.2 | |
| Multiple | 2.8 | 1.2 | |
| In relationship | Yes | 57.5 | 63.5 |
| Education yrs. mean(SD) | 14.3(2.4) | 14.8(2.5) | |
| Income | Under$30K | 33.6 | 24.4 |
| $30K-< $ 75K | 42.9 | 40.9 | |
| $75K-< $ 100K | 13.5 | 13.4 | |
| $100K-< $ 150K | 6.7 | 13.3 | |
| $150K-< $ 250k | 2.3 | 5.1 | |
| = $ 250k | 0.3 | 1.5 | |
| Declined | 0.6 | 1.3 | |
| Household #, mean(SD) | 14.3(2.4) | 14.8(2.5) | |
| Setting | Rural | 29.3 | 31.3 |
| Suburban | 46.9 | 46.6 | |
| Urban | 23.8 | 22.1 | |
| BMI, mean(SD) | 31.7(10.3) | -8.9 | |
| Exercise | 1 × /week | 12.6 | 9.5 |
| 2-4 × /week | 34.6 | 37.8 | |
| 5-7 × /week | 13.7 | 10.4 | |
| None | 39 | 42.4 | |
| Age at first menses | 12.6(1.7) | 12.4(1.7) | |
| Age at first birth | 23.1(5.7) | 24.5(5.8) | |
| Mother took DES | No | 63.9 | 57.2 |
| Yes | 1.5 | 4.5 | |
| I don't know | 34.6 | 38.3 | |
| Other Chronic Disease | 22.6 | 38.4 | |
BMI=Body Mass Index; DES=diethylstillboestrol
Table 2: Demographic and lifestyle variables for cases and controls.
Breast cancer history for the cases is presented in Table 3. The average time since diagnosis was approximately seven and a half years. Women were in their middle-age when diagnosed, most usually by a mammogram. Most cancers were below Stage 3 and less than 3 cm in size at their largest. The left breast was slightly more involved than the right. About 40% of the women had lymph node involvement. A majority of the women were treated with radiation with a smaller amount treated with chemotherapy, lumpectomy, and mastectomy. Eightyseven percent of the women were in remission. Twelve percent had a recurrence at some point in the past. Only 11% of the women were BRCA positive (Table 3).
| Factor | Level | Value |
|---|---|---|
| Time since diagnosis(months) | 88.8(86.2) | |
| Age at Diagnosis(years) | 49.7(11.2) | |
| Method of First Diagnosis | Manual Exam | 479(46.0%) |
| Mammogram | 720(69.2%) | |
| Ultrasound | 286(27.5%) | |
| Don't know/not sure | 56(5.4%) | |
|
Highest stage ever |
Stage 0(in situ) | 173(16.6%) |
| Stage I | 289(27.8%) | |
| Stage II | 282(27.1%) | |
| Stage III | 166(15.9%) | |
| Stage IV | 75(7.2%) | |
| Largest Tumor Size | Smaller than 1 cm | 157(15.1%) |
| 1-1.9 cm | 203(19.5%) | |
| 2-2.9 cm | 175(16.8%) | |
| 3-3.9 cm | 120(11.5%) | |
| 4-4.9 cm | 42(4.0%) | |
| Larger than 5 cm | 101(9.7%) | |
| Don't know/not sure | 243(23.3%) | |
| Breast affected | Both | 65(6.2%) |
| Left | 508(48.8%) | |
| Right | 468(45.0%) | |
| Lymph Node Involvement | 419(40.2%) | |
| Treatment | Remove tumor | 114(11.0%) |
| Radiation | 618(59.4%) | |
| Lumpectomy | 455(43.7%) | |
| Chemotherapy | 496(47.6%) | |
| Mastectomy | 458(44.0%) | |
| Hormone Therapy | 344(33.0%) | |
| Remission | 907(87.1%) | |
| Recurrence | 127(12.2%) | |
| Time since last mammogram(months) | 28.0(50.9) | |
| BRCA positive | 114(11.0%) | |
Table 3: Breast cancer history of cases.
The logistic regression results examining the relationship between demographics and breast cancer demonstrated that increasing the age at first live birth (β=0.03, p=0.0027) and years of education (β=0.06, p=0.03) slightly increased the odds of breast cancer holding the other covariates fixed. The presence of major health problems (β=0.83, p<0.00005), hormone therapy (β=0.58, p<0.00005) and the use of diethylstilbestrol (β=1.13, p<0.00005) increased the odds of breast cancer holding the other covariates fixed (Table 4 in the Supplementary Material for detailed model results). All other demographics and breast cancer parameters were not significant.
| # | Items | Frequency |
Age event occurred | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-7 | Aug-18 | 19-90 | |||||||
| Ctl | Cse | Ctl | Cse | Ctl | Cse | Ctl | Cse | ||
| 1 | I didn’t have enough to eat | 1.3(0.8) | 1.3(0.8) | 16 | 14.8 | 16.3 | 16.9 | 16.3 | 15.9 |
| 2 | I knew that there was someone to take care of me | 4.3(1.1) | 4.3(1.1) | 92.4 | 90.2 | 87.4 | 83.7 | 77.5 | 77.2 |
| 3 | People in my family called me things like "stupid”, “lazy” or “ugly” | 1.8(1.2) | 1.9(1.3) | 26.7 | 28 | 37.5 | 36.9 | 26.1 | 26.2 |
| 4 | My parents were too drunk or high to take care of the family | 1.4(0.9) | 1.4(0.9) | 16.3 | 16.6 | 16.7 | 18.6 | 9.1 | 11.9 |
| 5 | There was someone in my family who helped me feel important or special | 3.9(1.2) | 3.9(1.3) | 87.9 | 86.3 | 86.8 | 83.5 | 84.2 | 81.9 |
| 6 | I had to wear dirty clothes | 1.3(0.7) | 1.2(0.7) | 12 | 12.5 | 12.5 | 12.2 | 9.2 | 8.8 |
| 7 | I felt loved | 4.0(1.2) | 4.0(1.2) | 88.2 | 86.6 | 81.8 | 77.5 | 83.2 | 83.2 |
| 8 | I thought that my parents wished I had never been born | 1.6(1.2) | 1.6(1.2) | 21.7 | 21.1 | 27.9 | 27.1 | 18.5 | 16.3 |
| 9 | I got hit so hard by someone in my family that I had to see a doctor or go to the hospital | 1.1(0.5) | 1.2(0.7) | 7.1 | 10.4 | 8.8 | 12.1 | 10 | 12.7 |
| 10 | There was nothing I wanted to change about my family | 3.0(1.5) | 2.9(1.4) | 57.9 | 53.9 | 64.4 | 64.3 | 62.3 | 58.9 |
| 11 | People in my family hit me so hard that it left me with bruises or marks | 1.5(1.1) | 1.6(1.1) | 21.3 | 20.7 | 25.6 | 25.6 | 14.8 | 15.6 |
| 12 | I was punished with a belt, a board, a cord (or Some other hard object) | 2.2(1.3) | 2.1(1.3) | 45 | 41.3 | 43.9 | 40.8 | 8.4 | 10.2 |
| 13 | People in my family looked out for each other | 3.8(1.3) | 3.9(1.2) | 85.2 | 86 | 81.9 | 81.7 | 81.5 | 79.5 |
| 14 | People in my family said hurtful or insulting things to me | 2.2(1.3) | 2.3(1.3) | 33.6 | 34.7 | 51.7 | 49.4 | 46.3 | 43.5 |
| 15 | I believe that I was physically abused | 1.7(1.2) | 1.7(1.2) | 22.3 | 20.4 | 25.8 | 25.6 | 22.5 | 19.5 |
| 16 | I had the perfect childhood | 3.0(1.4) | 3.0(1.3) | 68.2 | 70.6 | 58.1 | 56.8 | 55.3 | 56.8 |
| 17 | I got hit or beaten so badly that it was noticed by someone like a teacher, neighbour, or doctor | 1.3(0.8) | 1.3(0.8) | 11 | 11.8 | 13.1 | 14.9 | 13.7 | 13.4 |
| 18 | Someone in my family hated me | 1.7(1.2) | 1.8(1.2) | 20.8 | 22.3 | 27.2 | 29 | 27.7 | 28.2 |
| 19 | People in my family felt close to each other | 3.7(1.3) | 3.7(1.3) | 83.8 | 82.2 | 77.7 | 74.4 | 75.2 | 72.9 |
| 20 | Someone tried to touch me in a sexual way or tried to make me touch them | 1.7(1.1) | 1.7(1.2) | 21 | 22.1 | 32.9 | 32.1 | 20 | 18.5 |
| 21 | Someone threatened to hurt me or tell lies about me unless I did something sexual with them | 1.3(0.8) | 1.3(0.9) | 10.8 | 12.8 | 15.4 | 17.1 | 10.4 | 11.9 |
| 22 | I had the best family in the world | 3.4(1.4) | 3.3(1.4) | 74.9 | 76.6 | 66.1 | 66.9 | 69.1 | 69.8 |
| 23 | Someone tried to make me do sexual things or watch sexual things | 1.5(1.0) | 1.6 (1.1) | 16.4 | 18.3 | 27.1 | 26.3 | 19.4 | 17.9 |
| 24 | Someone molested me (took advantage of me sexually) | 1.6(1.2) | 1.7(1.2) | 18.2 | 20.1 | 26.9 | 26.8 | 16.8 | 15.6 |
| 25 | I believe that I was emotionally abused | 2.2(1.4) | 2.2(1.5) | 32.4 | 32.1 | 43.1 | 43.4 | 43.5 | 40.9 |
| 26 | There was someone to take me to the doctor if I needed it | 4.5(1.0) | 4.5(1.0) | 95.8 | 93.7 | 93.7 | 91.7 | 87.4 | 86.1 |
| 27 | I believe that I was sexually abused | 1.7(1.2) | 1.7(1.3) | 19.2 | 19.9 | 27.2 | 25.9 | 18 | 16 |
| 28 | My family was a source of strength and support | 3.7(1.3) | 3.7(1.3) | 82.7 | 81.7 | 77.1 | 74.4 | 78.2 | 76.4 |
Note: Frequency is denoted as means (standard deviations). Range of frequency variables=1-5. 1=never true; 2=rarely true; 3=sometimes true; 4=often true; 5=very often true. Age values are percentages of total respondents who endorsed the item
Table 4: Childhood Trauma Questionnaire item and subscale values.
All models below control for demographic variables and results assume holding all other covariates fixed. For all remaining logistic regression models, the age at first live birth, major health problems, diethylstilbestrol use, hormone therapy, education, income, and race were consistently selected as important predictors and had very similar coefficient estimates (Tables 1-6 in the Supplementary Material for detailed model results).
Childhood trauma
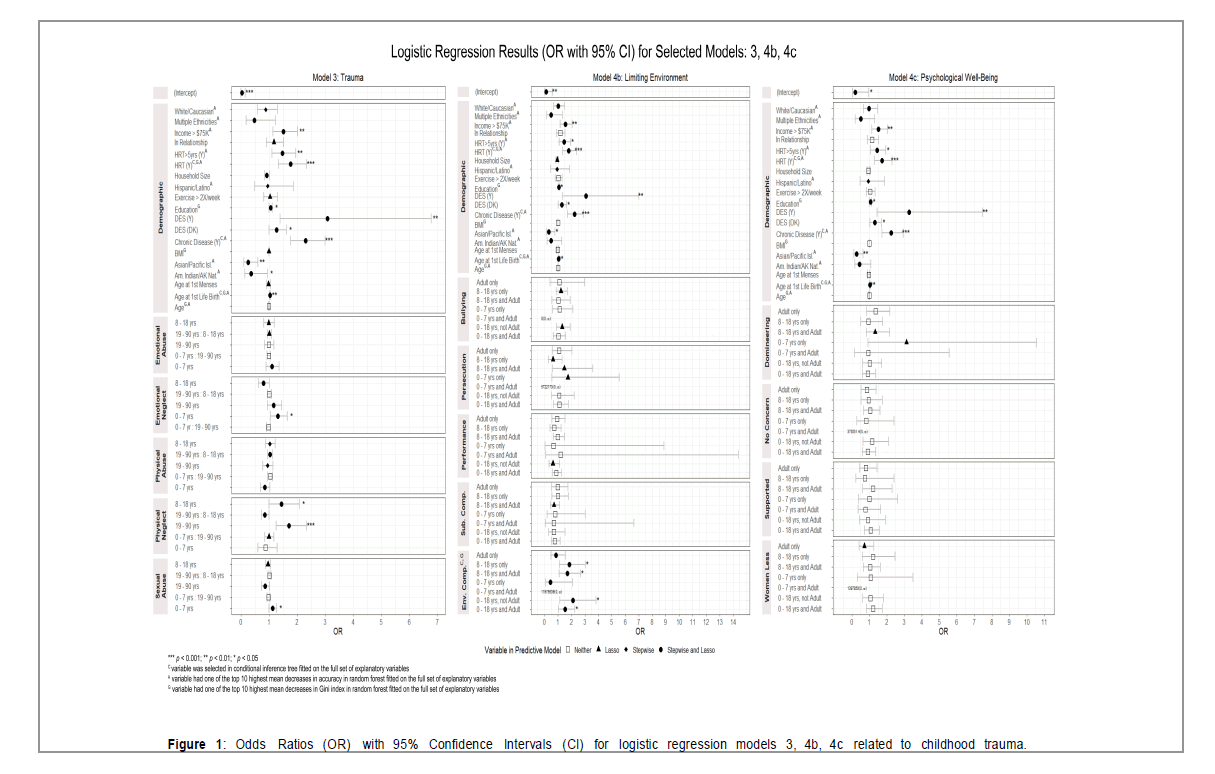
Means and standard deviations of Childhood Trauma Questionnaire (CTQ) items and the number of participants endorsing each item at three age categories are presented in Table 4. CTQ subscale means and standard deviations for controls and cases were as follows: Denial ctl-6.4(2.6), cse-6.2(2.4); Emotional Abuse ctl-7.4(4.3), cse- 7.6(4.2); Emotional Neglect ctl-10.8(5.5), cse-10.9(5.5); Physical Abuse Ctl- 6.7(3.7), cse-6.7(3.9) Physical Neglect ctl-8.4(1.8), cse-8.5(1.8) Sexual Abuse ctl-7.8(4.9), cse-8.0(5.2). These subscales did not explain breast cancer status nor were they significant predictors (Table 1 in the Supplementary Material for detailed model 1 results). The agebased values did have significant findings. Emotional neglect 0-7 was associated with increased odds (OR 1.32, z=2.44, p=0.01) and was a predictor (stepwise regression, lasso). Physical neglect 8-18 (OR 1.45, z=2.00, p=0.05) and 19-90 (OR 1.72, z=3.44, p=0.006) their interaction and sexual abuse 0-7 and 19-90 were predictors (Figure 1 below and Table 3 in the Supplementary Material for detailed model 3 results) (Table 4).
Frequency is denoted as means (standard deviations). Range of frequency variables=1-5. 1=never true; 2=rarely true; 3=sometimes true; 4=often true; 5=very often true. Age values are percentages of total respondents who endorsed the item.
The frequency means, and standard deviations for the INS Items and ACE #7 are displayed in Table 5. The frequency of competitive environment (INS9) was a predictor (lasso). A one-unit increase was associated with an 8% increase in the odds of breast cancer (z=1.51, p=0.13; Table 2 in the Supplementary Material for detailed model 2 results). The age-based values also had significant findings. A. Adverse family experiences (INS#7,13,ACE#7)-INS7/INS13 Having a tortured relationship with the mother and father in childhood and adulthood was a predictor (lasso). B. Environment (INS#1,2,6,8,9)-INS9 Competitive environment was a predictor (stepwise regression, lasso). Childhood 0- 18 and adulthood (OR1.52,z=2.13,p=0.03); Childhood 0-7 only (OR 2.1,z=2.42,p=0.016); Childhood 8-18 and adulthood (OR1.7,z=2.32, p=0.02); Childhood 8-18 only(OR 1.84,z=2.40,p=0.016). C. Adverse psychological experiences (INS#3-5,10-12)-Important lasso predictors were: INS 10 girls and women thought of as less than men as a child 0- 7 and adult and INS 4 having domineering people around you as a child 0-7 and adult. (Figure 1 and Tables 4a-4c, in the Supplementary Material for detailed model 4 results) (Table 5 and Figure 1).
| # | Items | Frequency | Age event occurred | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-7 | Aug-18 | 19-90 | |||||||
| Ctl | Cse | Ctl | Cse | Ctl | Cse | Ctl | Cse | ||
| ACE#7 I witnessed my mother, or mother figure being physically abused in some way (e.g. pushed, grabbed, slapped, repeatedly hit, threatened with a gun or knife)? | 1.4(1.0) | 1.4(1.0) | 17.7 | 19.5 | 17.1 | 20.2 | 10.2 | 9.8 | |
| 1 | I experienced bullying in school or my community when I was growing up | 2.2(1.3) | 2.2(1.3) | 31.9 | 33 | 52.3 | 52.2 | 18.5 | 19.5 |
| 2 | I experienced religious, spiritual or political persecution while growing up | 1.4(0.9) | 1.4(1.0) | 12.2 | 14.7 | 15.2 | 18.3 | 14.7 | 15.6 |
| 3 | I felt a sense of isolation, loneliness and undervalued | 2.5(1.4) | 2.4(1.4) | 32.4 | 34.2 | 55 | 55.5 | 47.8 | 49 |
| 4 | I felt that people around me were impulsive and domineering | 2.1(1.3) | 2.1(1.3) | 29.7 | 32 | 40.8 | 42.7 | 37 | 36 |
| 5 | I didn’t feel like people had sympathy or concern for my suffering | 2.2(1.3) | 2.1(1.3) | 25.6 | 28.3 | 41.2 | 43.3 | 36.9 | 39.9 |
| 6 | I felt that people were always focused on what I did or my performance rather than who I really was | 2.4(1.3) | 2.3(1.3) | 31.1 | 31.6 | 49.3 | 49.4 | 44.7 | 44.3 |
| 7 | I had a very tortured relationship with my mother or mother figure | 2.0(1.3) | 2.0(1.4) | 23.2 | 24.9 | 37.7 | 39.4 | 28.6 | 32.4 |
| 8 | I was very competitive and felt that I had to win in order to survive | 1.9(1.2) | 1.8(1.2) | 21.4 | 19.9 | 33.8 | 33.1 | 30.5 | 30.1 |
| 9 | My environment was very competitive and performance and appearance meant everything | 2.2(1.3) | 2.0(1.2) | 26 | 24.4 | 46.2 | 40.4 | 35.9 | 34 |
| 10 | Women and girls are not as important and less valuable than men | 2.0(1.3) | 2.0(1.3) | 27.3 | 28.7 | 38.6 | 36.6 | 33.5 | 33.3 |
| 11 | My thoughts and opinions were not honoured or acknowledged | 2.4(1.3) | 2.4(1.4) | 38.7 | 36.5 | 51.2 | 50.8 | 43.8 | 44.3 |
| 12 | I felt like I was supported and could work with others | 3.8(1.2) | 3.7(1.3) | 77.5 | 79 | 78.8 | 78.1 | 84.1 | 83 |
| 13 | I had a very tortured relationship with my father or father figure | 1.9(1.3) | 1.8(1.3) | 23 | 25.1 | 33.5 | 36.4 | 22.7 | 24.1 |
Table 5: INS Items and ACE #7 IONS Developed Items (INS) and Adverse Childhood Experience (A CE) questionnaire item #7 values.
Adverse life experiences
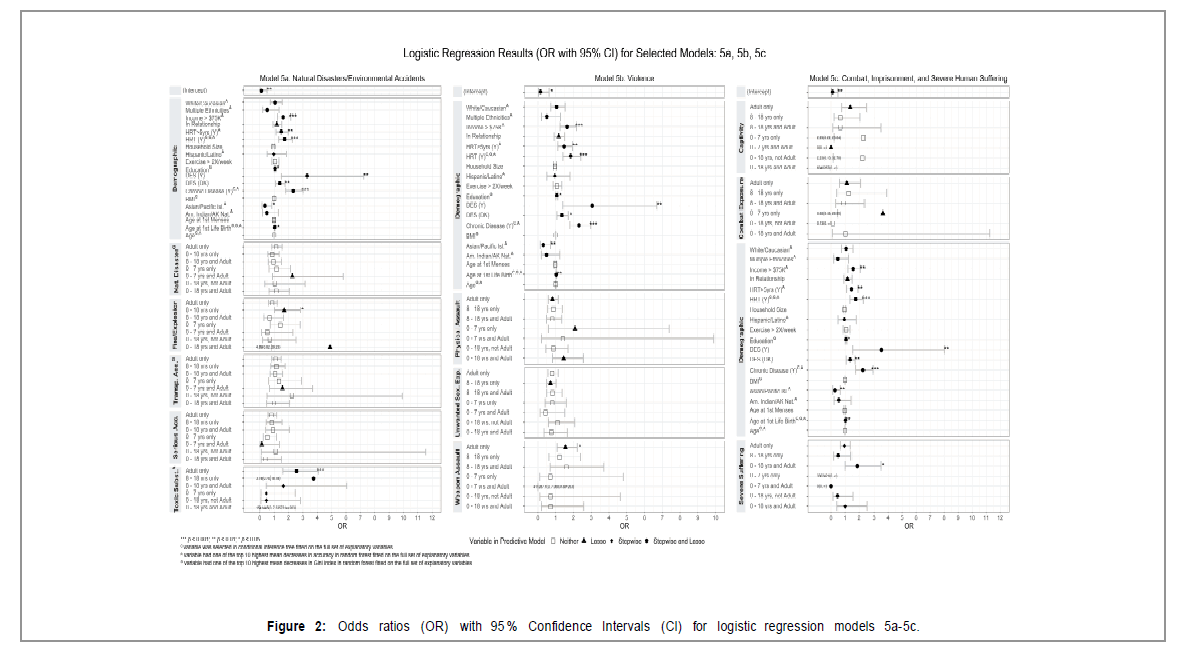
The frequency means, and standard deviations for the Life Events Checklist (LEC) are displayed in Table 6. For the items on natural disasters and/or environmental accidents (LEC#1-5), LEC2 fires or explosions 0-18, adult were significant and lasso-selected predictors (OR 1.7, z=2.03, p=0.04); LEC5 Exposure to a toxic substance 0-18, adult were also (OR 2.6, z=3.94, p=0.0001). For items on Violence (LEC#6-9), LEC7 Assault with a weapon 0-7 and adult (OR 1.55, z=2.39, p=0.017) were significant and lasso-selected predictor. For the items on combat, imprisonment, and severe human suffering (LEC#10-13), LEC13 Severe human suffering as a child 8-18 and adult were significant (OR 1.88, z=1.98, p=0.047) and also predictors in the step wise selection and the predictive lasso models.
For the items on indirect suffering, specifically suffering on account of the life experience of another (LEC#14-17), LEC14 “Sudden violent death experienced as a child and adult,” LEC16 “Serious injury you caused as a child but not an adult,” and LEC17 “Other stressful experiences as a child” were lasso predictors. One part of the LEC asks about adverse events related to the person’s job. The model assessing these variables found that LEC6 Physical assault and LEC13 Severe human suffering were predictors (stepwise regression, lasso).
(Figure 2 and Tables 5a-5d, and 6 in the Supplementary Material for detailed model results) (Table 6 and Figure 2)
| # | Items | Frequency | Age event occurred | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-7 | Aug-18 | 19-90 | |||||||
| Ctl | Cse | Ctl | Cse | Ctl | Cse | Ctl | Cse | ||
| 1 | Natural disaster (for example, flood, hurricane, tornado, earthquake) | 32.6 | 33.9 | 10.3 | 12.4 | 20.4 | 21 | 31.8 | 35.7 |
| 2 | Fire or explosion | 13.2 | 13.9 | 4 | 5.8 | 8.1 | 10.8 | 15.4 | 17.3 |
| 3 | Transportation accident (for example, car accident, boat accident, train wreck, plane crash) | 50.3 | 57.8 | 7 | 8.6 | 23.5 | 27.1 | 42 | 48 |
| 4 | Serious accident at work, home, or during recreational activity | 16.1 | 15.6 | 3.7 | 3.3 | 6.2 | 8.3 | 15.9 | 18.4 |
| 5 | Exposure to toxic substance (for example, dangerous chemicals, radiation) | 4.3 | 10.4 | 1 | 1.4 | 1.5 | 3.3 | 6.1 | 12.6 |
| 6 | Physical assault (for example, being attacked, hit, slapped, kicked, beaten up) | 36 | 35.3 | 10 | 10.8 | 21.2 | 21.4 | 26 | 27.7 |
| 7 | Assault with a weapon (for example, being shot, stabbed, threatened with a knife, gun, bomb) | 13.3 | 14.4 | 1.5 | 1.7 | 4.9 | 5.9 | 14.3 | 17.7 |
| 8 | Sexual assault (rape, attempted rape, made to perform any type of sexual act through force or threat of harm) | 27.7 | 29.7 | 7.7 | 9.6 | 18 | 20.4 | 17.8 | 18.2 |
| 9 | Other unwanted or uncomfortable sexual experience | 37.1 | 36.3 | 9.6 | 9.6 | 22.9 | 22.5 | 22.5 | 23.1 |
| 10 | Combat or exposure to a war-zone (in the military or as a civilian) | 1.2 | 1.5 | 0.6 | 1.2 | 2.4 | 3.3 | 4.8 | 6.9 |
| 11 | Captivity (for example, being kidnapped, abducted, held hostage, prisoner of war) | 4.4 | 3.6 | 0.4 | 1.2 | 1.8 | 2.1 | 4.2 | 5.3 |
| 12 | Life-threatening illness or injury | 18.7 | 60.6 | 3.5 | 4.8 | 8.1 | 6.8 | 24 | 59.2 |
| 13 | Severe human suffering | 8.7 | 10 | 3.3 | 3.8 | 7.1 | 9 | 15.8 | 19.1 |
| 14 | Sudden violent death (for example, homicide, suicide) | 5.2 | 5 | 1.3 | 2.3 | 7.9 | 9.5 | 18.1 | 24.3 |
| 15 | Sudden accidental death | 6.4 | 6.3 | 2.4 | 1.5 | 8.1 | 10.7 | 22.3 | 26.2 |
| 16 | Serious injury, harm, or death you caused to someone else | 1.8 | 2.1 | 0.9 | 0.6 | 1.5 | 1.3 | 3.2 | 4.1 |
| 17 | Any other very stressful event or experience | 38.6 | 47.1 | 8.5 | 8.5 | 16.2 | 17.5 | 39.6 | 44.8 |
Table 6: Life events checklist item frequencies.
Finally, three models evaluated the impact scores of the LEC. For example, participants marked if the event happened to them, they witnessed it or heard about it. When these variables were assessed with breast cancer status, there was a quadratic (z=-2.26, p=0.024) and polynomial (fourth-degree; z=-2.11, p=0.035) relationship between the impact of LEC2 “Fire and explosion” and the log odds of breast cancer and the stepwise model included LEC5 “Exposure to toxic substance.” The stepwise selected predictive model included LEC9 “Unwanted sexual experience” and the cubic and polynomial (4th degree) relationships were present in the lasso model. The lasso models also included multiple non-linear relationships between the LEC variables and breast cancer status.
(Tables 7a-7d in the Supplementary Material for detailed model results).
Evaluating all trauma and adverse life experiences together
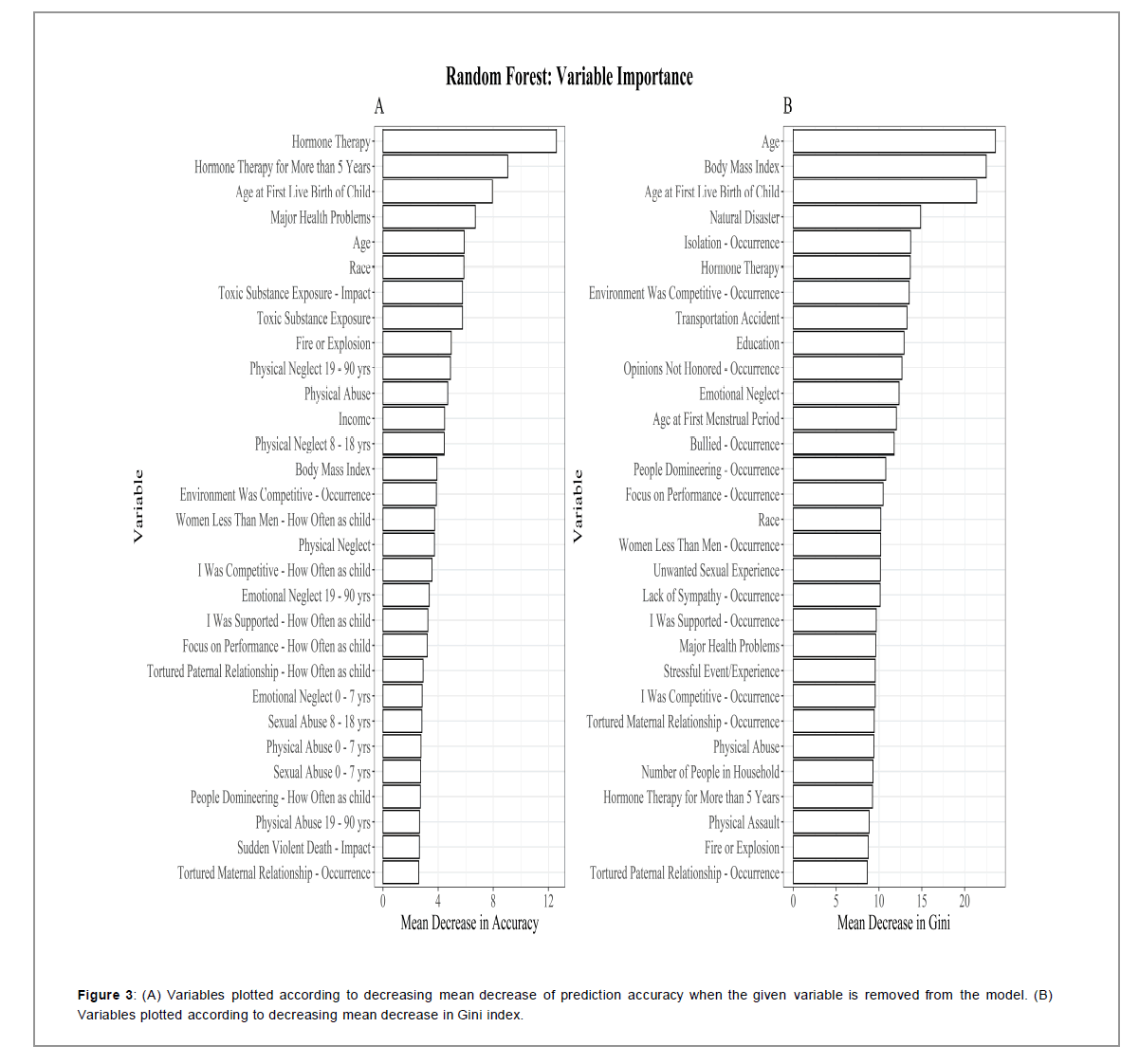
The conditional inference tree’s first binary split was based on hormone therapy. Subjects who took hormone replacement therapy were further split by the presence of major health issues/chronic diseases. Those without major health issues were split based on a competitive environment in childhood, adulthood or both. The tree did not result in any pure nodes. (Figure 1 in the Supplementary Material for detailed model results) [38,39].
Age at first live birth of a child, income, hormone therapy, and education were important variables in random forest construction, similar to the regression models (Figure 3). There was a large decrease in prediction accuracy when hormone therapy and the impact of toxic substance exposure were excluded from the model. Additionally, natural disasters, isolation, opinions not honored, and competitive environment were important in the mean Gini index decreases (Figure 3) [40-42].
Conclusion
This study explored if childhood and adult trauma are predictors for developing breast cancer. Trauma is multifaceted, with physical and emotional components, and our survey captured information across trauma’s many dimensions.
When examining the effect of demographic variables on breast cancer status, we found that age at first live birth, major health problems, DES use, hormone therapy, education, income, and race were consistently selected as important predictors and had very similar coefficient estimates in all models. Breast cancer status was associated with increased age at first live birth, years of education, and income. Fixing all other demographic variables, having an income over $75K was associated with a 62% increase in breast cancer odds. American Indian and Asian/Pacific Islander women had lower odds of breast cancer than African American counterparts. Major health problems and hormone replacement therapy and mother’s use of diethylstilbestrol double and triple the odds of breast cancer in the presence of the other demographic variables, respectively. These results reflect similar findings in other breast cancer studies evaluating demographic and health variables [38,39].
When evaluating trauma and adverse events in the separate models, a competitive environment and severe human suffering demonstrated a relationship between occurring as a child and then as an adult, with a subsequent breast cancer diagnosis. Emotional neglect (0-7), physical neglect (8-18), sexual abuse (0-7), experiencing a fire or explosion (8-18), exposure to a toxic substance (19-90), assault with a weapon (19-90), severe human suffering as a child (8-18) and adult, and a competitive environment in childhood and adulthood were associated with increased odds of breast cancer. Perhaps one or more of these variables are different facets of one or multiple childhood experiences. Whereas the Life Events Checklist asks about specific events, the Childhood Trauma Questionnaire and IONS Developed items speak to emotional experiences. For example, a competitive environment in youth and adulthood may very well be associated with emotional and physical neglect and severe human suffering. Sexual abuse from 0-7 can lead to physical neglect at 19–90 and be associated with feelings of emotional neglect from 0-7. Although correlative, these results highlight the potential impact of emotional trauma in childhood on breast cancer development in adulthood. Identifying specific subgroups of experiences that increase breast cancer risk when examining all the variables’ inferential capacity together in the conditional inference tree was moderately successful, but none of the modes were pure. Given that many cases would share similar difficult childhood experiences with controls, this is not surprising.
Fire or explosion occurrence from 8-18 was associated with increased breast cancer odds. Furthermore, there was a quadratic and polynomial trend in impact related to breast cancer. Essentially, the association between the impact of fire/explosion and the log odds of breast cancer (in the presence of the remaining covariates) was nonlinear. While trauma from fire or explosions could certainly cause posttraumatic stress disorder and subsequent strain, it is uncertain why this trauma was specifically noted. Adult exposure to toxic substances was associated with increased breast cancer odds, although this is likely due to participants reflecting on their chemotherapy experience. Assault with a weapon as an adult was also associated with increased breast cancer odds. Interestingly, several non-linear terms were selected as predictors in the lasso models of adverse life events, which support primarily Non-linear and potentially complex relationships between the impact of these various items and breast cancer. This finding and the possible mechanisms that generate it merit further investigation.
Perhaps the most substantial results in this study were from the IONS developed item: “My environment was very competitive and performance and appearance meant everything.” A competitive environment in childhood and adulthood was associated with increased breast cancer risk and one of the few variables selected in the conditional inference tree build. Only four out of 110 variables were selected for the tree, demonstrating its potential importance in all the predictor variables examined. Competitive environment was also in the top 10 most important variables for the ran dom forest, more important than education, race, and major health problems. As far as we know, this is the first study to evaluate the subjective experience of competition in women with breast cancer during various age ranges. Competition negatively influences hormones in other arenas like competitive sports. Interestingly, competition is positively related to the person’s perception of whether the opponent was more of a challenge than expected and is negatively related to losing [40].
Study limitations
There are several limitations to this study that should be considered when viewing and interpreting the results. The analysis is exploratory and correlative, and thus, cause and effect cannot be definitively determined. Like all cross-sectional studies, our data reflects one snapshot in time rather than a prospective longitudinal study of our cohort. Finally, the survey was subjective and queried experiences many decades ago, and data likely includes memory bias. Regardless, this study has generated results that can be further explored.
Clinical implications
Overall, this exploratory study’s results suggest that potential stress associated with competitive lifestyles, and possibly physical and emotional neglect accompanying these lifestyles, and the stress of severe human suffering in childhood experienced again in adulthood, may increase breast cancer risk. The link between stress and health is explored in psychoneuroimmunology, with hormonal and inflammatory processes as possible mediators between them. Stress can lead to an adult proinflammatory phenotype characterized by a heightened stress response and higher serum levels of arachidonic acid, cytokines, and chemokines, leading to carcinogenesis. Perhaps experiencing the high levels of stress of a traumatic event, As well as any posttraumatic stress that may develop afterward, affect the body’s innate ability to fight burgeoning cancers, resulting in higher breast cancer risk. Additionally, exposure to chemical substances, either via hormone therapy, drugs such as diethylstilbestrol, or chemicals possibly released in fires/ explosions, may play some role in breast cancer risk. Future research should further evaluate this study’s results and the information gleaned from them introduced into preventative and treatment practices.
Data Availability
The data that support the findings of this study are openly available http://doi.org/10.6084/m9.figshare.11922942.
Conflicts of Interest
All authors have no conflict of interest.
Funding Statement
The Stanny Foundation funded this study. The sponsor provided preliminary ideas for the study’s general research question but had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Acknowledgment
Supplementary Material includes one PDF document with detailed model output tables and conditional inference tree figure.
References
- Diet, nutrition, physical activity and breast cancer survivors (2014) World cancer research fund. American institute for cancer research 55.
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69: 7-34
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, et al. (2016) Body fatness and cancer-viewpoint of the iarc working group. N Engl J Med 375: 794-798.
- Colditz GA (2000) Cumulative risk of breast cancer to age 70 years according to risk factor status: Data from the nurses health study. Am J Epidemiol 152: 950-964.
- Menarche, (2012) Menopause and breast cancer risk: Individual participant meta-analysis, including 118964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13: 1141-1151.
- Kelsey J, Gammon M, John E (1993) Reproductive factors and breast cancer. Epidemiol Rev 15: 36-47.
- Collaborative group on hormonal factors in breast cancer (2001) Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58209 women with breast cancer and 101986 women without the disease. The Lancet 358: 1389-1399.
- Price MA, Tennant CC, Smith RC, Butow PN, Kennedy SJ, et al. (2001) The role of psychosocial factors in the development of breast carcinoma: Part I. The cancer prone personality. Cancer 91: 679-685.
- Sawada T, Nishiyama T, Kikuchi N, Wang C, Lin Y, et al. (2016) The influence of personality and perceived stress on the development of breast cancer: 20-year follow-up of 29098 Japanese women. Sci Rep 6: 32559.
- Minami Y, Hosokawa T, Nakaya N, Sugawara Y, Nishino Y, et al. (2015) Personality and breast cancer risk and survival: The miyagi cohort study. Breast Cancer Res Treat 150: 675-684.
- Nakaya N, Bidstrup PE, Saito-Nakaya K, Frederiksen K, Koskenvuo M, et al. (2010) Personality traits and cancer risk and survival based on finnish and swedish registry data. Am J Epidemiol 172: 377-385.
- Lemogne C, Consoli SM, Geoffroy-Perez B, Coeuret-Pellicer M, Nabi H, et al. (2013) Personality and the risk of cancer: A 16-year follow-up study of the GAZEL cohort 75: 262-271.
- Ports KA, Holman DM, Guinn AS, Pampati S, Dyer KE, et al. (2019) Adverse childhood experiences and the presence of cancer risk factors in adulthood: A scoping review of the literature from 2005 to 2015. J Pediatr Nurs 44: 81-96.
- Anda RF, Butchart A, Felitti VJ, Brown DW (2010) Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med 39: 93-98.
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, et al. (2004) The interrelatedness of multiple forms of childhood abuse, neglect and household dysfunction. Child Abuse Negl 28: 771–784.
- Jewkes RK, Dunkle K, Nduna M, Jama PN, Puren A (2010) Associations between childhood adversity and depression, substance abuse and HIV and HSV2 incident infections in rural South African youth. Child Abuse Negl 34: 833-841.
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, et al. (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. Am J Prev Med 14: 245-258.
- Alcalá HE, Mitchell EM, Keim-Malpass J (2018) Heterogeneous impacts: Adverse childhood experiences and cancer screening. Cancer Causes Control 29: 343-351.
- Heim C, Shugart M, Craighead WE, Nemeroff CB (2010) Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol 52: 671-690.
- Janusek LW, Tell D, Albuquerque K, Mathews HL (2013) Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain Behav Immun 30: S149-S162.
- Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, et al. (2017) Adverse childhood or adult experiences and risk of bilateral oophorectomy: A population based case control study. BMJ Open 7: e016045.
- Ryan GL, Mengeling MA, Summers KM, Booth BM, Torner JC, et al. (2016) Hysterectomy risk in premenopausal-aged military veterans: Associations with sexual assault and gynecologic symptoms. Am J Obstet Gynecol 214: 352.e1-352.e13.
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, et al. (2003) Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl 27: 169-190.
- Gray MJ, Litz BT, Hsu JL, Lombardo TW (2004) Psychometric properties of the life events checklist. Assessment 11: 330-341.
- Cann A, Calhoun LG, Tedeschi RG, Taku K, Vishnevsky T, et al. (2010) A short form of the posttraumatic growth inventory. Anxiety Stress Coping 19582640 23: 127-137.
- Charan J, Biswas T (2013) How to calculate sample size for different study designs in medical research? Indian J Psychol Med 35: 121.
- Pourhoseingholi MA, Vahedi M, Rahimzadeh M (2013) Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench 6: 14-17.
- Hartig F (2019) DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models.
- Venables W, Ripley B. Modern applied statistics with s. Springer Verlag. N Y 2002.
- Friedman J, Hastie T, Tibshirani R (2010) Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33: 1.
- Hastie T, Tibshirani R, Friedman J (2009) The elements of statistical learning: Data mining, inference and prediction. Springer.
- Lee JD, Sun DL, Sun Y, Taylor JE (2016) Exact post-selection inference with application to the lasso. Ann Stat 44: 907-927.
- Hothorn T, Zeileis A (2015) Party kit: A modular toolkit for recursive partytioning in r. J Mach Learn Res 16: 3905-3909.
- Hothorn T, Hornik K, Zeileis A (2006) Unbiased recursive partitioning: A conditional inference framework. J Comput Graph Stat 15: 651-674.
- Liaw A, Wiener M (2002) Classification and regression by randomforest. R News 2: 18-22.
- James G, Witten D, Hastie T, Tibshirani R (2013) An introduction to statistical learning. Springer.
- Rudolph A, Song M, Brook MN, Milne RL, Mavaddat N, et al. (2018) Joint associations of a polygenic risk score and environmental risk factors for breast cancer in the breast cancer association consortium. Int J Epidemiol 47: 526-536.
- Arthur R, Wassertheil-Smoller S, Manson JE, Luo J, Snetselaar L, et al. (2018) The combined association of modifiable risk factors with breast cancer risk in the women’s health initiative. Cancer Prev Res 11:317-326
- Bateup HS, Booth A, Shirtcliff EA, Granger DA (2002) Testosterone, cortisol, and women’s competition. Evol Hum Behav Cancer Prev. Res 23: 181-192
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R (2002) Psychoneuroimmunology and psychosomatic medicine: Back to the future. Psychosom Med 64: 15-28.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 49: 1603-1616.
Citation: Wahbeh H, Heinz B, Fry N, Wojakowski M (2021) Exploring Lifetime Experiences of People with Breast Cancer: A Cross-Sectional Study. J Oncol Res Treat 6:160. DOI: 10.4172/aot.1000160
Copyright: © 2021 Wahbeh H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3417
- [From(publication date): 0-2021 - Nov 10, 2025]
- Breakdown by view type
- HTML page views: 2481
- PDF downloads: 936