Research Article Open Access
Features of N-Glycosylation of Immunoglobulins from Knockout Pig Models
Marjorie Buist1, Emy Komatsu1, Paul G Lopez1, Lauren Girard1, Edward Bodnar1, Apolline Salama2,3, David H Sachs4, Cesare Galli5,6,7,8, Andrea Perota5, Sophie Conchon2, Jean-Paul Judor2, Jean-Paul Concordet9, Giovanna Lazzari5,6, Jean-Paul Soulillou2,8 and Hélène Perreault1*
1Department of Chemistry, University of Manitoba, Canada
2INSERM UMR 10-64, Institut de Transplantation Urology Nephrology (ITUN), Université de Nantes, France
4Massachusetts General Hospital, Harvard University, Cambridge, MA, USA
5Avantea Laboratory of Reproductive Technologies, Cremona, Italy
6Avantea Foundation, Cremona, Italy
7Department of Veterinary Medical Sciences, University of Bologna, Ozzano Emilia, Italy
8Translink Framework Program (FP7), Padova, Italy
9Université Paris Descartes, Paris, France
- *Corresponding Author:
- Hélène Perreault
Department of Chemistry, University of Manitoba
144 Dysart Road Winnipeg, Manitoba Canada R3T
Tel: 12044747418
Fax: 12044747608
E-mail: 2N2Helene.Perreault@umanitoba.ca
Received date: July 23, 2016; Accepted date: August 04, 2016; Published date: August 08, 2016
Citation: Buist M, Komatsu E, Lopez PG, Girard L, Bodnar E, et al. (2016) Features of N-Glycosylation of Immunoglobulins from Knockout Pig Models. J Anal Bioanal Tech 7:333. doi: 10.4172/2155-9872.1000333
Copyright: © 2016 Buist M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
For the first time, the N-glycosylation patterns of immunoglobulin G (IgGs) isolated from the serum of two varieties of knockout pigs (lacking N-glycolylneuraminic acid (Neu5Gc) and/or α 1,3 galactose) were examined for the presence of potential glycan xenoantigens and compared to N-glycosylation patterns obtained for wild-type (WT) pig IgGs. Glycopeptide analysis was chosen over glycan release, as protein-A eluates from pig serum may contain IgA and IgM as shown previously. The experiments focused on the analysis of tryptic glycopeptides EEQFNSTYR and AEQFNSTYR from IgGs, and excluded IgA and IgM, in which N-glycosylated peptides have different sequences and masses. WT pig IgG glycopeptides showed the presence of N-glycolylneuraminic acid (Neu5Gc) and absence of N-acetylneuraminic acid (Neu5Ac). Released glycans from the protein-A eluate, however, showed the presence of both types of sialic acids, allowing Neu5Ac to be attributed to IgA and/or IgM. The WT IgG samples also showed the presence of glycans that could by composition have been α-galactosylated, but treatments with α- and β-galactosidases produced inconclusive results as to the linkage nature of the terminal Gal residues. Single knockout (α-Gal transferase) pig IgG was shown to contain Neu5Gc residues, and there was a definite absence of α-Gal. Double knockout pigs (DKO for α-Gal transferase and cytidine monophosphate-A-acetylneuraminic acid hydroxylase (CMAH)) showed the definite absence of α-Gal and Neu5Gc. Instead of the latter, Neu5Ac residues were observed. Further investigation into the sialylation patterns of WT and DKO pig IgGs consisted of esterifying the glycopeptides to allow the detection and differentiation of α-2,3 and α-2,6 sialic acid-galactose linkages. Fucosylation levels were also compared between IgG species.
Keywords
Immunoglobulin; Knockout pig; Xenoantigen; MALDIToF- MS; Sialylation; Galactosylation
Introduction
Animal biological products offer possible clinical opportunities such as xenotransplantation in order to remedy to the frequent shortage of human organs. In the clinical arena, animal derived engineered tissues such as tendons [1], scaffolds [2], heart valves [3] or even polyclonal IgGs [4] have been designed and used. Although pigs have been considered as candidates of choice for these purposes, several immunological challenges still create obstacles to the grafting processes and cause potential concerns where animal derived products are used in humans. Indeed, a strong antibody-mediated response shortens the lifetime of xenografts [5], and this is also observed following grafting of engineered pig skin [6] or infusion of foreign immunoglobulins (IgGs) [7,8]. Genetically engineered donor pigs have thus been designed in order to eliminate the expression of xenogenic antigens α1,3 galactose (αGal) [9] and/or N-glycolylneuraminic acid (Neu5Gc) [10], found in glycoproteins and other glycoconjugates expressed in wild-type animals and considered as major xenoantigenic barriers [10]. A recent report showed that representative glycoproteins from wild-type pigs, IgGs, contain Neu5Gc, although no αGal was detected with the methods used [11].
Information specific to these IgGs is important, as clinical applications of xenotransplantation are not restricted to using organs or tissues but could also concern the use of specific molecules such as IgGs. It is expected that modifying the glycans on these antibodies can reduce the immunogenicity of polyclonal IgGs [8] which still can modify the course of Ebola infection in guinea pigs in passive immunotherapy [4]. Indeed, by knocking-out both the genes responsible for the expression of α-galactosyltransferase (GT) and cytidine monophosphate-Aacetylneuraminic acid hydroxylase (CMAH), the latter’s function being to add a glycolyl to N- acetylneuraminic acid (Neu5Ac) [12], it is highly likely that these IgGs will have a much lower immunogenic potential [10], despite the fact that they can still prolong survival of EBOV- infected guinea pigs as well as decrease the extent of EBOV replication in these animals [4].
Single α-galactosyltransferase knock-out (GTKO) pig models have also been used in different studies (reviewed in [13]). As there is still antibody-mediated xenograft rejection of organs from GTKO pig organs in non-human primates, this rejection can be directed to non-Gal pig proteins and carbohydrate antigens, a situation likely predictable if such grafts would have been done in humans. The analysis of glycans from pig GTKO tissues did not result in the identification of new antigens, however high levels of N-glycolylneuraminic acid (Neu5Gc) were detected in glycoproteins and glycolipids [13].
The purpose of this article is to characterize the N-glycosylation of IgGs isolated from the serum of double KO pigs (DKO, GT+CMAH KO) and GTKO pigs. This has not been reported before. Mass spectrometry is used as the main detection method, after extensive sample preparation as discussed herein. Although Burlak and coworkers profiled the serum N-glycome of these same two types of KO pigs [14], information specific to IgGs is also of primary importance given their potential utilisation in clinical applications, as mentioned above [4]. For whole serum N-glycomes, it was highlighted that DKO pig glycoproteins had more mannosylated, xylosylated, corefucosylated and truncated N-glycans than domestic WT pigs [14]. Reflection of these findings on IgGs isolated from pig serum may bring further insight on the immunogenic properties of these modified antibodies.
Experimental
Materials
Wild-type pigs of the strain Landrace × Large White of 25 kg were used (EARL du Pont Romain, Surzur, France) and were housed at the Large Animal Facility of the INSERM UMR1064 (Nantes University Hospital, France, agreement number: D44011). Whole blood was sampled, and serum was stored at -80°C until use for purification. Blood samples (100 mL) were also obtained from male GTKO and DKO pigs (14 months, weighing about 102 kg) [8]. All animal procedures were approved by the local ethic committee and carried out in accordance to DGL 116/92 for the Italian regulation. Trypsin Gold (MS Grade) was purchased from Promega (Madison, WI). Ammonium bicarbonate, trifluoroacetic acid (TFA), 2,5-dihydroxybenzoic acid (DHB), dithiothreitol (DTT) and iodiacetamide (IA) were purchased from Sigma-Aldrich (St. Louis, MO). Acetic acid (AcOH) was purchased from Fisher Scientific (Ontario, Canada), acetonitrile (ACN) was bought from EMD Millipore (Dermstadt, Germany) and ethanol (EtOH) was purchased from Commercial Alcohols (Ontario, Canada). C-18 cartridges were purchased from Phenomenex (Torrance, CA). Galactosidase α1-3,4,6 was obtained from New England Biolabs (Whitby, ON) and galactosidase β from bovine testes was obtained from Sigma-Aldrich.
Purification of porcine IgG: IgGs were purified from blood on a Protein-A column (high performance Sepharose™ (GE Healthcare)) using a low pressure chromatograph and 280 nm UV detection. The chromatograph allowed to record pH and conductivity. The IgGs were eluted with a solution of 0.1 M citric acid (pH 3), followed by immediate pH neutralization of the eluate to pH 7 - 7.4 with a solution of 1 M TRIS at pH 8. IgGs were then dialyzed against PBS 1X and their amount was assessed by UV spectrophotometry at 280 nm. In each case the final IgG amount was about 800 mg.
Tryptic digestion of IgGs: Digestion was conducted without prior reduction and alkylation. The Ab (75 μg) was reconstituted in 200 μL of 50 mM ammonium bicarbonate. Trypsin (1.5 μg) was added and digestion proceeded at 37°C for ~18 h.
HPLC fractionation of tryptic digestion mixtures: The digestion mixtures were injected on a Synergi C18 polar column (Phenomenex, Torrance, CA), and eluted with a gradient from 0 to 30% of acetonitrile in water at a flow rate of 0.25 mL/min. The HPLC system used was a Waters1525 binary pump equipped with a Waters 2707 autosampler and a Waters 2998 photodiode array detector. Fractions (2-min each) were collected, dried and resuspended in 3:7 ACN-water with 0.1% TFA for MALDI-MS analysis.
Alpha- and beta galactosidase digestions: For α1-3,4,6 galactosidase digestion, glycopeptides from HPLC fractionation (250 ng) were dried and resuspended in 15 μL of water, to which were added 3 μL of sodium phosphate buffer at pH 6, 3 μL of 1 mg/mL bovine serum albumin solution, and 3 μL of the enzyme solution provided by the manufacturer. The mixture was incubated for 17 h at 37°C, and digestion products were cleaned by HPLC using the method described above. For digestion with β-galactosidase, glycopeptides (250 ng) were resuspended in 13 μL of water, to which were added 12 μL of 100 mM sodium citrate/phosphate buffer at pH 4 and 6 μL of the enzyme solution provided by the manufacturer. The incubation time was 17 h at 37°C. Digestion products were cleaned by HPLC.
PNGase digestion: This procedure was initiated by the reduction and alkylation of 100 μg of antibody protein-A eluate. The protein sample was reconstituted in 10 μL of 50 mM ammonium bicarbonate buffer (pH 7.8). Dithiothreitol (DTT, 50 μL, 10 mM in buffer) was added and reduction was allowed to proceed at 50-60°C for 1 h. After cooling, iodoacetamide (IA, 40 μL, 10 mM in water) was added and the mixture was stored in the dark for 30 min for alkylation. The sample was then cleaned and desalted on a C18 cartridge, which was conditioned with 5 × 1 mL of ACN, then 5 × 1 mL of H2O. The protein sample was loaded and desalted with 5 × 1 mL of H2O. The protein was then eluted with 1.5 mL of 50:50 ACN:H2O+0.1% TFA, collected in an Eppendorf tube, and reconstituted in 40 μL of buffer. For PNGase F digestion, the enzyme (4 μL, at 10 units/μL) was added, and digestion proceeded 37°C for 18 h. Following the digest, the glycans were separated from the deglycosylated protein on a C18 cartridge. The column was conditioned with 5 × 1 mL of ACN+0.1% TFA and then with 5 × 1 mL of H2O+0.1% TFA. The sample was loaded and glycans were eluted with 3 mL of H2O+0.1% TFA and collected in two fractions. The glycan samples were resuspended in 20 μL of H2O.
Preparation of samples for MALDI-MS analysis: Fractions in 0.1% TFA in 30:70 ACN:water mixed with DHB matrix saturated solution in the same solvent at a 1:1 ratio. This mixture (1 μL) was spotted onto the stainless steel target and allowed to dry. It was estimated that for 100 μg of antibody, a maximum of 1.8 μg of glycopeptides was obtained, divided in 10 fractions, giving an average of ca. 0.18 μg per fraction.
Mass spectrometry: All MS analyses were performed on an UltrafleXtremeTM mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with LID-LIFTTM technology for tandem MS (MS/ MS) experiments. The following peptide calibration mixture was used (American Peptide Company, Vista, Ca): Bradykinin(1-7) 757.3992; angiotensin II 1046.542; angiotensin I 1296.685; substance P 1346.735; bombesin 1619.822; ACTH clip (1-17) 2093.086; ACTH clip (18-39) 2465.198; ACTH (1-39) 4539.267, where ACTH=adrenocorticotropic hormone and numbers are calculated m/z values of [M+H]+ ions. For sample preparation of this calibrant, 1 μL of a solution containing 0.125 μg/μL was mixed in the MALDI target with the sample volume of DHB matrix saturated solution. All experiments presented in this report were conducted in positive ionization reflector mode. As this study focuses on qualitative profiling, the mass accuracy was of the order of 150-200 ppm.
Results and Discussion
A previous study of IgG heavy chain glycosylation in WT pigs [11] revealed the presence of Neu5Gc on Fc glycans, whereas no α-galactosylated residues were identified, using a glycopeptide-based approach. The Asn-297 site tends to be frequently designated as the only glycosylation site in IgGs, however in as least 10-15% of polyclonal antibodies the variable region is also glycosylated [15]. Some studies have shown even higher glycosylation rates in the variable portion of polyclonal IgGs with up to to 30-40% [16,17]. Plomp et al. recently reported the detection of O-glycans in the hinge region of human IgG3 [18], and previous IgG glycopeptide studies (e.g., Ref. [11,19]) have found evidence of N-glycosylation in the variable region. Particular to pig IgG, no O-glycosylation was found in a 2014 study [20], and no detectable O- glycopeptides were listed in heavy chain tryptic digestion products [11]. As the N-linked glycans of serum proteins are thought to reflect organism-wide glycosylation patterns [14], the present study focuses on the characterization of only the Fc N-glycans of two KO pig models as compared to WT.
Glycoproteomic approach
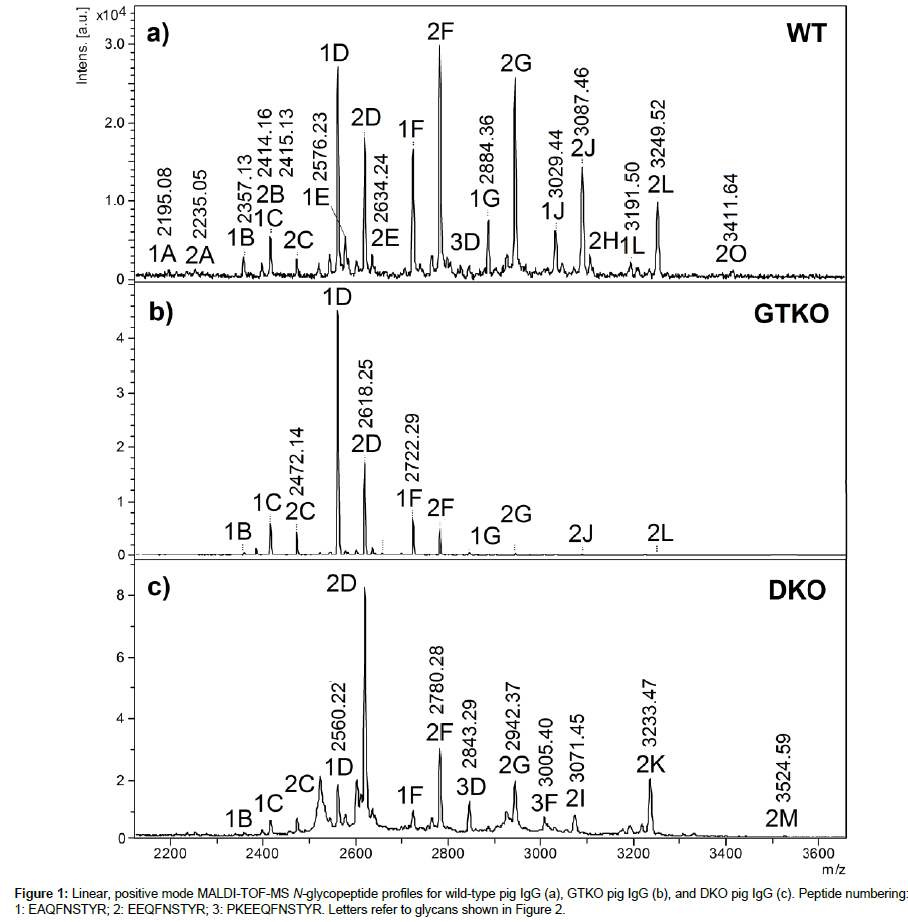
Study of N-glycopeptides from the Fc portion: Figure 1 shows a comparison between WT, GKO and DKO IgG N-glycans measured using glycopeptide profiling. The polyclonal nature of these IgG samples reflects itself in the observation of two main peptide chains bearing the N-glycans, EAQFNSTYR (1) and EEQFNSTYR (2), for which all equivalent glycoforms (letters referring to Figure 2) are separated by 58 m/z units, i.e., the difference between aspartic acid E and alanine A. These chains had been identified and sequenced in a previous report [11]. The relative abundances of these two chains will not be discussed, as it was shown that within the same race of WT pigs, different animals produced different ratios of EAQFNSTYR (subtype IgG6a) and EEQFNSTYR (subtypes IgG1a, IgG1b, IgG2a, IgG4a, IgG4b, IgG5a, IgG6b) in their IgG [11,21]. IgG subtypes and the deficiency/proficiency of, as well as their immunogenic and binding properties have been well characterized in humans [15], but not in pigs. Two other isobaric amino acid sequences could have been expected in this sample, i.e., EGQFNSTYR for subtype IgG2b and EEQFNSSYR for IgG 5b [21], however they were not observed.
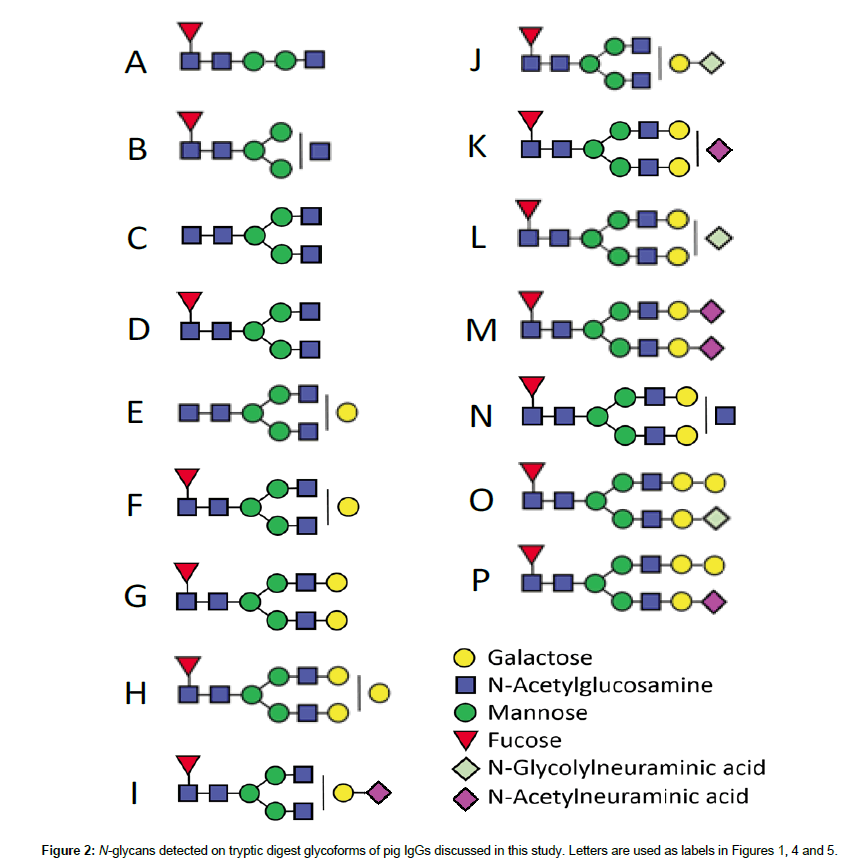
In Figure 1a, the main glycoforms observed are peptides bearing biantennary fucosylated glycans labelled 1D, 2D, 1F, 2F, 1G and 2G, with the number referring to the peptide chain and the letters, to the structures of Figure 2. Some minor glycoforms include xenoantigen Neu5Gc: 1J-2J, 1L-2L, and 2O. The latter (2O) could potentially include αGal, as well as glycoform 2H. These structures had not been reported in a first study [11] most possibly due to sample preparation procedures. The IgG chains had been first separated on gel followed by in-gel tryptic digestion, whereas in the present study the whole protein-A IgG eluate was directly subjected to tryptic digestion in solution. This method allowed to recover more material and hence the observation of these signals. However, repeated digestions with α- galactosidase did not modify species 2H and 2O. With β-galactosidase, both 2H and 2O were partially hydrolyzed to lower glycoforms, but the same situation was observed for peptides bearing F and G. Figure S1 shows the spectra before and after galactosidase treatments. These experiments with exoglycosidases therefore remained inconclusive. Other minor glycoforms found in Figure 1a are glycopeptides with truncated glycans A and B, and afucosylated glycans C and E.
Figure 1b shows the N-glycoforms obtained from the IgG of a GTKO pig. There is no significant variation in ions observed, for instance 1E and 2E are not as abundant as in Figure 1a, and species containing Neu5Gc are also in lower abundance. Peptides with glycans H and O were not detected.
The last spectrum (Figure 1c) corresponds to the tryptic N glycopeptides from the IgG of DKO pigs. A third peptide is also observed with sequence PKEEQFNSTYR (3), i.e., formed from a tryptic cleavage before proline, which is not common but has been reported [22]. Peaks corresponding to glycoforms of this peptide appear at m/z 2843 and 3005 and are labelled 3D and 3F. Of interest was that in the absence of Neu5Gc, replacement by Neu5Ac verified that no CMAH enzyme was available to convert Neu5Ac into Neu5Gc. This validated the DKO model, and indicated that no potentially diet derived Neu5Gc were present on the IgG from a classical pig diet. The spectrum emphasizes the sialylation of glycoforms, in this case 1I-2I, 1K-2K and 2M when referring to Figure 2.
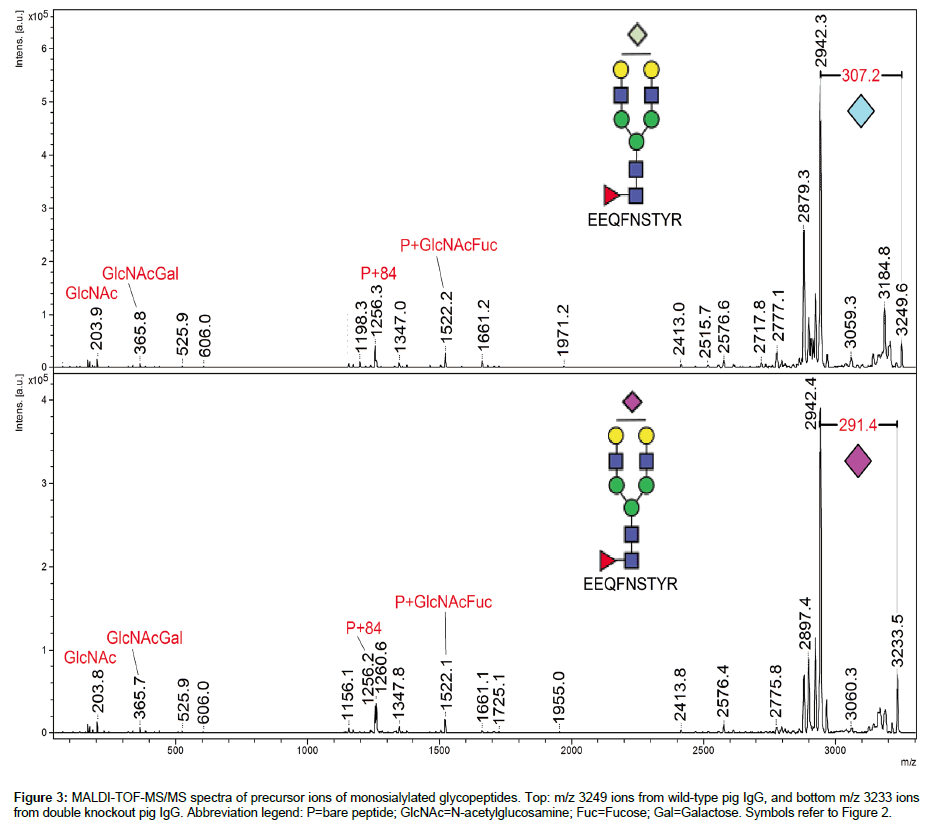
Figure 3 compares the tandem mass spectra obtained from the [M+H]+ precursor ions of glycoforms 2K and 2L. The prevalent feature of each spectrum is the loss of sialic acid (-307 for Neu5Gc, -291 for Neu5Ac). Otherwise, characteristic P+84 ions (where P=bare peptide) give access to the mass of the peptide [23] and thus to the mass of glycan component. These MS/MS spectra help confirm the presence of NeuGc in WT pig IgG and of NeuAc in DKO pig IgG. It was not possible to perform MS/MS for the GTKO pig IgG sample due to weakness of the signal.
Although Burlak et al. observed both Neu5Ac and Neu5Gc in the glycome of domestic pigs, the present study and previous work [11] show that it is not the case for IgG by itself. Burlak’s study also reported mostly Neu5Ac in DKO pigs with one instance of Neu5Gc being present [14]. In this study of glycopeptides, IgG from DKO pigs is shown to contain Neu5Ac exclusively. In humans, it has been shown that anti- Neu5Gc antibody is produced and that anti- Neu5Gc antibody response is induced after WT pig tissue grafts [13]. There is still uncertainty however about the pathogenicity of the human anti-Neu5Gc antibody xenografts using pig organs. Non-human primates used as models for such transplantations have shown anti-Gal immunity but, as expected, no production of anti-Neu5Gc antibodies [13]. In these models, posttransplantation antibody induction is rather directed to pig endothelial cells proteins and to a glycan due to the pig B4GALNT2 gene [24]. If this type of glycan is to be found in pig IgG, it would be on an O-site, in the hinge or Fab region, which were not specifically studied in this report. A more detailed study of Fab glycans, using papain to cleave the antibodies into two distinct portions [25] is underway and will be the object of a future manuscript.
Levels of fucosylation were compared in triplicate between WT and DKO samples. They were determined to be 14 ± 4% (WT) and 9 ± 1% (DKO), which could be determinant in explaining the observed higher toxicity dependence of DKO pig IgG vs WT [26,27]. On the other hand, DKO pig IgG has shown a higher complement (C1q) binding than WT pig IgG using BiacoreTM technology [27] which, according to the template of interactions proposed by Butler et al. [21], could relate to a higher IgG3 content in DKO samples. This could not be determined in this current glycopeptide study, however experiments using quantitative markers are underway to determine IgG3 in WT and DKO IgG samples.
There was thus no major variation in the predominant glycan structures observed in IgGs from WT, GTKO and DKO pigs in that they were mostly biantennary, core-fucosylated oligosaccharides. No significant increased abundance of mannosylated species was detected, as signaled by Burlak et al. in the glycome of DKO pig serum [14]. High mannose species (Man)5, monitored at m/z 2331 and 2389 for Peptides 1 and 2, were not observed in Figure 1a, but close inspection of an exploded view of the spectrum in (c) revealed a trace presence of these glycoforms. Glycoforms Man6 to Man9 were not observed at all in all spectra of Figure 1. As for truncated glycan forms noted by Burlak et al. in GTKO and DKO glycomes [14], they were not detected in this study, nor were xylose residues.
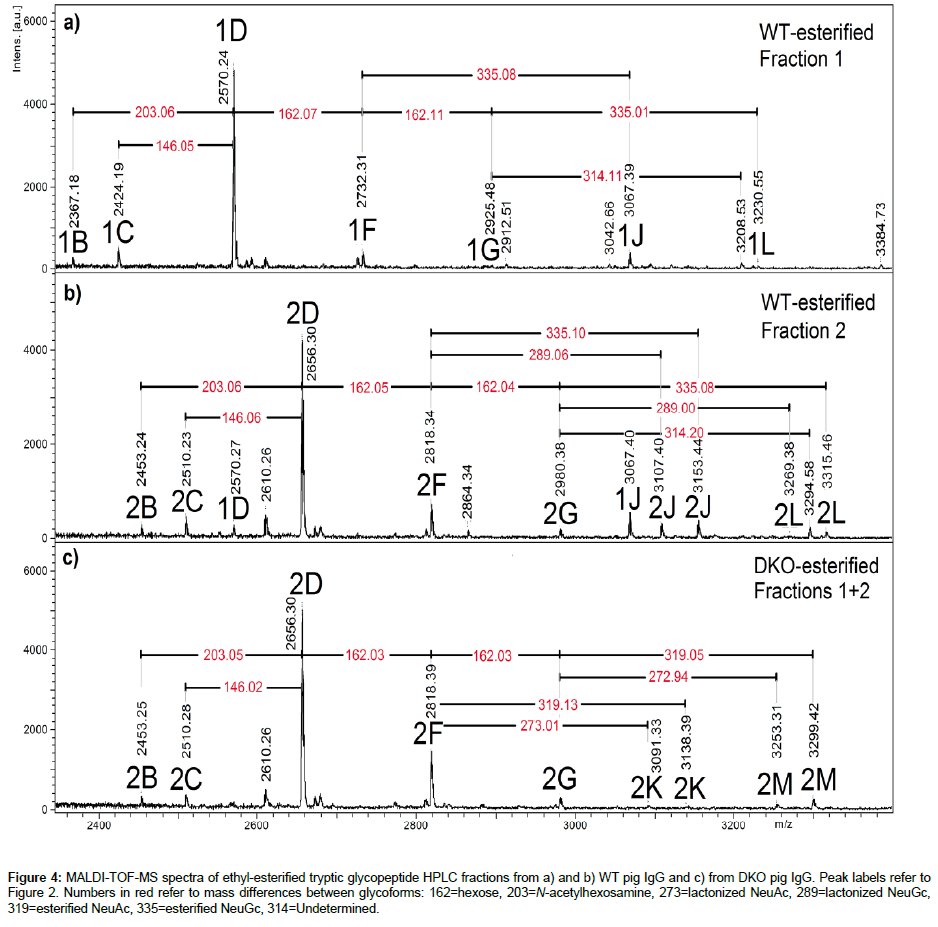
Another interesting aspect of terminal sialic acids is the linkage patterns to distal galactoses in the α2,3- or α2,6- position [28]. Sialylation has often been shown to enhance the half-lifes of glycoproteins, as sialic acid caps terminal galactose residues that are otherwise recognized by hepatic asialoglycoprotein receptors (ASGPR) [29]. It has been suggested that α 2,3 sialylation provides better half-life extension, as ASGPR recognizes Sia α 2,6Gal and Sia α 2,6GalNAc residues as well as Gal and GalNAc moieties [30,31]. In IgG samples, esterification of sialylated glycopeptides has allowed to differentiate these types of linkages [32,33], and this method was applied to asialylated pig IgG glycopeptides in a previous study [11], although the main purpose was to establish that peptides chains were EEQFNSTYR and EAQFNSTYR, E being reactive to esterification but not A. Glycopeptides from WT and DKO porcine IgG were subjected to this reaction and results are shown in Figure 4. Non-sialylated glycopeptides corresponding to Sequence 1 (EAQFNSTYR) saw their masses go up by 10 units (+28 for ethyl substitution, -18 for loss of H2O) [11,32]. Those corresponding to Sequence 2, EEQFNSTYR, showed an increment of 38 units (+(2 × 28)-18) [11,32]. Sialylated glycopeptides showed either an extra increment of 28 (335 instead of 307 for Neu5Gc, 319 instead of 291 for Neu5Ac) due to esterification of the carboxyl group or a loss of H2O (273 instead of 291 for Neu5Ac, 289 instead of 307 for Neu5Gc) due to lactonization. These mass differences are indicated by red numbers on the spectra. Some studies have pointed out that lactonization (-18) occurs for Sia α 2,3Gal linkages, while esterification takes place for Sia α 2,6Gal linkages, based on methods relying on methyl- and ethylesterification of carboxyl groups in glycans released from glycoprotein [28,32-35]. From this approach, used here for the first time with demonstrated lactonization of NeuGc, it can be observed in Figure 4 that IgGs from WT and DKO pigs exhibit both α 2,3 and α 2,6 linkages, for Neu5Gc (WT) and Neu5Ac (DKO). It is possible to assign semiquantitative figures to the abundances of each type by comparing peak areas. Overall for WT the α 2,6 to α 2,3 ratio was 1.7 and for DKO, 1.3, showing a small predominance of α 2,6 linkages. The half-life of the DKO pig IgG has been measured as normal (170 h) vs. WT IgG [27], which is consistent with these MS results.
Figure 3: MALDI-TOF-MS/MS spectra of precursor ions of monosialylated glycopeptides. Top: m/z 3249 ions from wild-type pig IgG, and bottom m/z 3233 ions from double knockout pig IgG. Abbreviation legend: P=bare peptide; GlcNAc=N-acetylglucosamine; Fuc=Fucose; Gal=Galactose. Symbols refer to Figure 2.
Figure 4: MALDI-TOF-MS spectra of ethyl-esterified tryptic glycopeptide HPLC fractions from a) and b) WT pig IgG and c) from DKO pig IgG. Peak labels refer to Figure 2. Numbers in red refer to mass differences between glycoforms: 162=hexose, 203=N-acetylhexosamine, 273=lactonized NeuAc, 289=lactonized NeuGc, 319=esterified NeuAc, 335=esterified NeuGc, 314=Undetermined.
These types of linkages were found to be important with respect to cell infectivities of human parainfluenza virus type 1 and type 3: Type 1 recognized only α2,3 sialic acid linkages as viral receptors, while type 3 recognized both α2,3 and α2,6 sialic acid linkages [36]. As the terminal and only (negatively) charged residue in Fc glycans, it has been suggested that sialic acids have the most effect on the Fc domain structure [15,37].
Glycomic approach
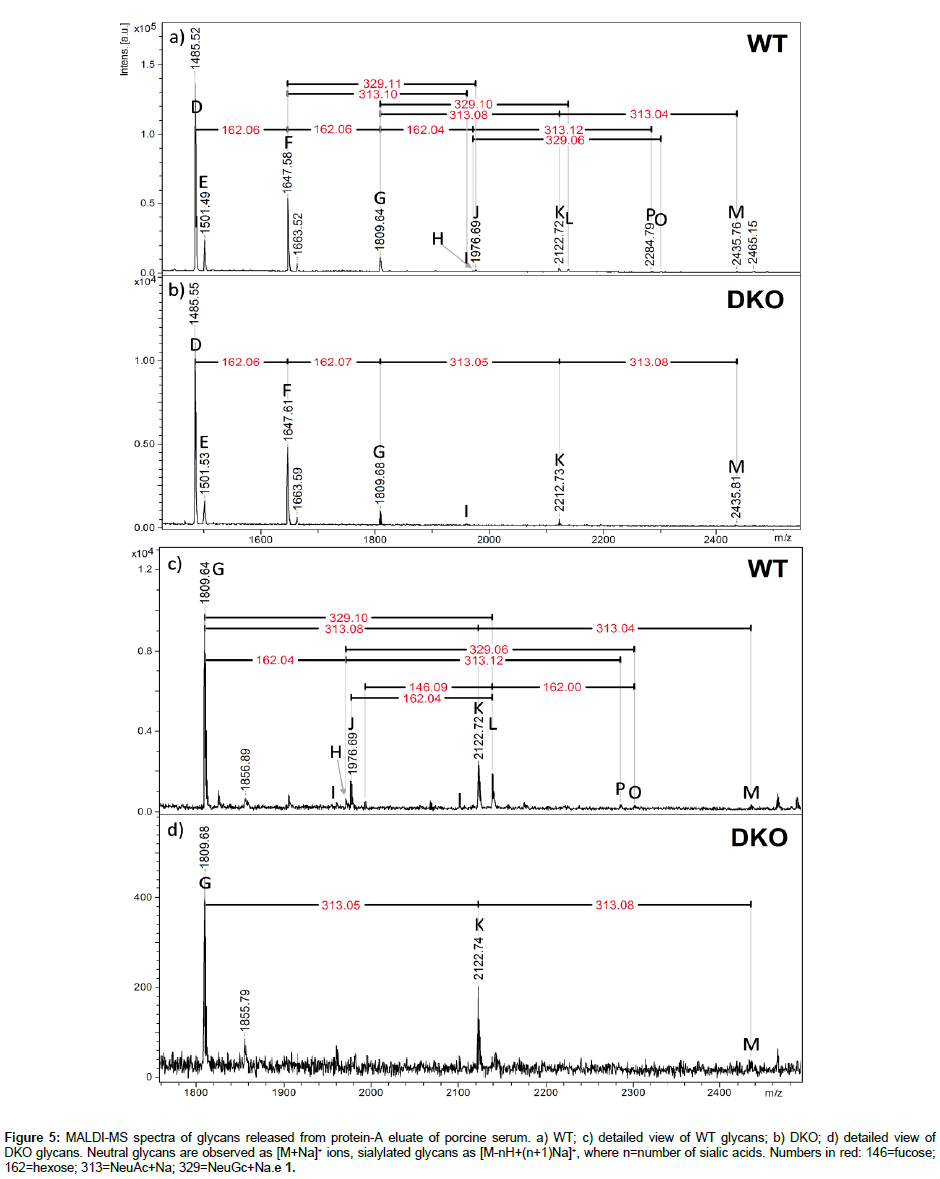
Study of N-glycans released from protein-A eluate: At the glycomic level, mass spectra of native glycans detached from full antibodies (WT and DKO) with PNGaseF are shown in Figure 5. These glycans were detached from the whole protein-A eluate, which also contains IgM and IgA [11], and thus are not uniquely related to the heavy chains of IgG. In Figure 5, neutral glycans are detected as [M+Na]+ ions, whereas monosialylated species produce [M-H+2Na]+ signals, and disialylated, [M-2H+3Na]+ [38]. The observation of glycans containing Neu5Ac in the WT (m/z difference of 313), not detected on specific IgG glycopeptides from the same antibody sample (Figure 1a), suggests that these glycans are foreign to the heavy chain of IgG. A study by Marco-Ramell et al. reported the presence of Neu5Ac in IgA glycans, but also in IgG [20], possibly released from the IgG Fab light chain portion. Fab vs. Fc glycans in the same antibody encompass some differences, especially in increased levels of bisection, galactosylation, and thereby sialylation [39,40]. For instance disialylation is rarely observed in Fc glycans but more frequently in Fab glycans. Reduced levels of fucosylation have also been observed from Fab to Fc glycans [39,40]. Comparing the glycans detected in Figure 5 with heavy chainspecific glycoforms of Figure 1 leads to the observation of disialylated (di-NeuAc, species M) glycans in the WT sample of Figure 5, which were not present in Figure 1. Glycans M were present in both Figures 1 and 5 in the case of the DKO sample. Significant changes in the WT sample between relative abundances of glycoforms/glycans suggest that a significant proportion of released glycans came from either the Fab domain, or IgM/IgA. There was more consistency for the DKO samples analyzed in Figures 1 and 5.
Figure 5: MALDI-MS spectra of glycans released from protein-A eluate of porcine serum. a) WT; c) detailed view of WT glycans; b) DKO; d) detailed view of DKO glycans. Neutral glycans are observed as [M+Na]+ ions, sialylated glycans as [M-nH+(n+1)Na]+, where n=number of sialic acids. Numbers in red: 146=fucose; 162=hexose; 313=NeuAc+Na; 329=NeuGc+Na.e 1.
MS/MS spectra were recorded to verify the presence of both Neu5Ac and Neu5Gc in the sample, as Figure S2 indicates. The mass selection tool of the UltraFleXtreme instrument was not able to completely isolate each of the m/z 2212 and 2228 precursor ions, resulting in overlapping MS/MS spectra which however show the respective loss of 313 (Neu5Ac+Na) and 329 (Neu5Gc+Na) from these two precursor masses.
Overall, results from the PNGase experiment help to emphasize the importance of glycosylation site specificity of analysis when studying glycopeptides. Where porcine IgG species contain variations of the Fc tryptic sequence EEQFNSTYR, IgA and IgM produce different tryptic peptides, according to UniprotKB accession numbers K7ZJP7 (IgM) and K7ZRK0 (IgA). The constant portion of porcine IgM has four consensus sequences for N-glycosylation which would be included in tryptic peptides ESLNISWTR and TSIVFSEIYANGTFGAR and two other longer peptides, whereas IgA’s conserved part would produce a peptide of sequence LAGKPTHVNVSVVMAEAEGICY.
Conclusions
This report gathers important information specific to polyclonal IgGs from KO pig species and makes the parallel with IgG from WT pigs. Modified glycans on these antibodies can modulate their immunogenicity in passive immunotherapy treatments. Knocking-out the gene responsible for αGal resulted in IgGs where in N-glycosylation no α Gal residues were found, and where Neu5Gc residues were present. Knocking-out both the genes for αGal and Neu5Gc produced IgGs in which no αGal is detected, and where Neu5Ac residues were detected. In WT and DKO pig IgG, an esterification reaction allowed to determine the binding pattern of sialic acids, which showed a greater proportion of α2,6 binding over α2,3, which could have important biological implications. The fucosylation level was also higher in the WT than in DKO IgG, which could be significant for toxicity dependence levels. Finally, this study emphasizes the specificity of using a glycoproteomic rather than strictly glycomic approach in the characterization of these antibodies, as N-glycans released from whole protein-A eluates showed compositions varying from those measured on IgG N-glycopeptides. Future work will focus on Fab N- and O-glycans.
Acknowledgments
This work was carried out under the European Union Seventh Framework Programme collaborative Project Translink (Grant agreement No. 603049). The authors thank the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN/170241-2011) and the Canadian Foundation for Innovation (Grant no. 23391) for funding this research. We also thank Bernard Martinet (Inserm 1064, Nantes) for preparing the protein A purification of pig IgGs.
References
- Na JY, Song K, Kim S, Lee HB, Kim JK, et al. (2014) Evaluation of porcine xenograft in collateral ligament reconstruction in beagle dogs. Res Vet Sci 97: 605-610.
- Hussein KH, Park KM, Kim HM, Teotia PK, Ghim JH, et al. (2015) Construction of a biocompatible decellularized porcine hepatic lobe for liver bioengineering. The International journal of artificial organs 38:96-104.
- Manji RA, Lee W, Cooper DK (2015) Xenograft bioprosthetic heart valves: Past, present and future. International journal of surgery (London, England) 23:280-284.
- Reynard O, Jacquot F, Evanno G, Mai HL, Salama S, et al. (2016) Anti-EBOV GP IgGs Lacking a1-3-Galactose and Neu5Gc Prolong Survival and Decrease Blood Viral Load in EBOV-infected Guinea Pigs. PloS one.
- Vadori M, Cozzi E (2014) Immunological challenges and therapies in xenotransplantation. Cold Spring Harb Perspect Med 4: a015578.
- Scobie L, Padler-Karavani V, Le Bas-Bernardet S, Crossan C, Blaha J, et al.(2013) Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. Journal of immunology 191:2907-2915.
- Couvrat-Desvergnes G, Salama A, Le Berre L, Evanno G, Viklicky O, et al. (2015) Rabbit antithymocyte globulin-induced serum sickness disease and human kidney graft survival. J Clin Invest 125: 4655-4665.
- GlobalSurg Collaborative (2016) Mortality of emergency abdominal surgery in high-, middle- and low-income countries. Br J Surg 103: 971-988.
- Galili U (2005) The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol 83: 674-686.
- Salama A, Evanno G, Harb J, Soulillou JP (2015) Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation 22: 85-94.
- Lopez PG, Girard L, Buist M, de Oliveira AG, Bodnar E, et al. (2016) Characterization of N-glycosylation and amino acid sequence features of immunoglobulins from swine. Glycoconj J 33: 79-91.
- Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, et al. (2013) Double knockout pigs deficient in N- glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 20:27-35.
- Byrne GW, McGregor CG, Breimer ME (2015) Recent investigations into pig antigen and anti-pig antibody expression. International journal of surgery (London, England) 23:223-228.
- [No authors listed] (2014) Retraction notice to Differential impact of diabetes and hypertension in the brain: adverse effects in white matter.Neurobiol Dis 68: 228.
- Burlak C, Bern M, Brito AE, Isailovic D, Wang ZY, et al. (2013) N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation 20:277-291.
- Vidarsson G, Dekkers G, Rispens T (2014) IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5: 520.
- Dwek RA, Lellouch AC, Wormald MR (1995) Glycobiology: 'the function of sugar in the IgG molecule'. J Anat 187: 279-292.
- Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, et al. (1997) Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry 36:1370-1380.
- Plomp R, Dekkers G, Rombouts Y, Visser R, Koeleman CA, et al. (2015) Hinge-Region O-Glycosylation of Human Immunoglobulin G3 (IgG3). Mol Cell Proteomics 14: 1373-1384.
- Lattová E, Kapková P, Krokhin O, Perreault H (2006) Method for investigation of oligosaccharides from glycopeptides: direct determination of glycosylation sites in proteins. Anal Chem 78: 2977-2984.
- Marco-Ramell A, Miller I, Nobauer K, Moginger U, Segales J, et al. (2014) Proteomics on porcine haptoglobin and IgG/IgA show protein species distribution and glycosylation pattern to remain similar in PCV2-SD infection. Journal of proteomics 101:205-216.
- Butler JE, Wertz N, Deschacht N, Kacskovics I (2009) Porcine IgG: structure, genetics, and evolution. Immunogenetics 61: 209-230.
- Rodriguez J, Gupta N, Smith RD, Pevzner PA (2008) Does trypsin cut before proline. J Proteome Res 7: 300-305.
- Krokhin O, Ens W, Standing KG, Wilkins J, Perreault H (2004) Site-specific N-glycosylation analysis: matrix-assisted laser desorption/ionization quadrupole-quadrupole time-of-flight tandem mass spectral signatures for recognition and identification of glycopeptides. Rapid communications in mass spectrometry: RCM 18:2020-2030.
- Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG (2014) Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation 21:543-554.
- Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, et al. (2007) Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology 17:104-118.
- Reynard O, Jacquot F, Evanno G, Mai HL (2016) Anti-EBOV GP IgGs Lacking α1-3-Galactose and Neu5Gc Prolong Survival and Decrease Blood Viral Load in EBOV-Infected Guinea Pigs. PLoS One 11: e0156775.
- L, Hervouet J, Minault D, Concordet JP, Dugast E, Vanhove B, et al. (2015) Immune phenotype and IgG characteristics of Neu5Gc and alpha-1-3-galdouble knock-out pigs. Xenotransplantation 22:S2-S47.
- Wheeler SF, Domann P, Harvey DJ (2009) Derivatization of sialic acids for stabilization in matrix-assisted laser desorption/ionization mass spectrometry and concomitant differentiation of alpha(2 --> 3)- and alpha(2 --> 6)-isomers. Rapid communications in mass spectrometry: RCM 23:303-312.
- Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G (1971) The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem 246: 1461-1467.
- Park EI, Mi Y, Unverzagt C, Gabius HJ, Baenziger JU (2005) The asialoglycoprotein receptor clears glycoconjugates terminating with sialic acid alpha 2,6GalNAc. Proc Natl Acad Sci USA 102: 17125-17129.
- [No authors listed] Clears glycoconjugates terminating with sialic acid alpha 2,6GalNAc. Proceedings of the National Academy of Sciences of the United States of America 102:17125-17129.
- Unverzagt C, André S, Seifert J, Kojima S, Fink C, et al. (2002) Structure-activity profiles of complex biantennary glycans with core fucosylation and with/without additional alpha 2,3/alpha 2,6 sialylation: synthesis of neoglycoproteins and their properties in lectin assays, cell binding, and organ uptake. J Med Chem 45: 478-491.
- Gomes de Oliveira AG, Roy R, Raymond C, Bodnar ED, Venkata ST, et al. (2015) A systematic study of glycopeptide esterification for the semi-quantitative determination of sialylation in antibodies. Rapid Commun Mass Spectrom 29: 1817-1826.
- de Haan N, Reiding KR, Haberger M, Reusch D, Falck D, et al. (2015) Linkage-specific sialic acid derivatization for MALDI-TOF-MS profiling of IgG glycopeptides. Anal Chem 87: 8284-8291.
- Harvey DJ (2005) Fragmentation of negative ions from carbohydrates: part 3. Fragmentation of hybrid and complex N-linked glycans. J Am Soc Mass Spectrom 16: 647-659.
- Reiding KR, Blank D, Kuijper DM, Deelder AM, Wuhrer M (2014) High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Analytical chemistry 86:5784-5793.
- Fukushima K, Takahashi T, Ito S, Takaguchi M, Takano M, et al. (2014) Terminal sialic acid linkages determine different cell infectivities of human parainfluenza virus type 1 and type 3. Virology 464-465: 424-431.
- Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV, et al. (2013) General mechanism for modulating immunoglobulin effector function. Proceedings of the National Academy of Sciences of the United States of America 110:9868-9872.
- Snovida SI, Chen VC, Krokhin O, Perreault H (2006) Isolation and identification of sialylated glycopeptides from bovine alpha1-acid glycoprotein by off-line capillary electrophoresis MALDI- TOF mass spectrometry. Analytical chemistry 78:6556-6563.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 181044
- [From(publication date):
October-2016 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 179973
- PDF downloads : 1071