Gastrointestinal (GI) Tract Microbes and Microbial Neurotoxins in the Human Central Nervous System (CNS) in Alzheimer’s Disease (AD)
Received: 03-Nov-2017 / Accepted Date: 09-Nov-2017 / Published Date: 16-Nov-2017 DOI: 10.4172/2161-0460.1000399
Abstract
Our ongoing appreciation of the magnitude and complexity of the human microbiome has resulted in a reassessment of many fundamental concepts of the contribution of the microbial community to neurological health and disease. The assumption of the privileged immunological and compartmentalized status of the human central nervous system (CNS) has been recently challenged in multiple investigations - particularly because microbial-derived nucleic acid sequences and highly neurotoxic and pro-inflammatory exudates representative of gastrointestinal (GI) tract Gram-negative anaerobic bacteria are showing up within CNS compartments. Unanticipated microbial presence has also recently been discovered in the anatomical regions of the CNS implicated in pro-inflammatory pathological signaling and neuro-immune disruptions that characterize progressive and lethal neurodegenerative diseases of the CNS such as Alzheimer’s disease (AD). This communication (i) will briefly review some very recent research on the contribution of the GI tract microbiome and microbial neurotoxins to inflammatory neurodegeneration in the CNS with emphasis on AD wherever possible; (ii) will review the evidence that the GI tract microbiome may have an increasing inter-relationship with the CNS via leaky barriers as we age; and (iii) will review recent experimental findings that support the intriguing possibility that the CNS may possess its own microbiome whose basal complexity is in part derived from the GI tract microbiome of the host
Overview
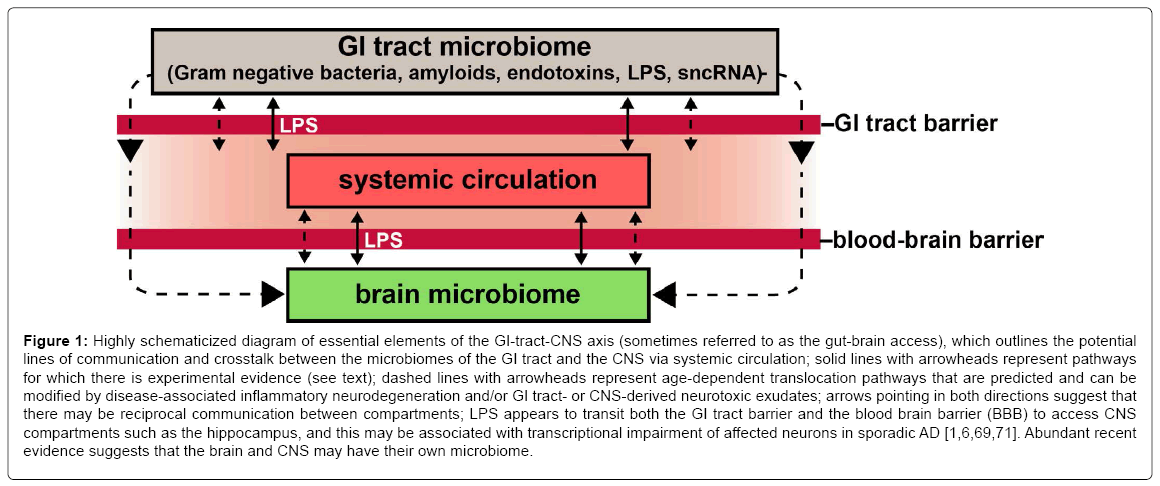
The microbiome of the healthy human gastrointestinal (GI) tract consists of a remarkably rich, diverse and dynamic community of microbiota consisting of virtually every classification of microbe known. These include, predominantly, aerobic, facultative and anaerobic bacteria of many different genera and species, viruses, protozoa, fungi and other microorganisms [1-6]. Normally the GI tract microbiome performs key functions in the maintenance of human health, including the biosynthesis of vitamins and vitamin precursors, protection against pathogen overgrowth, energy extraction and mobilization, the processing of dietary fiber and the modulation of the host-immune system [4,6-8]. There is emerging evidence that GI tract-derived bacterial exudates or GI tract bacteria and other microbes can find their way from the GI tract to compartments of the central nervous system (CNS) via a leaky epithelium of the GI tract and blood-brain barrier (BBB) via the systemic circulation (Figure 1). Normally, stringently compartmentalized bacteria and other microbial-derived neurotoxins may escape GI tract barriers and transit BBBs that become leaky or dysfunctional with age and/or disease [9-11]. Here we document several of the most recent illustrative and highly specific insights into the potential contribution of pathogenic microbes or their neurotoxic exudates from the GI tract to altered signaling across the GI-CNS axis and other GI tract-derived pathogenic components, including GI-tractderived nucleic acid sequences, to the development of AD and related forms of inflammatory neurodegeneration.
Figure 1: Highly schematicized diagram of essential elements of the GI-tract-CNS axis (sometimes referred to as the gut-brain access), which outlines the potential lines of communication and crosstalk between the microbiomes of the GI tract and the CNS via systemic circulation; solid lines with arrowheads represent pathways for which there is experimental evidence (see text); dashed lines with arrowheads represent age-dependent translocation pathways that are predicted and can be modified by disease-associated inflammatory neurodegeneration and/or GI tract- or CNS-derived neurotoxic exudates; arrows pointing in both directions suggest that there may be reciprocal communication between compartments; LPS appears to transit both the GI tract barrier and the blood brain barrier (BBB) to access CNS compartments such as the hippocampus, and this may be associated with transcriptional impairment of affected neurons in sporadic AD [1,6,69,71]. Abundant recent evidence suggests that the brain and CNS may have their own microbiome.
Emerging Evidence For Pathogenic GI Tract-CNS Communication In AD
Although we are still in the relatively early stages of 16S RNA sequencing analysis, nucleic acid sequence acquisition, bioinformatics and immunocytochemistry of the healthy, aging control human GI tract microbiome versus the GI tract microbiome in AD researchers have recently reported: (i) that RNA sequencing of the V4 region of the 16S rRNA gene in control (n=25) and AD (n=25) patients indicated that sequences representing a total of 4.8 million sequence reads [mean ± one standard deviation (SD) of ~96,000 ± 32,000 reads/participant] were clustered into operational taxonomic units (OTUs; an operational definition used to classify groups of closely related individuals) at 97% similarity [3,5]; (ii) that the final OTU dataset for the control and AD groups consisted of 972 OTUs classified into 95 genera, 46 families, 24 orders, 17 classes and 9 phyla (5); (iii) that there are about ~1000 bacterial species in a typical healthy human GI tract microbiome with a ‘GI tract microbial core’ of the genus Bacteroides and Firmicutes predominating, and with Proteobacteria, Verrumicrobia, Actinobacteria, Fusobacteria and Cyanobacteria making up the remainder [3,5,6,12-14]; (iv) that at least 13 of 95 microbial genera examined exhibit differential abundance between AD and age-matched control populations [4- 6,12]; (v) that there are consistent trends observed between relative GI tract bacterial abundance and CSF biomarkers of AD neuropathology [12,15]; (vi) that 16S rRNA next generation sequencing analysis has identified multiple microbial-derived nucleic acids in the AD brain, some of which are known to have pathogenic potential [9]; (vii) that remarkably, the abundance and complexity of microbial populations or their exudates in the GI tract is an approximate reflection of CNS microbial complexity, including microbiome-derived nucleic acid sequences and Gram negative-derived neurotoxins of the GI tract microbiome, such as lipopolysaccharide (LPS; [6,9,15-18]); and (viii) that there is a decrease in richness and diversity of GI tract bacteria in AD compared to age-matched controls, a finding that parallels results observed in other GI, vascular or neurological conditions linked to GI tract microbiome alterations, including those associated with obesity, diabetes, inflammatory bowel disease, systemic inflammation and Parkinson’s disease [9,19-22]. Related to the points above, overall the GI tract microbiome of AD patients was found to exhibit highly selective abundance and compositional differences in genus and species from control age- and sex-matched individuals and exhibited phylum- and genus-wide differences in bacterial abundance including a significantly increased abundance of Bacteroidetes and decreased abundance of Firmicutes and Bifidobacterium in the AD-affected brain [9,14-17,23-26]. Very recently, at least 2 independent publications have shown an enrichment of LPS within the neocortex or hippocampus of ADaffected brain [6,27-29].
Bacteroidetes and Bacterioides Fragilis Abundance and Proliferation in AD
A commensal genus of non-endospore-forming, rod-shaped bacillus, Bacteroidetes are a human distal-GI-tract-abundant microbe whose membranes contain sphingolipids or glycosylceramides; these complex lipids play important roles in signal transmission and cell recognition and are an unusual feature of obligately anaerobic Gram negative microbes [9,16,22]. Generally microbial Bacteroidetes species such as Bacteroides fragilis (B. fragilis) appear to be generally beneficial to human health via their ability to cleave dietary fiber into digestible short-chain fatty acids, their production of useful polysaccharides, vitamins and their precursors, volatile fatty acids and other nutrients, however, when they escape the confines of a healthy GI tract microbiome they elicit inflammatory systemic pathology with substantial morbidity and mortality [15,22-24,26]. It is well known that diet has a role in regulating the composition, complexity and speciation of the GI tract microbiome - for example species of Bacteroidetes proliferate in animal models fed high fat-cholesterol (HF-C) diets deprived of dietary fiber [25]. Besides their prodigious output of LPS, endotoxins generated by B. fragilis are a leading cause of anaerobic bacteremia and systemic inflammatory distress through the production of the highly pro-inflammatory zinc metalloproteinase B. fragilis toxin (BFT) fragilysin [24,26]. Recently it has been shown that BFT effectively disrupts epithelial cells of the GI tract barrier via cleavage of the E-cadherin, a synaptic adhesion zonula adherens protein essential for barrier exclusion functions [26,27].
Of further related interest are the recent observations: (i) that the increased abundance of gram-negative GI tract bacteria such as B. fragilis in AD patients appears to result in increased generation and translocation of LPS and other Bacteroides-derived neurotoxins from the GI tract into the systemic circulation, which in turn may contribute to AD neuropathology through the release of pro-inflammatory cytokines, systemic inflammation, an increase in GI-tract or BBB permeability or other AD-relevant pathogenic mechanisms [9,29-32], and (ii) that an increase in Bacteroidetes in the GI tract is also associated with Parkinson’s disease (PD; [33]) and with sporadic AD hippocampus and neocortex, two anatomical regions targeted by the AD process [6,34-36]. Importantly, while only the inflammatory potential of LPS towards primary human neuronal-glial (HNG) co-cultures have been studied and quantified by the induction of the pro-inflammatory NFkB p50/p65 complex, Bacteroidetes species are capable of secreting an unusually complex array of highly lethal neurotoxins including amyloids, sncRNAs and endotoxins which, when released from the confines of the healthy GI tract, are systemically pathogenic and can be highly detrimental to the homeostatic function of human CNS neurons [15].
Bacterial Nucleic Acid Sequences In CNS Compartments
Interest in the potential role of the microbiome in human health and disease has rapidly expanded over the last several years with the advent of novel 16S RNA sequencing methodologies and bioinformatics technologies that interrogate the genetics of very complex microbial communities without the need for classical microbiological assays. As fore-mentioned, microbes such as B. fragilis and Escherichia coli (E. coli), abundant anaerobic Gram-negative bacilli of the human GI-tract microbiome, appear to direct critical regulatory roles of pathogenicity through the stress-induced secretion of a complex mixture of bacterial amyloids, endotoxins and exotoxins, ‘microRNA-like’ sncRNAs and LPS. Recently work from several independent groups has further described the presence of intact bacteria, bacterial-derived nucleic acid sequences and/or bacterial-derived neurotoxins such as a highly pro-inflammatory LPS that is associated with neuronal parenchyma and in particular the neuronal nuclei of anatomical regions of the ADaffected brain exhibiting characteristic neuropathology [1,9,28,29,34-39]. Interestingly, the close association of bacterial LPS with neuronal nuclei may prevent the efficient export of messenger RNA (mRNA) from a highly active neuronal genome resulting in the down-regulation of gene expression in AD as is widely observed [6,15]. The strikingly large and unexpected bacterial loads of GI-tract-derived bacteria or their neurotoxic exudates within AD tissues are strongly suspected to upset the efficient operation of these normally highly metabolically active repositories of genetic information.
Lipopolysaccharides (LPS) in the Brain and CNS
Lipopolysaccharides (LPSs), also known as lipoglycans, are 10-20 kDa structural components of the outer leaflet of the outer membrane of Gram-negative bacteria and consist of 3 ‘modular’ components: (i) a hydrophobic lipid domain known as lipid A, that is responsible for the toxic properties of the molecule; (ii) a hydrophilic core polysaccharide chain; and (iii) a repeating hydrophilic O-antigenic oligosaccharide side chain specific to the bacterial serotype [40-42]. The toxicity and inflammatory potential of different bacterial LPSs vary depending on the composition of these 3 modular components; for example the LPS of the anaerobic B. fragilis (BF-LPS), is a remarkably pro-inflammatory glycolipid, perhaps the most pro-inflammatory LPS known, and capable of triggering systemic inflammation and the release of proinflammatory cytokines after translocation across the GI tract into systemic circulation [23,34-36,43]. These neurotoxic glycolipids are shed into the extracellular space, play key pathological roles in hostpathogen interactions, pro-inflammatory signaling and the activation of the innate-immune system of the host [23,44-46]. As an abundant obligate anaerobe resident of the distal human GI tract microbiome BFLPS is unusually immunogenic and highly pro-inflammatory toward human neurons in primary culture [8,15,24,37,47-50]. It has been very recently shown (i) that both LPS and BF-LPS are abundant in anatomical regions of the human brain’s limbic system in AD, including the hippocampus and neocortex that exhibit focused neuropathology and an intense inflammatory response as is characteristic of the AD process [6,28,29]; and (ii) that BF-LPS is an extremely potent inducer of pro-inflammatory gene signaling pathways, as quantified by the evolution of the pro-inflammatory transcription factor NF-kB p50/p65 complex HNG cells in primary co-culture [51]. To further cite several highly relevant research investigations: (i) using immunocytochemistry E. coli K99 pili protein and E. coli LPS levels were found to be significantly greater in AD compared to control brains, finding that in AD LPS co-localized with Aβ1-40/42-positive amyloid plaques surrounding cerebral vessels [29]; (ii) using an immunocytochemical approach Zhao et al. have discovered LPS in very short post-mortem interval (PMI) AD hippocampus (1-3 h PMI) to levels up to 36-fold over age-matched controls [34-36]; (iii) co-incubation of Aβ peptide with LPS potentiates amyloidogenesis and fibrillogenesis (amyloid fiber formation; [34,52]); (iv) systemic injection of LPS in wild-type and transgenic AD mice results in greater amyloid deposition and tau pathology [53]; and (v) a selective enrichment of LPS specifically associated with the neuronal nuclear membrane in AD was found to be correlated to a down-regulation in the output of transcription products from neuronal nuclei ([34]; manuscript under review). This suggests that GI-tract microbiome-derived LPS may be an important initiator and/or significant contributor to failure in adequate gene expression the AD CNS including those genes involved in the modulation of inflammatory signaling (Figure 1; manuscript submitted). In humans, intestinal permeability increases with age and elderly individuals show an association between increased LPS-binding protein (a marker of microbial translocation) and inflammation [5,10,31] and gram-negative E. coli fragments co-localize with amyloid plaques [29]. Taken together these results suggest that the increased abundance of Bacteroides in patients with AD may result in an increased translocation of LPS from the gut to the systemic circulation, which in turn may exacerbate AD pathology through enhanced pro-inflammatory signaling or related pathogenetic mechanisms [5,10,54,55]. These findings further suggest that LPS and perhaps other bacterial-derived amyloids, sncRNAs, endotoxins and neurotoxins are localized to the same anatomical regions involved in AD-type neuropathology and these may be significant initiators or progressive contributors to inflammatory degeneration, amyloidogenesis and/or an altered innate-immune response in the AD CNS (Figure 1; [5,34,54,55]).
Thanatomicrobiome
Evidence for the considerable biological and biophysical efforts in keeping the GI tract microbiome contained within GI tract compartments, and from expansion beyond its normal boundaries, comes from the analysis of the human GI tract microbiome and CNS at the time of death. Evidence for the possible existence of a brain microbiome comes from the study at the point of death that the thanatomicrobiome (after the Greek ‘thanatos’=death; the microbiome that appears and subsequently proliferates at the time of death) plays a primary role in the rapid decomposition and decay of host tissues [34,56-61]. In the Western world death is defined as the complete loss and irreversible absence of brain activity including involuntary activities (such as heartbeat and breathing) necessary to sustain life [56-58]. To satisfy the legal criterion of brain death, two isoelectric electroencephalograms (flat-line or zero line EEG; ZLEEG) must be demonstrated 24 h apart [56-60]. As a human body decomposes, microbes proliferate in the brain and other organs in a time-dependent manner and as a human body decays the thanatomicrobiome shifts in its microbial character from that normally encountered in the healthy GI tract microbiome [34,57,58]. At this point in time the microbial species that constitute the thanatomicrobiome ‘core’ begin to rapidly proliferate in large part due to of a dysfunctional innate-immune system. Ongoing work from temporal and PMI studies on the thanatomicrobiome across defined PMIs further indicate that the majority of the microbes within the human body and those which propagate most rapidly at the time of death are obligate anaerobes that begin to non-randomly proliferate from the GI tract continuing throughout the human organs over the PMI [57-60]. Kinetic considerations and limitations involving the thanatomicrobiome support the idea of a brain microbiome due to the rapidity in which microbial populations appear and proliferate within the brain and CNS suggest that there would not be enough time for GI tract bacteria to cross both the GI tract and BBB to enter these CNS compartments to proliferate, so they may be already be resident within the confines of the BBB and/or compartments of the CNS [6]. Further support originates from the observation that blood-borne microbes are not appreciably elevated at early time-points of the PMI ([60,61]; unpublished observations; manuscript in preparation).
Leaky Gastrointestinal (GI) Tract and Blood-Brain Barriers (BBB)
There are energetically costly efforts in keeping the GI tract and BBB intact and properly functioning; however both the epithelial barriers of the GI and the BBB become significantly more ‘leaky’ and permeable to neurotoxins over the course of aging [10,11]. This agerelated increase in permeability appears to make the normally protected CNS compartments more exposed and potentially susceptible to neurotoxins generated by dietary and/or environmental pathogens and GI tract-resident microbes normally contained within the GI compartment [10,11,62]. Moreover (i) dietary, environmental and pathological infections, including chronic bacterial or viral influences, or disease-related toxins such as LPS, sncRNA and the 42-amino acid amyloid beta (Aβ42) peptide can progressively and permanently alter blood-brain barrier permeability and thereby facilitate cerebral vascular dysfunction and cerebral colonization by opportunistic microbes as we age [11,63]; and (ii) microbial dysbiosis in the GI tract microbiome can induce inflammatory signaling detrimental to both GI tract and BBB dysfunction that is associated with the pathogenesis of obesity, type 2 diabetes and AD [11,22,64]. Indeed, one major contributing factor to AD pathogenesis is cerebral vascular dysfunction due in part to the loss of the protective function of the BBB and impaired clearance of excess neurotoxic Aβ peptides that induce perivascular association with amyloid peptides, vascular perturbation and altered neurovascular function [63,64]. The relationship between the prevalence of AD and vascular permeability factors along the microbiota-GI-tract-CNS axis as a bidirectional communication system that also includes the supplementary influences of neural, immune, endocrine, and metabolic pathways is exceedingly complex and currently not fully understood. A more complete understanding of the underlying mechanisms that maintain GI tract and BBB homeostasis should provide novel mechanistic insights and more efficacious therapeutic strategies ultimately useful in the clinical management of AD [11,63,64].
Unresolved Issues
GI tract microbes, and especially Gram negative anaerobic bacteria such as B. fragilis, besides secreting a highly pro-inflammatory BF-LPS and a B. fragilis-derived fragilysin also secrete a complex mixture of additional endotoxins, amyloids and sncRNAs which have been only partially characterized [9,34,51]. Altogether the one thousand or so species of bacteria in the human GI tract might be expected to secrete exceedingly complex mixtures of neurotoxins of many different types and compositions whose actions might exhibit synergism in their neurotoxic potential and abilities to transverse normally protective physiological barriers [1,51,65]. An alternative, yet, highly speculative view is that the human CNS may have its own highly compartmentalized microbiome, which could also explain the presence of abundant bacterial secretory components in the brain as well as multiple forms of microbial-derived nucleic acid sequences [22,50,65,66]. Virtually nothing in known about plant derived microbiomes, plant microRNAs and viroids, natural sncRNAs widely distributed throughout the plant kingdom which are ingested in a regular human diet and how they may contribute to the human microbiome in health and disease [17,65]. Importantly, this short review has focused on bacteria as the major component of the GI tract microbiome; it has not addressed the potential contribution of viruses, protozoa, fungi and other microorganisms to functions along the GI-tract-CNS axis, but they might be appreciable [1-6,67-71].
Lastly, comprehensive knowledge of the number and abundance of each organ’s thantomicrobiome signature could be useful to forensic microbiologists as a novel source of data for estimating PMI and agonal processes. These data combined with RNA sequencing and robust bioinformatics analysis should also be invaluable in aiding researchers who use post-mortem tissues in their research work and in the study of microbial speciation in the GI tract, forensic criminology, and the study of microbiome-host genetics, espcially in the later stages of life.
Conclusion
Our progress in understanding the biophysics, molecular-genetics and neurobiology of the human GI-tract-CNS axis continues to evolve. What we clearly understand is that the human CNS is under constant assault by a wide array of extrinsic, intrinsic and opportunistic neurotrophic microbes and pathogens including bacteria, virus, fungus, nucleic-acid free prions, viroids or sncRNAs found both in the environment and those that are ingested and/or generated from within and normally contained within the GI tract microbiome. Remarkably, virtually every type of microbe known has been implicated in contributing to the susceptibility and pathogenesis of the AD process [17,65]. The concern of contamination of short PMI brain tissues with GI tract microbes is not likely because typically, in our hands and at our institution, and in the brain tissue banks used in these studies the brain is the first organ to be removed from the deceased patient at autopsy while the rest of the tissues and organs remain intact ([56,58]; unpublished observations). GI-tract and BBB barrier permeability factors are normally highly effective natural biophysical barriers encountered along the microbiota-GI tract-brain axis as a bidirectional communication system for microbial and/or neurotoxin translocation, however their selective screening for small molecules and the biophysics of the translocation mechanism in aging and neurologically diseased brains are currently not completely understood. These physiological barrier systems also involve other neural, immune, endocrine, and metabolic signaling pathways that is exceedingly complex in both structure and function. A more complete understanding of the underlying mechanisms that maintain GI tract and BBB homeostasis should provide novel mechanistic insights and therapeutic strategies useful in understanding basic aging mechanisms and in the clinical management of AD [11,22,64]. These very recent findings continue to add AD to the growing list of diseases associated with alterations in GI tract-CNS microbial communication along the microbiota-gut-brain axis, as well as suggest that bacterial communities along this axis may provide novel targets for highly effective therapeutic intervention. A possible causal relationship between GI tract abundant microbes such as B. fragilis, other potentially pathogenic GI tract microbiota, their neurotoxic exudates that drive inflammatory signaling and immune disruptions and AD is certainly in need of further in depth investigation.
References
- Bhattacharjee S, Lukiw WJ (2013) Alzheimer's disease and the microbiome. Front Cell Neurosci 7: 153.
- Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, et al. (2014) Pathogenic microbes, the microbiome and Alzheimer's disease (AD). Front Aging Neurosci 6: 127.
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K (2014) Gut microbes and the brain: Paradigm shift in neuroscience. J Neurosci 34: 15490-15496.
- Quigley EMM (2017) Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep 17: 94.
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, et al. (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7: 13537.
- Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol 7: 318.
- Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK (2010) Host-bacterial symbiosis in health and disease. Adv Immunol 107: 243-274.
- Ghaisas S, Maher J, Kanthasamy A (2016) Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther 158: 52-62.
- Emery DC, Shoemark DK, Batstone TE, Waterfall CM, Coghill JA, et al. (2017) 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front Aging Neurosci 9: 195.
- Tran L, Greenwood-Van Meerveld B (2013) Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci 68:1045-1056.
- Chakraborty A, de Wit NM, van der Flier WM, de Vries HE (2017) The blood brain barrier in Alzheimer’s disease. Vascul Pharmacol 89: 12-18.
- Jovel J, Patterson J, Wang W, Hotte N, O'Keefe S, et al. (2016) Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol 7: 459.
- Lloyd-Price J, Abu-Ali G, Huttenhower C (2016) The healthy human microbiome. Genome Med 8: 51.
- Lukiw WJ (2016) Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer's disease. Front Microbiol 7: 1544.
- Hill JM, Bhattacharjee S, Pogue AI, Lukiw WJ (2014) The gastrointestinal tract microbiome and potential link to Alzheimer's disease. Front Neurol 5: 43.
- Hill JM, Lukiw WJ (2016) MicroRNA (miRNA)-mediated pathogenetic signaling in Alzheimer’s disease (AD) Neurochem Res 41: 96-100.
- Jaber V, Zhao Y, Lukiw WJ (2017) Alterations in micro RNA-messenger RNA (miRNA-mRNA) coupled signaling networks in sporadic Alzheimer’s disease (AD) hippocampal CA1. J Alzheimers Dis Parkinsonism 7: 312.
- Dicksved J, Halfvarson J, Rosenquist M, Järnerot G, Tysk C, et al. (2008) Molecular analysis of the gut microbiota of identical twins with Crohn's disease. ISME J 2: 716-727.
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, et al. (2009) A core gut microbiome in obese and lean twins. Nature 457: 480-484.
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, et al. (2015) Colonic bacterial composition in Parkinson's disease. Mov Disord 30: 1351-1360.
- Jiang C1, Li G2, Huang P1, Liu Z1, Zhao B1 (2017) The gut microbiota and Alzheimer's disease. J Alzheimers Dis 58: 1-15.
- Hofer U (2014) Microbiome: B. fragilis and the brain. Nat Rev Microbiol 12: 76-77.
- Fathi P, Wu S (2016) Isolation, detection and characterization of enterotoxigenic Bacteroides fragilis in clinical samples. Open Microbiol J 10: 57-63.
- Heinritz SN, Weiss E, Eklund M, Aumiller T, Heyer CM, et al. (2016) Impact of a high-fat or high-fiber diet on intestinal microbiota and metabolic markers in a pig model. Nutrients 8.
- Choi VM, Herrou J, Hecht AL, Teoh WP, Turner JR, et al. (2016) Activation of Bacteroides fragilis toxin by a novel bacterial protease contributes to anaerobic sepsis in mice. Nat Med 22: 563-567.
- Seong E, Yuan L, Arikkath J (2015) Cadherins and catenins in dendrite and synapse morphogenesis. Cell Adh Migr 9: 202-213.
- Zhan LS, Davies SS (2016) Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med 8: 46
- Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, et al. (2016) Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87: 2324- 2332.
- Romond MB, Colavizza M, Mullié C, Kalach N, Kremp O, et al. (2008) Does the intestinal bifidobacterial colonisation affect bacterial translocation? Anaerobe 14: 43-48.
- Man AL, Bertelli E, Rentini S, Regoli M, Briars G, et al. (2015) Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci 129: 515-527.
- Zhao Y, Dua P, Lukiw WJ (2015) Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer’s disease (AD). J Alzheimers Dis Parkinsonism 5: 177.
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, et al. (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30: 1351-1360.
- Zhao Y, Cong L, Jaber V, Lukiw WJ (2017) Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer's disease brain. Front Immunol 8: 1064.
- Zhao Y, Lukiw WJ (2015) Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer's disease (AD). J Nat Sci 1: e138.
- Foster JA, Lyte M, Meyer E, Cryan JF (2016) Gut microbiota and brain function: An evolving field in neuroscience. Int J Neuropsychopharmacol 19: pyv114.
- Bagyinszky E, Giau VV, Shim K, Suk K, An SSA, et al. (2017) Role of inflammatory molecules in the Alzheimer's disease progression and diagnosis. J Neurol Sci 376: 242-254.
- McManus RM, Heneka MT (2017) Role of neuroinflammation in neurodegeneration: New insights. Alzheimers Res Ther 9: 14
- Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D (2016) Lipopolysaccharide transport and assembly at the outer membrane: The PEZ model. Nat Rev Microbiol 14: 337-345.
- Morita-Takemura S, Nakahara K, Tatsumi K, Okuda H, Tanaka T, et al. (2016) Changes in endothelial cell proliferation and vascular permeability after systemic lipopolysaccharide administration in the subfornical organ. J Neuroimmunol 298: 132-137.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, et al. (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761-1772.
- Hill JM, Lukiw WJ (2015) Microbial-generated amyloids and Alzheimer's disease (AD). Front Aging Neurosci 7: 9.
- Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human GI-tract microbiome and their potential impact on Alzheimer’s disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol 7: 318.
- Maldonado RF, Sá-Correia I, Valvano MA (2016) Lipopolysaccharide modification in gram-negative bacteria during chronic infection. FEMS Microbiol Rev 40: 480-493.
- Alkasir R, Li J, Li X, Jin M, Zhu B (2016) Human gut microbiota: The links with dementia development. Protein Cell 8: 90-102.
- Rogers MA, Aronoff DM (2016) The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect 22: 178e1-178e9.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK (2016) The central nervous system and the gut microbiome. Cell 167: 915-932.
- Shivaji S (2017) We are not alone: A case for the human microbiome in extra intestinal diseases. Gut Pathog 9: 13.
- Lukiw WJ (2016) The microbiome, microbial-generated pro-inflammatory neurotoxins and Alzheimer’s disease. J Sport Health Sci 5: 393-396.
- Asti A, Gioglio L (2014) Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis 39: 169-179.
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM (2005) Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci 25: 8843-8853.
- Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, et al. (2003) Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis 14: 133-145.
- Â Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, et al. (2016) Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev 74: 624-634.
- Â Goila A, Pawar M (2009) The diagnosis of brain death. Indian J Crit Care Med 13: 7-11.
- Â Javan GT, Finley SJ, Abidin Z, Mulle JG (2016) The thanatomicrobiome: A missing piece of the microbial puzzle of death. Front Microbiol 7: 225.
- Â Can I, Javan GT, Pozhitkov AE, Noble PA (2014) Distinctive thanatomicrobiome signatures found in the blood and internal organs of humans. J Microbiol Methods 106: 1-7.
- Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, et al. (2016) Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev 74: 624-634.
- Â Lukiw WJ, LeBlanc HJ, Carver LA, McLachlan DR, Bazan NG (1998) Run-on gene transcription in human neocortical nuclei - implications for neurodegenerative disease. J Mol Neurosci 11: 67-78.
-  Brenner SR (2013) Blue-green algae or cyanobacteria in the intestinal microflora produce neurotoxins such as beta-N-methylamino-L-alanine which may be related to development of amyotrophic lateral sclerosis, AD and Parkinson’s dementia-complex. Med Hypotheses 80: 103.
- Goila A, Pawar M (2009) The diagnosis of brain death. Indian J Crit Care Med 13: 7-11.
- Javan GT, Finley SJ, Abidin Z, Mulle JG (2016) The thanatomicrobiome: A missing piece of the microbial puzzle of death. Front Microbiol 7: 225.
- Â Zhao Y, Cong L, Lukiw WJ (2017) Plant and animal microRNAs (miRNAs) and their potential for inter-kingdom communication. Cell Mol Neurobiol.
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, et al. (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30: 350-358.
- Â Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, et al. (2017) Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol Life Sci.
- Can I, Javan GT, Pozhitkov AE, Noble PA (2014) Distinctive thanatomicrobiome signatures found in the blood and internal organs of humans. J Microbiol Methods 106: 1-7.
- Â Kumar PS (2017) From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J Physiol 595: 465-476. Â
- Zhao Y, Cong L, Lukiw WJ (2017) Lipopolysaccharide (LPS) encapsulation of neuronal nuclei in sporadic Alzheimer’s disease (AD) neocortex is linked to an impairment of transcriptional output. Frontiers in Aging Neuroscience.
- Â Negi S, Singh H, Mukhopadhyay A (2017) Gut bacterial peptides with autoimmunity potential as environmental trigger for late onset complex diseases: In-silico study. PLoS ONE 12: e0180518.
Citation: Zhao Y, Cong L, Jaber V, Lukiw WJ (2017) Gastrointestinal (GI) Tract Microbes and Microbial Neurotoxins in the Human Central Nervous System (CNS) in Alzheimer’s Disease (AD). J Alzheimers Dis Parkinsonism 7: 399. DOI: 10.4172/2161-0460.1000399
Copyright: © 2017 Zhao Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7425
- [From(publication date): 0-2017 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 6360
- PDF downloads: 1065