Gastrointestinal (GI)-Tract Microbiome Derived Neurotoxins and their Potential Contribution to Inflammatory Neurodegeneration in Alzheimer’s Disease (AD)
Received: 04-May-2021 / Accepted Date: 18-May-2021 / Published Date: 25-May-2021 DOI: 10.4172/2161-0460.1000525
Abstract
The human gastrointestinal (GI)-tract microbiome is a rich, complex and dynamic source of microorganisms that possess a staggering diversity and complexity. Importantly there is a significant variability in microbial complexity even amongst healthy individuals-this has made it difficult to link specific microbial abundance patterns with age-related neurological disease. GI-tract commensal microorganisms are generally beneficial to human metabolism and immunity, however enterotoxigenic forms of microbes possess significant potential to secrete what are amongst the most neurotoxic and pro-inflammatory biopolymers known. These include toxic glycolipids such as lipopolysaccharide (LPS), enterotoxins, microbial-derived amyloids and small non-coding RNA. One major microbial species of the GI-tract microbiome, about ~100-fold more abundant than Escherichia coli in deep GI-tract regions is Bacteroides fragilis, an anaerobic, rod-shaped Gram-negative bacterium. B. fragilis can secrete: (i) a particularly potent, pro-inflammatory and unique LPS subtype (BF-LPS); and (ii) a zinc-metalloproteinase known as B. fragilis-toxin (BFT) or fragilysin. Ongoing studies indicate that BF-LPS and/or BFT disrupt paracellular-and transcellular-barriers by cleavage of intercellular-proteins resulting in 'leaky' barriers. These barriers: (i) become defective and more penetrable with aging and disease; and (ii) permit entry of microbiome-derived neurotoxins into the systemic-circulation from which they next transit the blood-brain barrier and gain access to the CNS. Here LPS accumulates and significantly alter homeostatic patterns of gene expression. The affinity of LPS for neuronal nuclei is significantly enhanced in the presence of amyloid beta 42 (Aβ42) peptides. Recent research on the appearance of the brain thanatomicrobiome at the time of death and the increasing likelihood of a complex brain microbiome are reviewed and discussed. This paper will also highlight some recent advances in this extraordinary research area that links the pro-inflammatory exudates of the GI-tract microbiome with innate-immune disturbances and inflammatory-signaling within the CNS with reference to Alzheimer's disease (AD) wherever possible.
Keywords: Aging; Alzheimer’s disease; Bacteroides fragilis; Brain microbiome; Dysbiosis; Lipopolysaccharide; microRNA-146a; miRNA-155; Necrobiome; NF-kB; Reactive oxygen species; Thanatomicrobiome
Introduction and Overview
The human GI-tract microbiome
Consisting of about ~1014 microbes the gastrointestinal (GI)- tract microbiome comprises the largest repository of microorganisms anywhere in the human body. The unified human gastrointestinal genome (UHGG) collection has recently classified 204,938 non- redundant genomes from 4,644 GI-tract prokaryotes that encode >170 million protein sequences [1]. About ~98% of this microbiome are made up of several thousand genetically diverse bacterial species that form a complex, dynamic, and highly interactive microbial community [1-3]. While the GI-tract microbiome consists chiefly of aerobic and anaerobic bacteria, archaebacteria, fungi, protozoa, viruses and other microorganisms make up the remainder [4-7]. In general, this abundant, diverse and complex array of commensal microorganisms, with the highest known density of microbes found anywhere in nature (approaching ~1011 microbes per gm of stool [8]; contribute to the digestive, metabolic, nutritional and immune health of the host including protection against foreign microbial pathogens. However under non- homeostatic conditions or when stressed, or with aging and disease, or with diets low in fiber and high in fat and cholesterol these same microorganisms are capable of becoming dangerous ‘enterotoxigenic’ microbes generating some of the most potent pro-inflammatory neurotoxins known [7,9-16]; see below). There has recently been an expanding recognition of the ability of neurotoxins released by GI-tract resident microbes to influence metabolic and neuro-immune functions in health and disease well beyond the confines of an intact GI-tract [7,12,13,16,17]. As humans age, senescence of the GI-tract barrier integrity and immune system appears to result in an increased systemic microbial and/or microbial-derived neurotoxin burden as host-cell mediated biophysical barriers become “leaky” and innate-immune responses progressively decline. Maintaining the fidelity and integrity of biophysical barriers-including, prominently, the GI-tract barrier, and the blood-brain barrier (BBB)-against an increasing microbial pressure is essential to exclude an increasing bacterial load, in keeping GI-tract- derived neurotoxins like LPS and other microbial toxins out of the portal and/or systemic circulation, and in minimizing neurotoxin access to the brain and CNS compartments. In fact GI-tract microbiome-derived neurotoxin entry into the systemic circulation via leaky biophysical barriers and the induction of ‘systemic inflammation’: (i) appears to be a precursor event to the onset of inflammatory neurodegeneration in the brain and CNS [7,18-20]; and (ii) an important step in establishing pathways of pathological communication between the GI-tract, the systemic circulation and central innate-immune systems involved in progressive age-related neurological disease [17,18,21-24]. Multiple independent reports support the observation of early GI-tract disruption, increased pro-inflammatory neurotoxins in the systemic circulation and/or BBB disruption in AD brain long before proinflammatory neurodegenerative changes and cognitive impairment occur [12,20, 25-29].
Major GI-tract Microbiome Bacterial Divisions-Bacteroides and Firmicutes
There are about ~52 major divisions of bacteria currently recognized in nature, however evolution has selected just 2 of these major divisions-Bacteroidetes and Firmicutes-to populate and form the ‘bacterial core’ the human GI-tract microbiome [30-33]. The gene set of the GI-tract microbiome has been estimated to consist of about ~2.23 × 107 genes, or about ~839 times larger than the total number of genes in the human genome [1,14,32-35]. This large and diverse microbial population makes an important contribution to human metabolism, immune and endocrine health and neurobiology by contributing enzymes and metabolic factors that are not encoded by the human genome. In the healthy gut Gram-negative Bacteroidetes and Gram-positive Furmicutes coexist symbiotically, generating essential vitamins such as vitamin K, as well as most of the water‐soluble B vitamins including cobalamin, folate, pyridoxine, riboflavin and thiamine, bile acids, complex sugars, polysaccharides and carbohydrates, polyphenols and cofactors, process dietary constituents such as phytochemicals, and digest dietary fiber, via the anaerobic fermentation of cellulose, lignin, and pectin into small chain fatty acids (SCFAs; chiefly acetate, propionate, and butyrate). SCFAs may enter the systemic circulation directly; many of these are precursors that play key roles in neuro-immunoendocrine regulation [36,37]. Theseremarkable capabilities have made these 2 phyla especially suitable to support healthy human digestion, immunology and immunoregulation, lipid metabolism, physiology, biochemistry, neurochemistry, neuroendocrinology and neurobiology [1,13,30,37-43]. Of Bacteroidetes and Furmicutes, the phyla Bacteroidetes, represents the largest class of obligate anaerobic Gram-negative bacteria of the human GI-tract ileum and upper large intestine where a favorable pH of 6-7.5 and obligate anaerobic conditions coexist [15,44-47]. It should be kept in mind that as for all GI-tract microbes, various strains of Bacteroides: (i) exhibit significant variations in abundance, stoichiometry, anaerobic and pH requirements along the ~7-meter length of the human GI-tract [48-52]; and (ii) interestingly, exhibit a basal variation in abundance, speciation and complexity amongst individual humans [1,33]. It is tempting to speculate that a certain composition and make-up of the GI-tract microbiome by all resident microbes may predispose humans to health or disease throughout the time course of life.
Strains of the Genus Bacteroides-the Good, the Bad and Bacteroides fragilis
In contrast to their beneficial roles in the GI-tract microbiome, the genus Bacteroides have potential to release a remarkable and extraordinarily complex array of neurotoxins-several of the most widely recognized are lipopolysaccharide (LPS), truncated forms of LPS molecules known as lipooligosaccharides (LOS) and related enterotoxins, bacterial amyloids and small non-coding RNA (sncRNA), some of the later which resemble mammalian microRNAs (miRNAs) in their structure, molecular-genetic function and neurochemistry [7,12,21,53-58]. Strains of the abundant, GI-tract resident, obligate anaerobe bacillus Bacteroides are distinguished in part by their biosynthetic capabilities to synthesize and secrete unique forms of these exceptionally potent neurotoxins, the most well characterized are: (i) a Bacteroides fragilis-specific strain of LPS known as the glycolipid subtype BF-LPS, smaller and more compact than most known forms of Gram-negative bacterial-derived LPS; and (ii) a hydrolytic, zinc-dependent metalloproteinase toxin known as Bacteroides fragilis toxin (BFT) or fragilysin. Strains of Bacteroides that do not secrete BF-LPS and/or BFT that are generally beneficial to human health are termed non-toxigenic B. fragilis (NTBF), while those that do secrete LPS, BF-LPS, BFT (fragilysin) and other neurotoxic compounds are termed enterotoxigenic B. fragilis (ETBF; [59,60]). Recent characterization of BF-LPS and fragilysin have shown them to be: (i) unusually detrimental to the growth and health of cultured human brain cells in primary culture; (ii) toxic to transgenic murine models for age-related neurodegeneration including transgenic murine models of AD (TgAD; such as the 5 × FAD model; [61]); and (iii) probably the most pro-inflammatory microbial-derived endogenously-sourced lipoglycans and enterotoxins known [12,14, 41,42,54,57,61,62]. Indeed these secreted neurotoxic glycolipid and metalloproteinase molecules are deleterious to multiple aspects of microbiome-human brain interactions, including their ability to: (i) alter GI-tract and blood-brain barrier (BBB) structure, integrity and permeability; (ii) induce a dyshomeostasis in the systemic circulation involving biofluids such as cerebrospinal fluid (CSF), the glymphatic and meningeal lymphatic systems of the brain and CNS, and blood serum that can potentially allow these toxins to circulate throughout the body, brain neurovasculature and CNS compartments [63,64] (https://www.meliordiscovery.com/in-vivo-efficacy-models/lps-systemic-inflammation/); (iii) trigger alterations in central nervous system (CNS) and peripheral nervous system (PNS) homeostasis and equilibrium, including that of the retina and visual system [20,64,65](https://www.meliordiscovery.com/in-vivo-efficacy-models/lps-systemic-inflammation/); (iv) induce synaptic disruption and disorganization [42,66]; and (v) promote a progressive age-related inflammatory degeneration within CNS and PNS compartments [7,12,66,67]. LPS, BF-LPS and BFT: (i) have also been found to be not only the most pro-inflammatory lipoglycans and endogenously-generated enterotoxins anywhere in the human body but also among the most barrier-disruptive, reactive oxygen species (ROS)-and inflammatory cytokine-generating toxins so far identified; and (ii) have been shown to participate in inflammation-induced alterations in synaptic plasticity and altered cognition in transgenic murine models of neurodegeneration including TgAD models [7,12,41-43,54,55,62,66,68,69].
When these highly toxic exudates of enterotoxigenic strains of B. fragilis escape the microbial-dense environment of the human GI-tract they can produce substantial systemic inflammatory pathology with significant mortality and morbidity. ETBF can induce and support a “smoldering” systemic infection if displaced into the bloodstream or surrounding tissue following septic disease including septicemia, trauma including chronic traumatic encephalopathy (CTE) and/or surgery [5,6,29,70]. Indeed ETBF proliferation, LPS, BF-LPS and fragilysin have long been known to associate with: (i) generalized bacteremia, anaerobic bacteremia, sepsis, systemic inflammatory distress, systemic infection, systemic inflammation and diarrheal disease; (ii) GI-tract and multiple pro-inflammatory colon and colorectal cancers [54,71]; (iii) brain and intra-abdominal abscess including intra-tumoral abscess [72,73]; (iv) cellulitis, colitis, diabetic ulcer, diarrhea, necrotizing fasciitis and peritonitis [74]; (v) the development of neurological diseases involving inflammatory neurodegeneration in part via the disruption of epithelial cell-based GI-tract barriers via cleavage of the synaptic adhesion zonula adherens protein E-cadherin, and entry of neurotoxins across biophysical barriers including the BBB [75]; and (vi) microglial-mediated innate-immune responses, detoxifying and phagocytic mechanisms, and the promotion of amyloidogenesis-these are all characteristic of the progressive inflammatory aspects of neurodegeneration [25,56,57,76-79]. Very recently LPS-induced systemic inflammation has been associated with synaptic loss and cognitive decline in multiple human neurological disorders and in transgenic animal models, and a role for LPS-mediated microglial release of pro-inflammatory cytokines such as IL-1β, based on both in vivo and primary culture studies in vitro [7,12,42,61,62, 69,79,80].
It has been further established that enterotoxigenic strains of B. fragilis can rapidly proliferate in the mammalian GI-tract both in the absence of adequate dietary fiber and in the presence of high-fat cholesterol diets [12,14,27,81]. This proliferation enhances the intestinal abundance of B. fragilis and hence the potential of this Gram-negative obligate anaerobic species to secrete its formidable array of neurotoxic exudates. Both BF-LPS and fragilysin can leak through the normally protective mucosal barriers of the GI-tract intestinal endothelium to induce substantial inflammatory pathology both systemically and after BBB transit into vulnerable CNS compartments, including, prominently the parenchyma of neocortical brain cells [27,41,42,56,57,61,70,79].
Bacterial LPS-Proliferation by Inducers and Dietary Fiber
LPS molecules are amphipathic glycoconjugates consisting of a hydrophobic lipid domain attached to a core oligosaccharide and a distal polysaccharide with a lipid component that determines endotoxic activities and induces potent pathophysiological effects, are concentrated and shed from the outer membrane of Gram-negative bacteria [67,82]. The generation, abundance and secretion of these dynamic molecular glycolipid assemblies from Gram-negative bacteria can be stimulated by (i) proliferation of the Gram negative bacterial source itself; (ii) by neurotoxic ROS-inducing metallotoxins such as aluminum and other stressors [19,83]; and (iii) by other inducers, including those in the diet, such high fat-cholesterol (HF-C) consumption and insufficient dietary fiber, and by other unhealthy lifestyle factors [12,14,69,80,82-84]. Dietary deficiency-induced neurotoxicity of LPS are mediated in part by macrophages and microglia through the actions of tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β) and reactive oxygen species (ROS), which may be three of the most important endogenous mediators of the toxic pathophysiological effects of LPS [67,83,85]. Each Gram-negative bacillus, indeed each GI-tract resident microbe has the potential to secrete a slightly different LPS and/or neurotoxin assortment with slightly different lipid and oligosaccharide domain structures, abundances, activities, molecular properties and toxicities [12,53-55,57,67,82,86]. The modulatory effect of dietary fiber on the propagation of these microbial species and their neurotoxins remain incompletely understood. Different fiber types such as soluble versus insoluble dietary fibers appear to have different effects on the propagation of these enterotoxigenic microbes in the GI-tract and their microbe-derived neurotoxin-mediated effects, as well as on the proliferation and maintenance of GI-microbial diversity [12,14,48,81].
Generation Of Bacteroidetes-Derived Neurotoxins
As discussed above, the proliferation and absolute abundance of B. fragilis and B. fragilis-derived neurotoxins in the human GI-tract microbiome appears to be regulated in large part by the intake of dietary fiber, such that diets low in soluble fiber tend to proliferate anaerobic Gram-negative bacterial species and their exudates from the genus Bacteroides and others [7,12,14,15, 55,81, 87-90]. Post ingestion of dietary fibers into the GI-tract lead to their rapid catabolization into short-chain fatty acids (SCFAs), volatile fatty acids, polysaccharides and other digested components in part through the biosynthetic capability of multiple GI-tract microbial species including B. fragilis [54,71,87]. As described above, when B. fragilis or B. fragilis-derived neurotoxins escape the microbe-dense environment of the GI-tract they can induce substantial systemic inflammatory pathology with significant sickness, morbidity and mortality [7,12, 56,76,91]. In mammalian animal models multiple species of Bacteroidetes have been shown to propagate in mammalian models fed high fat-cholesterol (HFC) diets deprived of sufficient intake of dietary fiber suggesting that dietary fiber may have a significant role in regulating the abundance, complexity, speciation and and stoichiometry of microbial constituents of the GI-tract microbiome and their secretory products, including those of B. fragilis [12,14,81,91,92]. It is important to point out that: (i) that many other types of GI-tract resident Gram negative bacteria and/or other microbes also secrete a bewildering complexity of LPS and enterotoxins that are highly complex in their composition and may be synergistic in their neurotoxicity; (ii)that all of these broad spectrum of neurotoxins are endogenously sourced; and (iii) that they are constantly available with variable abundance throughout the lifespan of the host organism [7,12,54,81,87, 91-93] (Figure 1).
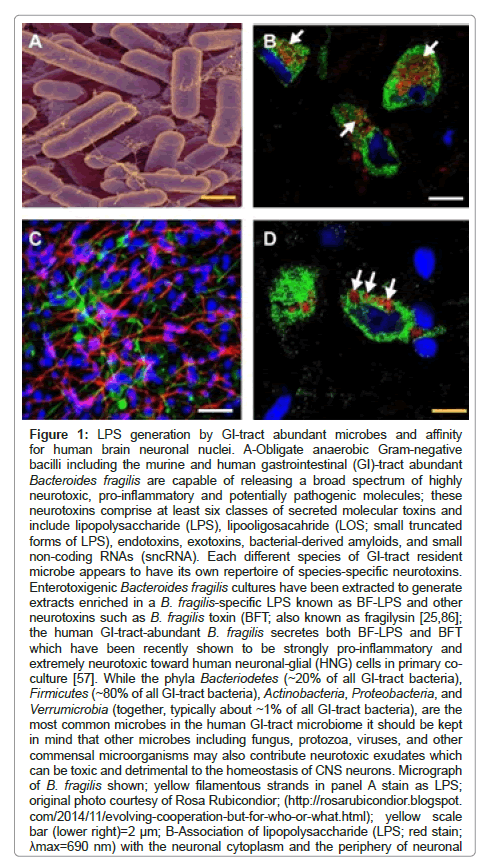
Figure 1: LPS generation by GI-tract abundant microbes and affinity for human brain neuronal nuclei. A-Obligate anaerobic Gram-negative bacilli including the murine and human gastrointestinal (GI)-tract abundant Bacteroides fragilis are capable of releasing a broad spectrum of highly neurotoxic, pro-inflammatory and potentially pathogenic molecules; these neurotoxins comprise at least six classes of secreted molecular toxins and include lipopolysaccharide (LPS), lipooligosacahride (LOS; small truncated forms of LPS), endotoxins, exotoxins, bacterial-derived amyloids, and small non-coding RNAs (sncRNA). Each different species of GI-tract resident microbe appears to have its own repertoire of species-specific neurotoxins. Enterotoxigenic Bacteroides fragilis cultures have been extracted to generate extracts enriched in a B. fragilis-specific LPS known as BF-LPS and other neurotoxins such as B. fragilis toxin (BFT; also known as fragilysin [25,86]; the human GI-tract-abundant B. fragilis secretes both BF-LPS and BFT which have been recently shown to be strongly pro-inflammatory and extremely neurotoxic toward human neuronal-glial (HNG) cells in primary coculture [57]. While the phyla Bacteriodetes (~20% of all GI-tract bacteria), Firmicutes (~80% of all GI-tract bacteria), Actinobacteria, Proteobacteria, and Verrumicrobia (together, typically about ~1% of all GI-tract bacteria), are the most common microbes in the human GI-tract microbiome it should be kept in mind that other microbes including fungus, protozoa, viruses, and other commensal microorganisms may also contribute neurotoxic exudates which can be toxic and detrimental to the homeostasis of CNS neurons. Micrograph of B. fragilis shown; yellow filamentous strands in panel A stain as LPS; original photo courtesy of Rosa Rubicondior; (http://rosarubicondior.blogspot. com/2014/11/evolving-cooperation-but-for-who-or-what.html); yellow scale bar (lower right)=2 µm; B-Association of lipopolysaccharide (LPS; red stain; ?max=690 nm) with the neuronal cytoplasm and the periphery of neuronal nuclei in AD neocortex; NeuN (neuron-specific green stain; ?max=520 nm) and DAPI (blue stain; ?max=470 nm); human superior temporal lobe AD neocortex (Brodmann A22); note affinity and polarized association of LPS for the neuronal nuclear envelope (white arrows; [93-97]); white scale bar (lower right)=20 µm; C-Control human neuronal-glial (HNG) cells in primary culture stained with antibody to glial fibrillary acidic protein (GFAP), a glial-specific cytoplasmic marker (green fluorescence; ?max=556 nm); an antibody to the neuron-specific cytoplasmic marker ßTUBIII, (red; ?max=702 nm), and with Hoescht 33258 to highlight the morphological features of both glial-and neuronal-cell nuclei (blue; ?max=461 nm; 2 weeks in culture; HNG cells are about ~60% neurons (red) and about ~40% astroglial (green); 20x magnification); human primary neuronal and glial “support” cell co-cultures are utilized because human neuronal cells do not culture well by themselves [25]; note large nuclear area, relative to both glial or neuronal cytoplasmic area, indicative of high levels of transcriptional activity; white scale bar (lower right)=20 µm; D-HNG (transplantation grade) cells in primary co-culture were used to study the dynamics of LPS association with neurons [4,62,47,92]. Dark blue stained spherical and oval bodies are DAPI-stained nonneuronal nuclei from astroglia; white arrows indicate punctate and perinuclear clustering of LPS and LPS affinity for the nuclear envelope as has been previously reported [25,26,30,46,47,89]; both in vivo analysis in AD brain (Figure 1B) and in vitro experiments using HNG cells in primary tissue culture (Figure 1D) show affinity of LPS for neuronal nuclei; yellow scale bar (lower right)=20 µm.
A-Obligate anaerobic Gram-negative bacilli including the murine and human gastrointestinal (GI)-tract abundant Bacteroides fragilis are capable of releasing a broad spectrum of highly neurotoxic, pro-inflammatory and potentially pathogenic molecules; these neurotoxins comprise at least six classes of secreted moleculartoxins and include lipopolysaccharide (LPS), lipooligosacahride (LOS; small truncated forms of LPS), endotoxins, exotoxins, bacterial-derived amyloids, and small non-coding RNAs (sncRNA). Each different species of GI-tract resident microbe appears to have its own repertoire of species-specific neurotoxins. Enterotoxigenic Bacteroides fragilis cultures have been extracted to generate extracts enriched in a B. fragilis-specific LPS known as BF-LPS and other neurotoxins such as B. fragilis toxin (BFT; also known as fragilysin [25,86]; the human GI-tract-abundant B. fragilis secretes both BF-LPS and BFT which have been recently shown to be strongly pro-inflammatory and extremely neurotoxic toward human neuronal-glial (HNG) cells in primary co-culture [57]. While the phyla Bacteriodetes (~20% of all GI-tract bacteria), Firmicutes (~80% of all GI tract bacteria), Actinobacteria, Proteobacteria, and Verrumicrobia (together, typically about ~1% of all GI-tract bacteria), are the most common microbes in the human GI-tract microbiome it should be kept in mind that other microbes including fungus, protozoa, viruses, and other commensal microorganisms may also contribute neurotoxic exudates which can be toxic and detrimental to the homeostasis of CNS neurons. Micrograph of B. fragilis shown; yellow filamentous strands in panel A stain as LPS; original photo courtesy of Rosa Rubicondior; (http://rosarubicondior.blogspot. com/2014/11/evolving-cooperation-but-for-who-or-what.html); yellow scale bar (lower right)=2 μm; B-Association of lipopolysaccharide (LPS; red stain; λmax=690 nm) with the neuronal cytoplasm and the periphery of neuronal nuclei in AD neocortex; NeuN (neuron-specific green stain; λmax=520 nm) and DAPI (blue stain; λmax=470 nm); human superior temporal lobe AD neocortex (Brodmann A22); note affinity and polarized association of LPS for the neuronal nuclear envelope (white arrows; [93-97]); white scale bar (lower right)=20 μm; C-Control human neuronal-glial (HNG) cells in primary culture stained with antibody to glial fibrillary acidic protein (GFAP), a glial-specific cytoplasmic marker (green fluorescence; λmax=556 nm); an antibody to the neuron-specific cytoplasmic marker βTUBIII, (red; λmax=702 nm), and with Hoescht 33258 to highlight the morphological features of both glial-and neuronal-cell nuclei (blue; λmax=461 nm; 2 weeks in culture; HNG cells are about ~60% neurons (red) and about ~40% astroglial (green); 20x magnification); human primary neuronal and glial “support” cell co-cultures are utilized because human neuronal cells do not culture well by themselves [25]; note large nuclear area, relative to both glial or neuronal cytoplasmic area, indicative of high levels of transcriptional activity; white scale bar (lower right)=20 μm; D-HNG (transplantation grade) cells in primary co-culture were used to study the dynamics of LPS association with neurons [4,62,47,92]. Dark blue stained spherical and oval bodies are DAPI-stained non-neuronal nuclei from astroglia; white arrows indicate punctate and perinuclear clustering of LPS and LPS affinity for the nuclear envelope as has been previously reported [25,26,30,46,47,89]; both in vivo analysis in AD brain (Figure 1B) and in vitro experiments using HNG cells in primary tissue culture (Figure 1D) show affinity of LPS for neuronal nuclei; yellow scale bar (lower right)=20 μm.
Bacteroidetes Neurotoxins, their Mechanism of Action and Alzheimer’s Disease (AD)
Over the last five years LPS and/or BF-LPS have been shown: (i) to associate with the periphery of neuronal nuclei in sporadic Alzheimer’s disease (AD) brain [3,83,94]; (ii) to promote the generation of reactive oxygen species (ROS), the inflammatory transcription factor NF-kB (p50/p65 complex) in human neuronal-glial cells in primary-culture [7,12,57,83]; (iii) to efficiently enter neurons with the assistance of Aβ42 peptides, perhaps as a consequence of Aβ42 peptides’ ‘pore-forming’ capabilities [95,96]; and (iv) to strongly induce the upregulation of the pro-inflammatory transcription factor NF-kB (p50/p65 complex) and transcription of a selective family of pro-inflammatory NF-kB-sensitive microRNAs (miRNAs) that include miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, and miRNA-155 [24,57,62, 83,97,98]. These miRNAs in turn ultimately bind to the 3-untranslated region (3-UTR) of multiple target messenger RNAs (mRNAs) in brain cells and thereby reduce their expression post-transcriptionally. Down-regulated mRNAs include those encoding complement factor-H (CFH) of the innate-immune system [83,99], an SH3-proline-rich multi-domain-scaffolding protein of the postsynaptic density (SHANK3; [98]); and the triggering receptor expressed in myeloid/microglial cells (TREM2) surface glycoprotein immune receptor [4,100,101]. The down-regulation and/or loss-of-function mutations of CFH, SHANK3 and/or TREM2 expression are also observed in AD brain and/or in transgenic murine models of AD (TgAD; [83,100,101]). Hence, an extremely toxic LPS normally confined to the GI-tract is capable of driving a NF-kB-miRNA-mediated deficiency and disruption in gene expression that contributes to alterations in synaptic-architecture and synaptic-deficits, amyloidogenesis, innate-immune defects, and progressive inflammatory signaling, all of which are characteristic of AD-type neuropathology and multiple states of brain cell degeneration. Hence BF-LPS as well as ‘generic LPS glycolipids’ from other Gram negative GI-tract resident bacilli represent an important pathogenic initiator component of an NF-kB-miRNA-mRNA signaling program that has potential to down regulate specific gene expression patterns known to be required for normal CNS homeostasis, and hence contribute progressively to AD-type neurodegenerative change.
LPS Entry Into CNS Neurons-Facilitation By Aβ42 Peptides
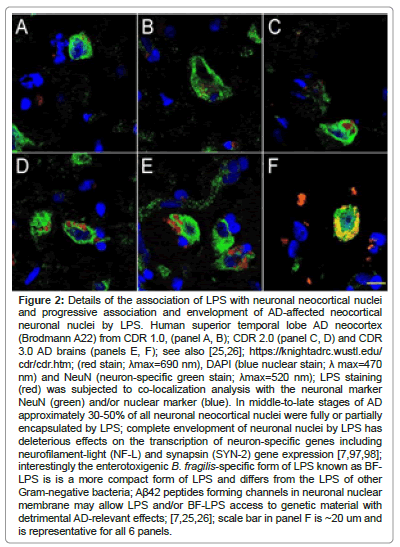
Recent reports have indicated a remarkably high affinity of microbiome-derived LPS for human brain neuronal nuclear membranes resulting in the complete envelopment of neuronal nuclei in AD brain neocortex, especially in the middle-to later-stages of AD (Figure 2). This ‘encapsulation of neuronal nuclei’ in AD brain by LPS has a disruptive effect on the generation of important neuron-specific transcription products that include the presynaptic phosphoprotein synapsin-2 (SYN-II) that associates with the cytoplasmic surface of synaptic vesicles, and the neurofilament light (NF-L) chain filament protein that maintains the overall shape and cytoarchitecture of the neuron [42,66,97,98,102].
Human superior temporal lobe AD neocortex (Brodmann A22) from CDR 1.0, (panel A, B); CDR 2.0 (panel C, D) and CDR 3.0 AD brains (panels E, F); see also [25,26]; https://knightadrc.wustl.edu/cdr/cdr.htm; (red stain; λmax=690 nm), DAPI (blue nuclear stain; λ max=470 nm) and NeuN (neuron-specific green stain; λmax=520 nm); LPS staining (red) was subjected to co-localization analysis with the neuronal marker NeuN (green) and/or nuclear marker (blue). In middle-to-late stages of AD approximately 30-50% of all neuronal neocortical nuclei were fully or partially encapsulated by LPS; complete envelopment of neuronal nuclei by LPS has deleterious effects on the transcription of neuron-specific genes including neurofilament-light (NF-L) and synapsin (SYN-2) gene expression [7,79]; interestingly the enterotoxigenic B. fragilis-specific form of LPS known as BF-LPS is is a more compact form of LPS and differs from the LPS of other Gram-negative bacteria; Aβ42 peptides forming channels in neuronal nuclear membrane may allow LPS and/or BF-LPS access to genetic material with detrimental AD-relevant effects; [7,25,26]; scale bar in panel F is ~20 um and is representative for all 6 panels.
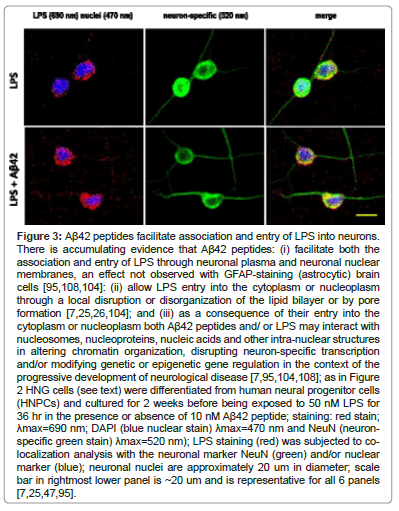
Probably the major biomarker for AD is the excessive abundance of amyloid beta (Aβ) peptides, both free and aggregated into senile plaque deposits in the parenchyma of the AD brain. Almost 30 years ago it was discovered that Aβ peptides support the formation of different types of atypical heterogeneous ionic pores and/or ion conducting channels spanning the lipid bilayer of the plasma membrane and that pore formation is linked to the pathogenicity and development of AD [95,103,104]. Since then these findings have been repeatedly supported by multiple independent reports regarding the ability of Aβ42 peptides [2 hydrophobic amino acid residues (isoleucine and alanine) longer at the C-terminal than the Aβ40 peptide] to form ~2.4-3.0 nm diameter pores through lipid bilayer membranes through which LPS and other neurotoxins may translocate across the cellular and/or nuclear plasma membrane [95,104-108]. Note that LPS has a MW ~10-20 kDa as an SDS-dissociated monomer with highly variable geometry and the basic monomeric shape of a flexible cylindrical molecule approximately 2.4 nm in diameter and 9.6 nm in length; the unique LPS of B. fragilis (BF-LPS) is significantly smaller than ‘generic LPS’ from other Gram negative bacteria such as Escherichia coli. There is evidence that Aβ42 peptides: (i) may facilitate the entry of both LPS through neuronal plasma and neuronal nuclear membranes by a localized membrane disruption or disorganization of the lipid bilayer [95,104,108]: (ii) may allow LPS transit into the cytoplasm or nucleoplasm through the lipid bilayer by pore formation [95,108]; and (iii) as a consequence of their entry into the cytoplasm or nucleoplasm both Aβ42 peptides and/or LPS may interact with nucleosomes, nucleoproteins, nucleic acids and other intra-nuclear structures in altering chromatin organization, disrupting transcription and/or modifying genetic or epigenetic gene regulation in the context of the development of disease [95,104,108] (Figure 3).
There is accumulating evidence that Aβ42 peptides: (i) facilitate both the association and entry of LPS through neuronal plasma and neuronal nuclear membranes, an effect not observed with GFAP-staining (astrocytic) brain cells [95,108,104]: (ii) allow LPS entry into the cytoplasm or nucleoplasm through a local disruption or disorganization of the lipid bilayer or by pore formation [7,25,26,104]; and (iii) as a consequence of their entry into the cytoplasm or nucleoplasm both Aβ42 peptides and/or LPS may interact with nucleosomes, nucleoproteins, nucleic acids and other intra-nuclear structures in altering chromatin organization, disrupting neuron-specific transcription and/or modifying genetic or epigenetic gene regulation in the context of the progressive development of neurological disease [7,95,104,108]; as in Figure 2 HNG cells (see text) were differentiated from human neural progenitor cells (HNPCs) and culture D for 2 weeks before being exposed to 50 nM LPS for 36 hr in the presence or absence of 10 nM Aβ42 peptide; staining: red stain; λmax=690 nm; DAPI (blue nuclear stain) λmax=470 nm and NeuN (neuron-specific green stain) λmax=520 nm); LPS staining (red) was subjected to co-localization analysis with the neuronal marker NeuN (green) and/or nuclear marker (blue); neuronal nuclei are approximately 20 um in diameter; scale bar in rightmost lower panel is ~20 um and is representative for all 6 panels [7,25,47,95].
Figure 2: Details of the association of LPS with neuronal neocortical nuclei and progressive association and envelopment of AD-affected neocortical neuronal nuclei by LPS. Human superior temporal lobe AD neocortex (Brodmann A22) from CDR 1.0, (panel A, B); CDR 2.0 (panel C, D) and CDR 3.0 AD brains (panels E, F); see also [25,26]; https://knightadrc.wustl.edu/ cdr/cdr.htm; (red stain; ?max=690 nm), DAPI (blue nuclear stain; ? max=470 nm) and NeuN (neuron-specific green stain; ?max=520 nm); LPS staining (red) was subjected to co-localization analysis with the neuronal marker NeuN (green) and/or nuclear marker (blue). In middle-to-late stages of AD approximately 30-50% of all neuronal neocortical nuclei were fully or partially encapsulated by LPS; complete envelopment of neuronal nuclei by LPS has deleterious effects on the transcription of neuron-specific genes including neurofilament-light (NF-L) and synapsin (SYN-2) gene expression [7,97,98]; interestingly the enterotoxigenic B. fragilis-specific form of LPS known as BFLPS is is a more compact form of LPS and differs from the LPS of other Gram-negative bacteria; Aß42 peptides forming channels in neuronal nuclear membrane may allow LPS and/or BF-LPS access to genetic material with detrimental AD-relevant effects; [7,25,26]; scale bar in panel F is ~20 um and is representative for all 6 panels
Figure 3: Aß42 peptides facilitate association and entry of LPS into neurons. There is accumulating evidence that Aß42 peptides: (i) facilitate both the association and entry of LPS through neuronal plasma and neuronal nuclear membranes, an effect not observed with GFAP-staining (astrocytic) brain cells [95,108,104]: (ii) allow LPS entry into the cytoplasm or nucleoplasm through a local disruption or disorganization of the lipid bilayer or by pore formation [7,25,26,104]; and (iii) as a consequence of their entry into the cytoplasm or nucleoplasm both Aß42 peptides and/ or LPS may interact with nucleosomes, nucleoproteins, nucleic acids and other intra-nuclear structures in altering chromatin organization, disrupting neuron-specific transcription and/or modifying genetic or epigenetic gene regulation in the context of the progressive development of neurological disease [7,95,104,108]; as in Figure 2 HNG cells (see text) were differentiated from human neural progenitor cells (HNPCs) and cultured for 2 weeks before being exposed to 50 nM LPS for 36 hr in the presence or absence of 10 nM Aß42 peptide; staining: red stain; ?max=690 nm; DAPI (blue nuclear stain) ?max=470 nm and NeuN (neuronspecific green stain) ?max=520 nm); LPS staining (red) was subjected to colocalization analysis with the neuronal marker NeuN (green) and/or nuclear marker (blue); neuronal nuclei are approximately 20 um in diameter; scale bar in rightmost lower panel is ~20 um and is representative for all 6 panels [7,25,47,95].
The GI-Tract Microbiome at the Cessation of Life-the Thanatomicrobiome
There are enormous biological, biophysical, immunological, physiological and bioenergetic efforts in keeping the GI-tract microbiome contained within GI-tract compartments and from expansion beyond its normal niche, and our understanding of this comes in part from analysis of the human microbiome that evolves at the time of death. At the cessation of life the generation of energy-containing nucleotide triphosphates ceases, nucleotide triphosphate stores such as those of ATP are rapidly depleted, circulatory systems involving the CSF, lymph and glymphatic system and blood flow ease and eventually terminate, the immune system falters and microbes proliferate throughout the body. In the healthy adult for example, internal organs that include the spleen, liver, heart, and brain are generally devoid of enterotoxigenic microbes such as ETBF because the innate-immune system or other microbial components keeps them in check. Relatively little is known on exactly what happens to the human GI-tract microbiome when the human host dies, and the process of human tissue decomposition is exceedingly complex. The concept of the thanatomicrobiome (thanatos, Greek for death; sometimes referred to as the ‘epinecrotic microbiome’ or ‘necrobiome’), defined as the composition, organization and expansion of the normal GI-tract microbiome and other microbial communities following cessation of all life and neurological activity, in part after the observation of a zero line electroencephalogram (ZLEEG) in multiple brain regions [109,110]. The community of microbial species associated with the decaying corpse remains a relatively recently appreciated area of scientific investigation from multiple neurological, forensic and neuropathological aspects. Emerging studies indicate that this series of catabolic events appears to begin in the ileocecal area of the GI-tract, spreading to the liver and spleen, continuing to the heart and brain [111-114]. Ongoing work from temporal studies on the thanatomicrobiome across a defined series of PMIs further indicate: (i) there is a constant struggle to contain GI-tract microbiome integrity and regulate specific bacterial abundance and complexity right up to the cessation of all life activity [111,112]; (ii) that the majority of the microbes within the human body and those which propagate most rapidly at the time of death are the obligate anaerobes (such as B. fragilis) that begin to non-randomly proliferate from deeper regions of the GI-tract continuing throughout the human organs over the PMI [112,113]; and (iii) that comprehensive knowledge of the number and abundance of each organ's microbial signature is critical to forensic microbiologists, neuropathologists, human microbiome and post-mortem tissue researchers as a new source of data for estimating microbial complexity and evolution over the PMI [1-3,112- 114]. These data combined with DNA-and RNA-based nucleic acid sequencing and bioinformatics are also essential in aiding researchers who use post-mortem tissues in their research work,in forensic criminology, and the study of microbial speciation, complexity and microbiome-host genetics in the later stages of life. The very rapid post-mortem appearance of multiple genus/species of microbes in the brain and CNS at the time of death lends credence to the concept that these microbes would not have enough time to transit from the microbiome of the GI-tract to the brain, especially when systemic circulation of all types have essentially ceased, and suggests that microbes are already present within the confines of the brain and CNS compartments [7,12,113,114].
A Brain Microbiome?
While multiple research reports provide abundant evidence that GI-tract microbiome-derived neurotoxins such as LPS, BF-LPS, fragilysin and other microbial-sourced toxins are fully capable of disrupting, damaging and crossing biophysical barriers there is also the possibility that the brain and CNS possesses its own microbiome. Indeed multiple CNS compartments such as the eye, derived from the neural crest cells: (i) appear to harbor their own microbiome; and (ii) there is an interesting association of dysbiotic changes in the GI-tract microbiome with extra-intestinal diseases such as ocular diseases include bacterial keratitis, fungal keratitis, uveitis, age-related macular degeneration and ocular mucosal diseases [115-117]. Some of the strongest support for a brain microbiome comes from studies on the thanatomicrobiome that evolves following cessation of all life-relevant biological, biochemical, neurochemical and neurophysiological activities [25,111-113, 118]. In a healthy adult, most internal organs such as the spleen, liver, heart, and brain are generally devoid of enterotoxigenic microbes because the innate-immune system and/or other microbial components keeps them in check, however, certain microbial species rapidly appear in the thanatomicrobiome of the brain post-mortem, faster than transport would allow these bacteria transit from the GI-tract into CNS compartments, suggesting that some type of microbial community already populates the brain and CNS. Widespread independent reports documenting: (i) the presence of microbes and/or their secreted neurotoxins, especially viral-and bacterial-derived neurotoxins and related components deep within the brain parenchyma, cytoplasm and nucleoplasm either free, plasma-membrane-bound or vesicle-bound further lend support to this intriguing idea; and (ii) 16S ribosomal RNA (rRNA) next generation sequencing analysis have repeatedly shown bacterial and microbial nucleic acids in post-mortem brain from AD patients [4-6,13,24,26,57,74,94,113,118,119].
Related to the idea of a brain microbiome is the assumption of the ‘privileged immunological status’ of the CNS also continues to be questioned, particularly in terms of inflammatory neurodegenerative diseases such as AD, as both microbial-derived nucleic acid sequences and/or noxious exudates representative of GI-tract Gram-negative bacteria and neurotropic viruses such as herpes simplex 1 (HSV-1) have found to be resident within CNS compartments. These compartments include, prominently, anatomical regions of the CNS involved in inflammatory and pathological signaling and neuro-immune disruptions that characterize the AD process [25,26,57,94,118,120-122]. For example, LPS has been recently localized to the same anatomical regions involved in AD-type neuropathology to levels of greater than 7-fold over control in the temporal lobe neocortex and at least ~20-to-25-fold over controls in the hippocampus, suggesting that GI-tract microbiome-derived LPS may be an important initiator and/or significant contributor to inflammatory degeneration in the AD affected CNS over a background of aging [7,12,25,26,47,118].
Unanswered Questions
A number of unanswered questions remain regarding GI-tract microbiome-derived microbial neurotoxins and their contribution to human neurological disease and more specifically to the development of progressive inflammatory neurodegeneration such as that observed during the onset, propagation and course of sporadic AD. These include: (i) the major risk factor for AD is age-is there a progressive age-related contribution of a single neurotoxin or cocktail of neurotoxins over the human lifespan that cause a slowly developing life-long neuronal damage to the point where there is sufficient brain cell damage or dropout to cause AD-type change and a failure in neuronal signaling and cognition as humans age?; (ii) related to the previous point-does life-long exposure to specific GI-tract derived infectious agents, their secreted neurotoxins and/or environmental toxins in our diet and/or environment predispose the brain and CNS to the development of AD at a later age?;(iii) what combinations of microbes and/or bacterial-and/or viral-based neurotoxins and perhaps other microbiome-derived toxins are the harmful to the CNS and PNS and which are the most efficient in inducing a progressive pro-inflammatory neurodegeneration such as that observed in AD?; (iv) are other potentially pathogenic transcription factors in addition to NFkB (p50/p65) and other pro-inflammatory pathology-associated microRNAs besides miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, and miRNA-155 involved in driving and supporting progressive neurodegenerative AD-type change and cognitive decline?; (v) do microbiome-derived neurotoxins or polymicrobial infections exhibit synergism in their toxicities toward neural cells of the PNS and CNS?; (vi) do prebiotics, nucleic acids, carbohydrates and/or specialized plant-based dietary fibers that nourish beneficial microbes already in the GI-tract, or probiotics, consisting of beneficial “health-and homeostasis-promoting” microbes have any role in AD onset or progression?; (vii) would it be possible to devise prebiotic, probiotic, anti-neurotoxin, anti-NF-kB (p50/p65), anti-microRNA, dietary fiber manipulation or related approaches, or combinations of these therapeutic strategies to benefit the clinical management of AD once the disease has already taken hold?; (viii) does the brain and CNS possess its own microbiome, and if so what is its composition, structure and function in neurological health and disease?; (ix) do specific combinations in individuals of species or stoichiometries of GI-tract microbes that include aerobic or anaerobic, Gram negative or Gram-positive bacteria, archaebacteria, fungi, protozoa, viruses and other microorganisms and/or their secreted neurotoxins favor the development of systemic inflammation, inflammatory neurodegeneration or other types of human disease?; (x) do multiple whole-body microbial infections earlier in life predispose to the development of AD at a later date?: and (xi) perhaps most importantly, could it be possible to tailor a life-long dietary intake and nutritional management strategy, perhaps along the lines of a ‘personalized medicine approach’, that creates a GI-tract microbiome that is conducive to lowered abundances of Gram-negative-derived neurotoxins and a dietary intake that effectively minimizes the risk of CNS-based age-related neuroinflammatory diseases such as AD?
Discussion and Summary
The human GI-tract microbiome remains a vast and understudied repository of microbial species, and pro-karyotes and their genes considerably outnumber the total number of host cells and host genes contained in the human genome [1,7,12,14,32-35,57]. This large and diverse microbial community makes an important contribution to human metabolism, innate-immune health and neurobiology by contributing a considerable number of enzymes and metabolic factors that are not encoded by the human genome. This genus-, species-and genetically-diverse microbial population: (i) forms a complex, dynamic, and highly interactive microbial community that plays major roles in digestion, nutrition, inflammation, growth, immunity against foreign pathogens and neurological disorders that include neurodegeneration [5,15,45,80]; (ii) has symbiotic associations and interactions with the host indispensable for human health and homeostatic physiological functions [13,22,38,123]; and (iii) exhibits fluctuations in individual abundance, speciation, stoichiometry and complexity in response to developmental stage, dietary factors, GI-tract disturbances, aging, and disease [16,38,46]. An imbalance in microbial populations in the GI-tract of AD patients has been reported in several studies, and this dysbiosis very likely increases the abundance of GI-tract microbiome-derived molecules that may be detrimental to the human metabolism and neurological health. The high variability in microbial complexity however, even amongst healthy individuals has made it difficult to link specific microbial abundance patterns with age-related neurological disease such as AD [15,33,43,118]. There is strengthening evidence that: (i) the ability of GI-tract microbiome-resident bacteria to influence neuro-immune functions well beyond the confines of the GI-tract; (ii) that changes are communicated to the brain and CNS through a GI-tract-CNS network, sometimes called the ‘gut-brain-axis’, in part via small signaling molecules such as SCFAs or other chemical messenger signaling systems; (iii) that microbial components of the GI-tract microbiome such as BF-LPS and/or the microbes themselves can transverse biophysical barriers without too much difficulty and contribute to AD-type change; and (iv) that specific GI-tract microbiome-derived neurotoxins have a strong pathological role in eliciting an up-regulation of ROS and pro-inflammatory NF-kB-miRNA-directed gene expression that is both AD-relevant and propagates the AD process [7,12,15,16,27]. Established pathways of gut-brain axis communication currently include the autonomic nervous system (ANS), the enteric nervous system (ENS), the neuroendocrine system, the immune system, the systemic circulation and vesicular trafficking [9,13,15,43,124-127]. Surprisingly, neuronal signaling pathways along the bidirectional gut-brain axis remain an understudied research area despite their important roles: (i) in coordinating metabolic-, nutritive-and neurobiological-functions, and (ii) in their functional disruption in chronic diseases such as metabolic syndrome, diabetes, obesity, anxiety, autoimmune-disease and stress-induced neuropsychiatric disease and neurodegenerative brain diseases such as AD [7,12,15,37,43,119, 117].
Conclusion
Lastly, an intriguing thanatomicrobiome develops in the postmortem human body and lack of metabolic, bioenergetic, immune and neuroendocrine systems facilitate microbial succession in the GI-tract, systemic circulation, brain and CNS compartments. The initial verification of prokaryotic 16S rRNA sequences within the AD brain using next generation sequencing, the appearance of LPS and other microbial-derived neurotoxins in brain tissues in AD, the occurrence of GI-tract-derived microbial species within the brain and relatively rapid appearance of a novel thanatomicrobiome in the brain after death distant from the abundant microbial species of the GI-tract microbiome suggests that a poorly understood and insufficiently characterized brain microbiome is most likely already there.
Acknowledgements
Sincere thanks are extended to Drs. Wayne Poon, Joshua Grill and Piotr N. Alexandrov for short post-mortem interval (PMI) human brain, eye and/or retinal tissues or extracts, stabilized RNA and DNA probes, nucleic acid hybridization work and initial data interpretation, and to Aileen I. Pogue and Darlene Guillot for expert technical assistance and medical artwork. Thanks are further extended to the many neuropathologists, physicians and researchers of Canada and the USA who have provided high quality, short PMI human CNS, eye and retinal tissues or extracted total brain and eye DNA and RNA for scientific study and quantitative analysis. Research on human brain, eye and host cell transcriptomics in the Lukiw laboratory involving total human RNA analysis and gene expression, microRNA (miRNA) and messenger RNA (mRNA) sequencing and complexity and array-based quantitation, the innateimmune response in AD and in other forms of neurological or retinal disease, amyloidogenesis and neuroinflammation was supported through an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness (RPB); the Louisiana Biotechnology Research Network (LBRN) and NIH grants NEI EY006311, NIA AG18031 and NIA AG038834 (WJL).
Data Availability Statement
The original contributions presented in the study are included in the manuscript text; further inquiries can be directed to the corresponding author/s.
Authors' Contributions
WJL and YZ conceived and wrote the paper; LA and TB participated in data collection and analysis; all authors read and approved the manuscript.
Conflict of Interest and Competing Interests
The authors declare that they have no conflict of interest of any kind with any of the material presented in this research report. The authors declare that they have no competing interests associated with any data in this report.
References
- Almeida A, Nayfach S, Boland M, Strozzi F, Beracochea M, et al. (2021) A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat Biotechnol 39: 105-114.
- Schloss PD, Girard RA, Martin T, Edwards J, Thrash JC (2016) Status of the archaeal and bacterial census: An update. mBio 7: e00201-216.
- Lukiw WJ, Pogue AI (2020) Vesicular transport of encapsulated microRNA between glial and neuronal cells. Int J Mol Sci 21: 5078.
- Bhattacharjee S, Lukiw WJ (2013) Alzheimer's disease and the microbiome. Front Cell Neurosci 7: 153.
- Hill JM, Bhattacharjee S, Pogue AI, Lukiw WJ (2014a) The gastrointestinal tract microbiome and potential link to Alzheimer's disease. Front Neurol 5: 43.
- Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, et al. (2014b) Pathogenic microbes, the microbiome, and Alzheimer's disease (AD). Front Aging Neurosci 6: 127.
- Lukiw WJ (2020a) Human gastrointestinal tract microbiome-derived pro-inflammatory neurotoxins from Bacteroides fragilis: Effects of low fiber diets and environmental and lifestyle factors. Integr Food Nutr Metab 7: 277.
- Allaband C, McDonald D, Vázquez-Baeza Y, Minich JJ, Tripathi A, et al. (2019) Microbiome 101: Studying, analyzing, and interpreting gut microbiome data for clinicians. Clin Gastroenterol Hepatol 17: 218-230.
- Forsythe P, Kunze WA, Bienenstock J (2012) On communication between gut microbes and the brain. Curr Opin Gastroenterol 28: 557-562.
- Collins SM, Kassam Z, Bercik P (2013) The adoptive transfer of behavioral phenotype via the intestinal microbiota: Experimental evidence and clinical implications. Curr Opin Microbiol 16: 240-245.
- Douglas-Escobar M, Elliott E, Neu J (2013) Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr 167: 374-379.
- Lukiw WJ (2020b) microRNA-146a signaling in Alzheimer's disease (AD) and prion disease (PrD) Front Neurol 11: 462.
- Rutsch A, Kantsjö JB, Ronchi F (2020) The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front Immunol 11: 604179.
- Tangestani H, Emamat H, Ghalandari H, Shab-Bidar S (2020) Whole grains, dietary fibers and the human gut microbiota: A systematic review of existing literature. Recent Pat Food Nutr Agric 11: 235-248.
- Shabbir U, Arshad MS, Sameen A, Oh DH (2021) Crosstalk between gut and brain in Alzheimer's disease: The role of gut microbiota modulation strategies. Nutrients 13: 690.
- Spichak S, Bastiaanssen TFS, Berding K, Vlckova K, Clarke G, et al. (2021) Mining microbes for mental health: Determining the role of microbial metabolic pathways in human brain health and disease. Neurosci Biobehav Rev 21: 103-112.
- Foster JA, Lyte M, Meyer E, Cryan JF (2016) Gut microbiota and brain function: An evolving field in neuroscience. Int J Neuropsychopharmacol 19: 114.
- Holmes C (2013) Review: Systemic inflammation and Alzheimer’s disease. Neuropathol Appl Neurobiol 39: 51-68.
- Pogue AI, Jaber V, Zhao Y, Lukiw WJ (2017) Systemic Inflammation in C57BL/6J mice receiving dietary aluminum sulfate; up-regulation of the pro-inflammatory cytokines IL-6 and TNFa, c-reactive protein (CRP) and miRNA-146a in blood serum. J Alzheimers Dis Parkinsonism 7: 403.
- Pires JM, Foresti ML, Silva CS, Rêgo DB, Calió ML, et al. (2020) Lipopolysaccharide-induced systemic inflammation in the neonatal period increases microglial density and oxidative stress in the cerebellum of adult rats. Front Cell Neurosci 14: 142.
- Hofer U (2014) Microbiome: B. fragilis and the brain. Nat Rev Microbiol. 12: 76-77.
- Serpente M, Bonsi R, Scarpini E, Galimberti D (2014) Innate immune system and inflammation in Alzheimer’s disease: From pathogenesis to treatment. Neuroimmunomodulation 21: 79-87.
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, et al. (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14: 388-405.
- Hill JM, Pogue AI, Lukiw WJ (2015) Pathogenic microRNAs common to brain and retinal degeneration; recent observations in Alzheimer’s disease and age-related macular degeneration. Front Neurol 6: 232.
- Zhao Y, Cong L, Lukiw WJ (2017a) Lipopolysaccharide (LPS) accumulates in neocortical neurons of Alzheimer's disease (AD) brain and impairs transcription in human neuronal-glial primary co-cultures. Front Aging Neurosci 9: 407.
- Zhao Y, Jaber V, Lukiw WJ (2017b) Secretory products of the human GI-tract microbiome and their potential impact on Alzheimer's disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol 7: 318.
- Zhao Y, Lukiw WJ (2018) Microbiome-mediated upregulation of microRNA-146a in sporadic Alzheimer's disease. Front Neurol 9: 145.
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, et al. (2015) Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85: 296-302.
- Montagne A, Zhao Z, Zlokovic BV (2017) Alzheimer's disease: A matter of blood-brain barrier dysfunction? J Exp Med 214: 3151-3169.
- Hill JM, Lukiw WJ (2015) Microbial-generated amyloids and Alzheimer's disease (AD) Front Aging Neurosci 7: 9.
- Zhao Y, Dua P, Lukiw WJ (2015) Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer's disease (AD) J Alzheimers Dis Parkinsonism 5: 177.
- Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, et al. (2016) The gut microbiota and host health: A new clinical frontier. Gut 65: 330-339.
- Tierney BT, Yang Z, Luber JM, Beaudin M, Wibowo MC, et al. (2019) The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe. 26: 283-295.
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, et al. (2001) The sequence of the human genome. Science 291: 1304-1351.
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, et al. (2012) Host-gut microbiota metabolic interactions. Science 336: 1262-1267.
- Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol 11: 25.
- Ojeda J, Ãvila A, Vidal PM (2021) Gut microbiota interaction with the central nervous system throughout life. J Clin Med 10: 1299.
- Sampson TR, Mazmanian SK (2015) Control of brain development, function, and behavior by the microbiome cell host microbe. Cell Host Microbe 17: 565-576.
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Â et al. (2018) Gut microbiota functions: Metabolism of nutrients and other food components. Eur J Nutr 57: 1-24.
- Batista CR, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira AC (2019) Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci 20: E2293.
- Sheppard O, Coleman MP, Durrant CS (2019) Lipopolysaccharide-induced neuroinflammation induces presynaptic disruption through a direct action on brain tissue involving microglia-derived interleukin 1 beta. J Neuroinflam 16: 106.
- Leblhuber F, Ehrlich D, Steiner K, Geisler S, Fuchs D, et al. (2021) The immunopathogenesis of Alzheimer's disease is related to the composition of gut microbiota. Nutrients 13: 361.
- Fallingborg J (1999) Intraluminal pH of the human gastrointestinal tract. Dan Med Bull 46: 183-196.
- Bhattacharjee S, Zhao Y, Dua P, Rogaev EI, Lukiw WJ (2016) microRNA-34a-mediated down-regulation of the microglial-enriched triggering receptor and phagocytosis-sensor TREM2 in age-related macular degeneration. PLoS One 11: e0150211.
- Zhang LS, Davies SS (2016) Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med 8: 46.
- Zhan X, Stamova B, Sharp FR (2018) Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer's disease brain: A review. Front Aging Neurosci 10: 42.
- Perez HJ, Menezes ME, d'Acâmpora AJ (2014) Intestinal microbiota. Acta Gastroenterol Latinoam 44: 265-272.
- Potgieter M, Bester J, Kell DB, Pretorius E (2015) The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev 39: 567-591.
- ZhaoY, Lukiw WJ (2015) Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer’s disease (AD). J Nat Sci 1: e138.
- Alkasir R, Li J, Li X, Jin M, Zhu B (2017) Human gut microbiota: The links with dementia development. Protein Cell 8: 90-102.
- Hu X, Wang T, Jin F (2016) Alzheimer’s disease and gut microbiota. Sci China Life Sci 59: 1006-1023.
- Mancuso G, Midiri A, Biondo C, Beninati C, Gambuzza M, et al. (2005) Bacteroides fragilis-derived lipopolysaccharide (LPS) produces cell activation and lethal toxicity via toll-like receptor 4. Infect Immun 73: 5620-5627.
- Sears CL (2009) Enterotoxigenic B. fragilis: A rogue among symbiotes. Clin Microbiol Rev 22: 349.
- Sears CL, Geis AL, Housseau F (2014) Bacteroides fragilis subverts mucosal biology: From symbiont to colon carcinogenesis. J Clin Invest 124: 4166-4172.
- Fathi P, Wu S (2016) Isolation, detection, and characterization of enterotoxigenic Bacteroides fragilis in clinical samples. Open Microbiol J 10: 57-63.
- Lukiw WJ (2016) Bacteroides fragilis, LPS and inflammatory signaling in Alzheimer’s disease. Front Microbiol 7: 1544.
- Valguarnera E, Wardenburg J (2020) Good gone bad: One toxin away from disease in Bacteroides fragilis. J Mol Biol 432: 765.
- Jawara Allen SH, Sears CL, Timp W (2019) Epigenetic changes induced by Bacteroides fragilis toxin. Infect Immun 87: e00447-18.
- Zamani S, Taslimi R, Sarabi A, Jasemi S, Sechi LA, (2020) Enterotoxigenic Bacteroides fragilis: A possible etiological candidate for bacterially-induced colorectal precancerous and cancerous lesions. Front Cell Infect Microbiol 9: 449.
- Barton SM, Janve VA, McClure R, Anderson A, Matsubara JA, et al. (2019) Lipopolysaccharide induced opening of the blood brain barrier on aging 5XFAD mouse model. J Alzheimers Dis 6: 503-513.
- Zhao Y, Lukiw WJ (2018) Bacteroidetes neurotoxins and inflammatory neurodegeneration. Mol Neuro 55: 9100.
- Chakraborty S, Zawieja SD, Wang W, Lee Y, Wang YJ, et al. (2015) Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am J Physiol Heart Circ Physiol 309: H2042-57.
- Noailles A, Maneu V, Campello L, Lax P, Cuenca N (2018) Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis 9: 350.
- Yang J, Malone F, Go M, Kou J, Lim JE, et al. (2020) Lipopolysaccharide-induced exosomal miRA-146a is involved in altered expression of Alzheimer's risk genes via suppression of TLR4 signaling. J Mol Neurosci.
- Lenz M, Eichler A, Vlachos A (2021) Monitoring and modulating inflammation-associated alterations in synaptic plasticity: Role of brain stimulation and the blood-brain interface. Biomolecules 11: 359.
- Sumi N, Nishioku T, Takata F, Matsumoto J, Watanabe T, et al. (2010) Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol Neurobiol 30: 247-253.
- Zhao Y, Cong L, Jaber V, Lukiw WJ (2017b) Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer's disease brain. Front Immunol 8: 1064.
- Kesika P, Suganthy N, Sivamaruthi BS, Chaiyasut C (2021) Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer's disease. Life Sci 264: 118627.
- Fox M, Knorr DA, Haptonstall KM (2019) Alzheimer's disease and symbiotic microbiota: An evolutionary medicine perspective. Ann N Y Acad Sci 1449: 3-24.
- Keenan JI, Aitchison A, Purcell RV, Greenlees R, Pearson JF (2016) Screening for enterotoxigenic Bacteroides fragilis in stool samples. Anaerobe 40: 50-53.
- Yeates KE, Halliday W, Miyasaki J, Vellend H, Straus S (2003) A case of 'circling seizures' and an intratumoral abscess. Clin Neurol Neurosurg 105: 128-131.
- Bajpai A, Prasad KN, Mishra P, Singh AK, Gupta RK, et al. (2014) Distinct cytokine pattern in response to different bacterial pathogens in human brain abscess. J Neuroimmunol 273: 96-102.
- Lobo LA, Benjamim CF, Oliveira AC (2016) The interplay between microbiota and inflammation: Lessons from peritonitis and sepsis. Clin Transl Immunology 5: e90.
- Remacle AG, Shiryaev SA, Strongin AY (2014) Distinct interactions with cellular E-cadherin of the two virulent metalloproteinases encoded by a Bacteroides fragilis pathogenicity island. PLoS One 9: e113896.
- Choi VM, Herrou J, Hecht AL, Teoh WP, Turner JR, et al. (2016) Activation of Bacteroides fragilis toxin by a novel bacterial protease contributes to anaerobic sepsis in mice. Nat Med 22: 563-567.
- Pierce JV, Bernstein HD (2016) Genomic diversity of enterotoxigenic strains of Bacteroides fragilis. PLoS One 11: e0158171.
- Shields CE, White JR, Chung L, Wenzel A, Hicks JL, et al. (2021) Bacterial-driven inflammation and mutant BRAF expression combine to promote murine colon tumorigenesis that is sensitive to immune checkpoint therapy. Cancer Discov.
- Zhao Y, Jaber VR, LeBeauf A, Sharfman NM, Lukiw WJ (2019) microRNA-34a mediated down-regulation of the post-synaptic cytoskeletal element SHANK3 in sporadic AD. Front Neurol 10: 28.
- Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, et al. (2020) The links between the gut microbiome, aging, modern lifestyle and Alzheimer's disease. Front Cell Infect Microbiol 10: 104.
- Heinritz SN, Weiss E, Eklund M, Aumiller T, Heyer CM, et al. (2016) Impact of a high-fat or high-fiber diet on intestinal microbiota and metabolic markers in a pig model. Nutrients 8: 317.
- Bertani B, Ruiz N (2018) Function and biogenesis of lipopolysaccharides. EcoSal Plus 8.
- Alexandrov P, Zhai Y, Li W, Lukiw W (2019) Lipopolysaccharide-stimulated, NF-kB-, miRNA-146a-and miRNA-155-mediated molecular-genetic communication between the human gastrointestinal tract microbiome and the brain. Folia Neuropathol 57: 211-219.
- Freudenberg MA, Galanos C (1990) Bacterial lipopolysaccharides: Structure, metabolism and mechanisms of action. Int Rev Immunol 6: 207-221.
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, et al. (1994) Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J 8: 217-225.
- Eidhin D, Mouton CA (1993) Rapid method for LPS preparation from Bacteroides FEMS Microbiol Lett 110: 133.
- Sears CL (2001) The toxins of Bacteroides fragilis Toxicon 39: 1737-1746.
- Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20: 145-155.
- Yang NJ, Chiu IM (2017) Bacterial signaling to the nervous system through toxins and metabolites. J Mol Biol 429: 587-605.
- Zhu X, Han Y, Du J, Liu R, Jin K, et al. (2017) Microbiota-gut-brain axis and the central nervous system. Oncotarget 8: 53829-53838.
- Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, et al. (2016) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49: 60-68.
- A Kohler C, Maes M, Slyepchenko A, Berk M, Solmi M, et al. (2016) The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: Mechanisms and pathophysiological role in Alzheimer's disease. Curr Pharm Des 22: 6152-6166.
- Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, et al. (2016) Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev 74: 624-634.
- Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, et al. (2016) Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87: 2324-2332.
- Lukiw WJ, Li W, Bond T, Zhao Y (2019) Facilitation of gastrointestinal (GI) tract microbiome-derived lipopolysaccharide (LPS) entry into human neurons by amyloid beta-42 (Aß42) peptide. Front Cell Neurosci 13: 545.
- Darling AL, Shorter J (2020) Atomic structures of amyloid-ß oligomers illuminate a neurotoxic mechanism. Trends Neurosci 43: 740-743.
- Lukiw WJ, Cong L, Jaber V, Zhao Y (2018) Microbiome-derived lipopolysaccharide (LPS) selectively inhibits neurofilament light chain (NF-L) gene expression in human neuronal-glial (HNG) cells in primary culture. Front Neurosci 12: 896.
- Zhao Y, Sharfman NM, Jaber VR, Lukiw WJ (2019) Down-regulation of essential synaptic components by GI-tract microbiome-derived lipopolysaccharide (LPS) in LPS-treated human neuronal-glial (HNG) cells in primary culture: Relevance to Alzheimer's disease (AD). Front Cell Neurosci 13: 314.
- Lukiw WJ, Surjyadipta B, Dua P, Alexandrov PN (2012) Common miRNAs target complement factor H regulation in Alzheimer's disease and in age-related macular degeneration. Int J Biochem Mol Biol 3: 105-116.
- Zhao Y, Jaber V, Lukiw WJ (2016) Over-expressed pathogenic miRNAs in Alzheimer's disease (AD) and prion disease (PrD) drive deficits in TREM2-mediated Aß42 peptide clearance. Front Aging Neurosci 8: 140.
- Deczkowska A, Weiner A, Amit I (2020) The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell 181: 1207-1217.
- Zhao Y, Jaber V, Alexandrov PN, Vergallo A, Lista S, et al. (2020) microRNA-based biomarkers in Alzheimer's disease (AD). Front Neurosci 14: 585432.
- Arispe N, Rojas E, Pollard HB (1993) Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc Natl Acad Sci USA 90: 567-571.
- Venko K, Novic M, Stoka V, Zerovnik E (2021) Prediction of transmembrane regions, cholesterol, and ganglioside binding sites in amyloid-forming proteins indicate potential for amyloid pore formation. Front Mol Neurosci 14: 619496.
- Bode DC, Freeley M, Nield J, Palma M, Viles JH (2019) Amyloid-ß oligomers have a profound detergent-like effect on lipid membrane bilayers, imaged by atomic force and electron microscopy. J Biol Chem 294: 7566-7572.
- Jang H, Arce FT, Lee J, Gillman AL, Ramachandran S et al. (2016) Computational methods for structural and functional studies of Alzheimer's amyloid ion channels. Methods Mol Biol 1345: 251-268.
- O¨sterlund N, Moons R, Ilag LL, Sobott F, Gra¨slund A (2019) Native ion mobility-mass spectrometry reveals the formation of ß-barrel shaped amyloid-ß hexamers in a membrane-mimicking environment. J Am Chem Soc 141: 10440-10450.
- Sun J, Shi Y, Yildirim E (2019) The nuclear pore complex in cell type-specific chromatin structure and gene regulation. Trends Genet 35: 579-588.
- Koenig MA, Kaplan PW (2019) Brain death. Handb Clin Neurol 161: 89-102.
- Aboubakr M, Alameda G (2021) Brain death criteria. Treasure Island (FL): StatPearls Publishing.
- Clement C, Hill JM, Dua P, Culicchia F, Lukiw WJ (2016) Analysis of RNA from Alzheimer's disease post-mortem brain tissues. Mol Neurobiol 53: 1322-1328.
- Javan GT, Finley SJ, Abidin Z, Mulle JG (2016) The thanatomicrobiome: A missing piece of the microbial puzzle of death. Front Microbiol 7: 225.
- Javan GT, Finley SJ, Tuomisto S, Hall A, Benbow ME, et al. (2019) An interdisciplinary review of the thanatomicrobiome in human decomposition. Forensic Sci Med Pathol 15: 75-83.
- Dash HR, Das S (2020) Thanatomicrobiome and epinecrotic community signatures for estimation of post-mortem time interval in human cadaver. Appl Microbiol Biotechnol 104: 9497-9512.
- Gomes JÃP, Frizon L, Demeda VF (2020) Ocular surface microbiome in health and disease. Asia Pac J Ophthalmol (Phila) 9: 505-511.
- Ranjith K, Sharma S, Shivaji S (2021) Microbes of the human eye: Microbiome, antimicrobial resistance and biofilm formation. Exp Eye Res 205: 108476.
- Shivaji S (2021) A systematic review of gut microbiome and ocular inflammatory diseases: Are they associated? Indian J Ophthalmol 69: 535-542.
- Huang H, Nguyen PT, Schwab NA, Tanner JJ, Price CC, et al. (2017) 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer's post-mortem brain. Front Aging Neurosci 9: 195.
- Salihoglu R, Önal-Süzek T (2021) Tissue microbiome associated with human diseases by whole transcriptome sequencing and 16S metagenomics. Front Genet 12: 585556.
- Bagyinszky E, Van Giau V, Shim K, Suk K, An SS, et al. (2017) Role of inflammatory molecules in the Alzheimer's disease progression and diagnosis. J Neurol Sci 376: 242-254.
- Itzhaki RF, Golde TE, Heneka MT, Readhead B (2020) Do infections have a role in the pathogenesis of Alzheimer disease? Nat Rev Neurol 16: 193-197.
- Sait A, Angeli C, Doig AJ, Day PJ (2019) Viral Involvement in Alzheimer's Disease. ACS Chem Neurosci 9.
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, et al. (2017) Gut microbiome alterations in Alzheimer's disease. Sci Rep 7: 13537.
- Camfield DA, Owen L, Scholey AB, Pipingas A, Stough C (2011) Dairy constituents and neurocognitive health in ageing. Br J Nutr 106: 159-174.
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108: 3047-3052.
- Aziz Q, Doré J, Emmanuel A, Guarner F, Quigley EM (2013) Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol Motil 25: 4-15.
- Burokas A, Moloney RD, Dinan TG, Cryan JF (2015) Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol 91: 1-62.
Citation: Lukiw WJ, Arceneaux LS, Li W, Bond T, Zhao Y (2021) Gastrointestinal (GI): Tract Microbiome-Derived Neurotoxins and their Potential Contribution to Inflammatory Neurodegeneration in Alzheimer’s Disease (AD). J Alzheimers Dis Parkinsonism 11: 525. DOI: 10.4172/2161-0460.1000525
Copyright: © 2021 Lukiw WJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3867
- [From(publication date): 0-2021 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 2955
- PDF downloads: 912