Glucagon-Like Peptide-1 Receptor Pet/Ct in Patients with and Without Post Gastric Bypass Hypoglycemia: A Prospective, Matched Case-Control Study
Received: 18-May-2022 / Manuscript No. JGDS-22-64178 / Editor assigned: 20-May-2022 / PreQC No. JGDS-22-64178 (PQ) / Reviewed: 03-Jun-2022 / QC No. JGDS-22-64178 / Revised: 08-Jun-2022 / Manuscript No. JGDS-22-64178 (R) / Published Date: 15-Jun-2022 DOI: 10.4172/2161-069X.1000686
Abstract
Purpose: The aim of this pilot study is to prospectively compare in vivo glucagon like peptide-1 (GLP-1) receptor expression in the duodenum and pancreas of patients with and without postprandial hypoglycemia after gastric bypass using GLP-1 receptor imaging with 68Ga-DOTA-exendin-4 PET/CT.
Methods: Five patients after Roux-Y-gastric bypass (RYGB) surgery with postprandial hypoglycemia (PGBH) and five patients after RYGB without PGBH matched for sex, age, operative procedure and postoperative interval underwent 68Ga-DOTAexendin- 4 PET/CT. Total 68Ga-DOTA-exendin-4 uptake in the duodenum and pancreas, defined as the sum of the standard uptake value (SUVsum) for each voxel in the volume of interest (VOI) was measured by two independent readers who were blinded for the clinical information. Additionally, three further patients with PGBH who received 68Ga-DOTA-exendin-4 PET/CT at our center were added to the analysis. Clinical follow up data was collected for all patients with PGBH.
Results: 68Ga-DOTA-exendin-4 PET/CT exhibited a greater range of total duodenal and pancreatic uptake (SUVsum) for matched hypoglycemic patients (2144-7802, median 3022) than for matched normoglycemic patients (5104-7507, median 7072), with no statistical significant difference of total uptake=total in vivo GLP-1 receptor expression (p=0.187). In one patient with PGBH a focal tracer uptake in the pancreatic tail was identified. Four patients with PGBH required ultimately a reversal of their gastric bypass surgery due to insufficient dietary and medical therapy of their hypoglycemic episodes.
Conclusion: 68Ga-DOTA-exendin-4 uptake appears not to be significantly different in patients with PGBH compared to matched normoglycemic patients but may reflect varying stages of the complex pathophysiology of postbariatric hypoglycemia.
Keywords: Post gastric bypass hypoglycemia; Late-dumping; Glp- 1 receptor expression; 68Ga-dota-exendin-4; Glp-1 receptor imaging
Introduction
Postprandial hypoglycemia after bariatric surgery is an endogenous form of hyperinsulinemic hypoglycemia [1,2]. It is a frequent, underdiagnosed, intricate medical complication, which impairs quality of life and jeopardizes the beneficial effects of bariatric surgery [2-4]. Several factors are important for the development, intensity and frequency of postprandial hypoglycemia and the multifactorial etiology may partly explain the lack of an effective medical therapy [1,5]. So far, no established diagnostic test for postprandial hypoglycemia after bariatric surgery exists, but the diagnosis is based on a typical clinical pattern and confirmatory postprandial glucose sampling in case of symptomatic hypoglycemia (often <3.0 mmol/l) with improvement of symptoms after ingestion of carbohydrates leading to normal glucose levels (Whipple’s triad) [2]. Whereas the majority of patients can be treated with dietary measures alone, some may require additional medical therapy, which is generally off label and based on an individual case finding strategy [1,2].
In patients who cannot sufficiently be treated with dietary measures, further evaluation is recommended to distinguish postprandial hypoglycemia caused by non-insulinoma pancreatogenous hypoglycemia syndrome from various entities such as insulinoma or nesidioblastosis, which may be successfully treated surgically [6-8].
Herein, glucagon like peptide-1 (GLP-1) receptor PET/CT has proved to facilitate the localization of insulinomas, which usually overexpress GLP-1 receptor in a high incidence and density [9-12]. Few studies have shown that GLP-1 receptor PET/CT can detect focal pancreatic islet cell hypertrophy/ hyperplasia in neonates/children (congenital hyperinsulinism) and adults indicating an overexpression of GLP-1 receptors in pancreatic islet cell hypertrophy/hyperplasia [13,14].
In the last decade, conflicting results on the expression of GLP-1 receptors of the pancreas in patients with postprandial hypoglycemia after bariatric surgery have been published. For example, Service et al. and Patti et al. suggested that postprandial hypoglycemia is caused by pancreatic β-cell hypertrophy and hyperplasia [15-17]. In contrast, Meier et al. argued that it is not accompanied by an increased β-cell mass [18]. Using autoradiography, Reubi and colleagues also documented no increase in GLP-1 receptor expression in surgically removed pancreatic tissue of patients with PGBH, indicating that in vivo imaging, using GLP1 receptor imaging may not be a suitable tool in this condition. Thus, it is of particular interest whether GLP-1 receptor PET/CT can also be used to quantitatively assess the GLP- 1 receptor expression of the entire pancreas and/or duodenum in patients by measuring total 68Ga-DOTA-exendin-4 uptake in a volume of interest.
The aim of this study was to quantitatively investigate the in vivo GLP-1 receptor expression of the duodenum and pancreas in RYGB patients with and without PGBH using 68Ga-DOTA-exendin-4 PET/CT and retrospectively collect data on all patients with PGBH who underwent 68Ga-DOTAexendin- 4 PET/CT at our center.
Materials and Methods
Participants
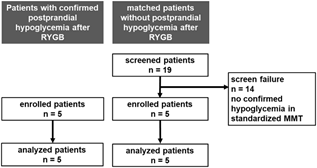
Five consecutive patients with confirmed postprandial hypoglycemia (PGBH) after gastric bypass surgery received 68Ga-DOTA-exendin-4 PET/ CT and five patients without postprandial hypoglycemia matched for sex, age, operative procedure and postoperative interval were prospectively included. Nineteen potential patients serving as controls and matched for age, gender, operative procedure, and postoperative interval, was screened in the outpatient clinic at the Division of Endocrinology, Diabetes and Metabolism at the University Hospital Basel (Figure 1). All potential controls underwent a standardized liquid mixed meal test (300 ml Ensure plus®, 60 g carbohydrates, 450 kcal). 14/19 patients showed symptomatic hypoglycemia and were, therefore, not considered as control patients. 5/19 patients remained asymptomatic and euglycemic during mixed meal testing and were, therefore, included in the study as matched controls and also received a 68Ga-DOTA-exendin-4 PET/CT using the same protocol as in the five patients with PGBH. Exclusion criteria were pregnancy, allergy to exendin- 4, renal (creatinine concentrations >140 μmol/L) and hepatic insufficiency (>3x increased levels of transaminases). All patients gave written informed consent. The study was approved by the local ethics committee (Ethikkommission Nordwest-und Zentralschweiz) and amended for five matched normoglycemic control patients (EKBB 163/12).
Imaging
piCHEM (Graz Austria), synthesized DOTA-exendin-4 ([Nle14, Lys40(Ahx-DOTA)NH2]-exendin-4, where Ahx=aminohexanoic acid and DOTA=1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) according to good manufacturing practice. Eckert and Ziegler (Berlin, Germany) supplied the 68Ge/68Ga generator (IGG100 or GalliaPharmTM). The synthesis of 68Ga-DOTA-exendin-4 is performed at the University Hospital of Basel. 68Ga-DOTA-exendin-4 was prepared on an automated synthesis unit (PharmTracer, Eckert and Ziegler, Berlin, Germany) by loading the eluate of the 68Ge/68Ga-generator onto a cation exchange column (Strata-XC, Phenomenex). 68Ga was eluted with a mixture of acetone/HCl (97.6%/0.02 N) directly into the reaction vial containing DOTA-exendin-4 in sodium acetate buffer (0.2 M, pH 4.0). DOTA-exendin-4 was labeled with 68Ga at 85°C within 300 sec. The product was purified by C8 solidphase extraction using 50% ethanol in water, followed by dilution with isotonic NaCl 0.9% and filtration using Millex-GV 0.22 μm sterile filters (Millipore). 68Ga-DOTA-exendin-4 was synthesized with a labeling yield of >95% and radiochemical purity >93%.
PET/CT was performed on two different scanners in supine position: PET/64-detector CT scanner (Discovery ST; GE Healthcare) and PET/128- detector CT scanner (Biograph mCT-X (128); Siemens Healthineers). Calibration of PET scanners and their cross-calibration was performed. One bed position of the upper abdomen was acquired during 8 min, 2.5 h after slow (5 min) intravenous injection of 88.7 ± 1.5 MBq (range 60-90 MBq) for hypoglycemic patients and 88.5 ± 35.6 MBq (range 38-114 MBq) for normoglycemic matches. PET images were reconstructed using an ordered subsets expectation maximization (OSEM) algorithm with 3 iterations and 25 subsets. An unenhanced low dose CT (120 kVp, 30-100 mAs) for attenuation correction and anatomic guidance was performed in all patients.
Total uptake measurements of pancreas and duodenum
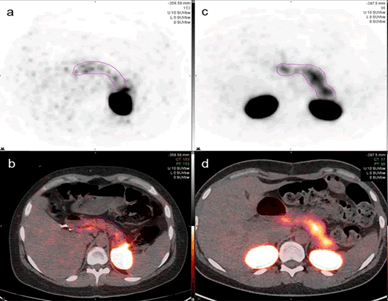
The total uptake of the pancreas and duodenum, defined as the sum of the standard uptake value (SUVsum) for each voxel in the volume of interest (VOI), was measured to compare the 68Ga-DOTA-exendin-4 uptake in both patient groups. The VOI was defined as the total volume of 68Ga- DOTA-exendin-4 uptake in the pancreas and duodenum (Figure 2). The upper SUV threshold was set at 10. Two independent readers (Reader 1 KA, board certified nuclear medicine physician with three years of experience after certification and Reader 2 PW, resident) who were blinded to patients' identity, clinical and laboratory information, carried out the uptake measurements with MIM Software, Version 7.0.1 using the universal PET/CT workflow. On consecutive, axial fused PET/CT images, a region of interest (ROI) was marked, including the entire duodenal and pancreatic tissue. After the ROIs were plotted, the VOI was calculated automatically. Due to the acquisition of an unenhanced low dose CT for anatomical correlation, differentiation between duodenum and pancreatic head was not possible in all patients. In patients where differentiation of the uptake in the pancreatic head and the duodenum was clearly possible, an additional VOI with the pancreatic uptake only was created and the duodenal uptake was excluded.
Statistical analysis
To compare the uptake of the two paired matched groups, Wilcoxon signed rank test and for comparison of unpaired groups, Mann-Whitney U test was performed. Due to the small sample size, tests for non-normally distributed data were used. An alfa level of 0.05 was regarded as statistically significant.
Results
Clinical characteristics of patients
Characteristics of hypoglycemic patients are summarized in Supplementary Table 1 and of normoglycemic matched patients in Supplementary Table 2. All participants were female. The median age was 37 years (range 28-51) for the hypoglycemic and 35 years (range 28-46) for the normoglycemic group. The median interval between surgery and PET-CT was 84 months (range 30-103 months) for the hypoglycemic and 74 months (range 29-102 months) for the normoglycemic group. Whipple triad was positive in all five hypoglycemic patients and negative in all five matched normoglycemic patients. Median preoperative body mass index (BMI) was 44.7 kg/m2 (36-51.1 kg/m2) for the hypoglycemic group and 38.3 kg/ m2 (34.8-51.7 kg/m2) for the normoglycemic group. Median postoperative BMI at the time of imaging was 25.5 kg/m2 (20.5-29.4 kg/m2) for the hypoglycemic group and 25.6 kg/m2 (24.9-39.7 kg/m2) for the normoglycemic group. Median age and range for the hypoglycemic patients including the three additional patients was 36 years (range 27-53), median interval between surgery and PET-CT 72 months (range 30-103).
Table 1: Uptake measurements of the hypoglycemic patients (reader 1=KA, reader 2=PW, N/A=not applicable).
| Pancreas and Duodenum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | ||
| Uptake | Reader 1 | 2780 | 1948 | 1874 | 3364 | 8259 | 2701 | 6743 | 8530 |
| Uptake | Reader 2 | 3264 | 2454 | 2414 | 3713 | 7345 | 2887 | 6145 | 8197 |
| Uptake | Mean | 3022 | 2201 | 2144 | 3539 | 7802 | 2794 | 6444 | 8363 |
| Pancreas only | |||||||||
| Patient 1 | Patient 2 | Patient 3 | Patient | Patient 5 | Patient 6 | Patient 7 | Patient 8 | ||
| Uptake | Reader 1 | N/A | N/A | N/A | N/A | 6427 | N/A | N/A | N/A |
| Uptake | Reader 2 | N/A | N/A | N/A | N/A | 5045 | N/A | N/A | N/A |
| Uptake | Mean | N/A | N/A | N/A | N/A | 5736 | N/A | N/A | N/A |
Table 2: Uptake measurements of the normoglycemic matched patients (reader 1=KA, reader 2=PW, N/A=not-applicable).
| Pancreas and Duodenum | ||||||
|---|---|---|---|---|---|---|
| Match 1 | Match 2 | Match 3 | Match 4 | Match 5 | ||
| Uptake | Reader 1 | 4483 | 7352 | 7266 | 7786 | 6056 |
| Uptake | Reader 2 | 5725 | 7455 | 6878 | 7227 | 5025 |
| Uptake | Mean | 5104 | 7404 | 7072 | 7507 | 5540 |
| Pancreas only | ||||||
| Match 1 | Match 2 | Match 3 | Match 4 | Match 5 | ||
| Uptake | Reader 1 | 2706 | 5392 | N/A | N/A | 5311 |
| Uptake | Reader 2 | 2992 | 6184 | N/A | N/A | 4034 |
| Uptake | Mean | 2849 | 5788 | N/A | N/A | 4672 |
Total uptake measurements of pancreas and duodenum
The median of the total uptake was 3022 (range 2144-7802) for matched hypoglycemic patients and 7072 (range 5104-7507) for matched control patients, respectively. The uptake of 68Ga-DOTA-exendin-4 in the pancreas and duodenum in patients with PGBH was not statistically different compared to the matched normoglycemic participants (p=0.187).
The total uptake in the pancreatic tissue and the duodenum by the two readers are shown in Table 1 for hypoglycemic and Table 2 for the normoglycemic patients and in Figure 3. Representative axial views of a patient from the hypoglycemic group (patient 3) and of the normoglycemic control group (match 3) are shown in Figure 2. For hypoglycemic patient 5 and normoglycemic matched patients 1, 2, and 5, it was possible to clearly differentiate the uptake of the duodenum from the pancreas and a separate VOI was drawn on the pancreatic uptake only.
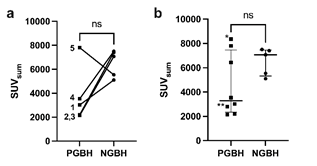
Figure 3: (a) SUVsum of pancreas and duodenum in patients (1-5) with post gastric bypass hypoglycemia (PGBH, rectangles) and their respective normoglycemic matches (NGBH, dots) as well as (b) with additional PGBH patients of clinical routine examinations of which patient 8 with diffuse pancreatic enhancement is indicated with one asterisk (*) and patient 6 with focal enhancement in pancreatic tail with two asterisks (**).
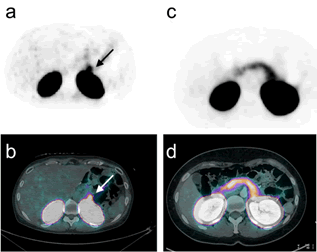
Whereas in the matched cohort 68Ga-DOTA-exendin-4 PET/CT did not show an increased focal or diffuse uptake in the pancreas, consistent with an insulinoma or nesidioblastosis (Figure 3), in one patient (#6) with PGBH a focal tracer uptake in the pancreas was observed (Figure 4).
When comparing total pancreatic and duodenal uptake of all eight patients with PGBH (median 3281, range 2144-8363) to the non-hypoglycemic group (median 7072, range 5104-7507), (Figure 3). No statistical significant difference was found (p=0.284) but a much greater range of tracer uptake was observed in patients with PGBH.
Clinical follow up of patients with PGBH
Data on clinical follow up of all patients with PGBH is depicted in Supplementary Table 1. In four of eight patients (#1, 2, 7 and 8) with PGBH who were insufficiently controlled with dietary and off label medications, a reversal operation of their gastric bypass and in patient 4 an extension of the common channel was performed which led to an improvement of the intensity and frequency of all their hypoglycemic episodes. Patient 6 with a possible focal nesidioblastosis refused to undergo hemipancreatectomy and could be treated successfully with a minimizer ring after several unsuccessful off label trials with sandostatin and diazoxide.
Discussion
To the best of our knowledge, this is the first article in which in vivo pancreatic and duodenal GLP-1R expression is compared in patients with and without PGBH using 68Ga-DOTA-exendin-4 PET/CT.
Postprandial hypoglycemia gained significant attention as an important medical complication after bariatric surgery. Especially for patients who are symptomatic despite intensified dietary and/or off label medical therapeutic measures, exclusion of surgically curable causes such as insulinoma or focal nesidioblastosis like lesions should be considered [6]. Thus, GLP- 1 receptor imaging may be a valuable tool for the identification of such alterations guiding therapeutic decisions [9,13].
In our study, one patient with PGBH showed a focal nesidioblastosis like pattern. However, there were no significant differences in the GLP-1 receptor expression in patients with and without PGBH, but patients with PGBH showed a much greater range of tracer uptake compared to normoglycemic patients after RYGB [19,20]. This is an intriguing finding and the pathophysiological background is ill-defined. It may be that patients with PGBH have a down regulation or internalization of GLP-1 receptors due to high postprandial peak glucose and/or GLP-1 levels, thereby explaining the lower pancreatic/duodenal 68Ga-DOTA-exendin-4 accumulation in some of our hypoglycemic patients compared to controls [21-24]. However, three patients with PGBH (#5, 7, and 8) had a pancreatic and duodenal 68Ga-DOTA-exendin-4 uptake comparable to normoglycemic counterparts. This may indicate that the disease is per se heterogeneous and/or may change during the course of the disease.
Additionally, there may be alternative explanations for this observation. In this study, imaging was performed in a non-fasted state due to the initial suspicion of an insulinoma in the hypoglycemic population and subsequently in the normoglycemic group. Postprandially, high endogenous GLP-1 levels may compete with 68Ga-DOTA-exendin-4 and thereby lead to GLP-1 receptor saturation and consequently low 68Ga-DOTA-exendin- 4 accumulation in the pancreas and duodenum.
Interestingly, Boss et al. described in a poster presentation a higher betacell mass using 68Ga-DOTA-exendin-4 PET/CT in patients with PGBH compared to patients with incomplete remission of their diabetes after gastric bypass surgery but did not find a correlation of beta-cell activity and beta-cell mass [25]. We would, therefore, argue that our data represent more likely snapshots of 68Ga-DOTA-exendin-4 uptake in patients with PGBH within a heterogeneous morphologic spectrum of GLP-1 receptor distribution in the pancreas and eventually duodenum reflected by the greater range of receptor distribution compared to normoglycemic patients after RYGB. This would add more conflicting data on hypertrophic beta-cells [16,26,27] as a reason for PGBH and other studies where these changes were not detected [18,26]. Post-bariatric hypoglycemia emerges over time after the bariatric procedure and its severity as well as the factors resulting in this disorder is multifaceted. Since we did not further characterize our patient’s immunometabolically, it remains elusive whether the patients differed substantially therein. Even though for five of our patients a well-controlled matching for sex, age, postoperative interval, and operative procedure, was performed, differences in limb length as well as other potentially contributing factors (incretins, inflammatory parameters, etc.) may explain our observations [5,28,29].
Our study also has limitations. It is a single center study with a small sample size and only female patients with postprandial hypoglycemia after gastric bypass surgery. However, among all bariatric procedures, this is the intervention with the highest rate of post-bariatric hypoglycemia and there is a female preponderance [1,30]. In addition, our hypoglycemic patients were strongly affected by post-bariatric hypoglycemia with frequent and severe hypoglycemic episodes and may, therefore, not reflect the majority of patients suffering from less severe symptoms.
In addition, a clear separation of pancreatic and duodenal parts was only possible in three of the non-hypoglycemic and in one hypoglycemic patient, limiting assertions on differences in pancreatic and duodenal GLP-1 receptor distribution. Furthermore, since none of the patients underwent a pancreatectomy, no histological data on ß-cell morphology is available.
Ongoing studies in patients undergoing bariatric surgery with diabetes or confirmed post-bariatric hypoglycemia (NCT03182192, NCT02542059) may shed further light on our first observations.
However, we would raise a word of caution when performing studies with GLP-1 receptor imaging in patients undergoing bariatric surgery in general or specifically in those with post-bariatric hypoglycemia. Patients should be very well metabolically characterized, including fasting and postprandial glucose, insulin and incretin levels as well as inflammatory parameters [5,31]. In addition, GLP-1 receptor imaging should be performed in a standardized way after a fasting period to avoid potential postprandial competition of endogenous GLP-1 levels which are high after bariatric surgery and may be high enough to explain different tracer distribution in those with postprandial hypoglycemia. Longitudinal follow up may be of particular importance to better characterize and differentiate GLP-1 receptor distribution after bariatric surgery to allow reliable assumptions about GLP-1 receptor imaging and its role in postprandial hypoglycemia, but must be balanced against radiation exposure.
Conclusion
GLP-1 receptor imaging with 68Ga-DOTA-exendin-4 PET/CT in patients with post gastric-bypass hypoglycemia was not significantly different from matched normoglycemic controls but revealed a greater variety of uptake compared to match non-hypoglycemic patients This may reflect a varying spectrum of GLP-1 receptor distribution in patients with PGBH and suggests a heterogeneous disease.
Acknowledgments
We thank all the patients who participated in the trial, the referring physicians and the local investigators who contributed to the trial, and the technicians who did the labelling and the scans.
Funding
The study was funded intramurally.
Competing Interests
All authors declare no competing interests.
Author Contributions
All authors planned the study. MH screened control patients and performed mixed meal testing. KA and PW did PET/CT readings. MH, KA, and PW did the analysis and wrote the first draft of the manuscript. All authors critically proved data, edited and approved the manuscript.
Data Availability
All data is available from the corresponding author on request.
Ethics Approval
The study was approved by the local ethics committee (Ethikkommission Nordwest-und Zentralschweiz, Basel, Switzerland, EKBB 163/12).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
All authors approved the manuscript for submission.
References
- Salehi M, Vella A, McLaughlin T, Patti ME (2018) Hypoglycemia after gastric bypass surgery: Current concepts and controversies. J Clin Endocrinol Metab 103(8):2815-26.
[Crossref] [Google Scholar] [Pubmed]
- Scarpellini E, Arts J, Karamanolis G, Laurenius A, Siquini W, et al. (2020) International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol 16(8):448-466.
[Crossref] [Google Scholar] [Pubmed]
- Emous M, Wolffenbuttel BHR, Totté E, Van Beek AP (2017) The short to mid-term symptom prevalence of dumping syndrome after primary gastric-bypass surgery and its impact on health-related quality of life. Surg Obes Relat Dis 13(9):1489-500.
[Crossref] [Google Scholar] [Pubmed]
- Varma S, Clark JM, Schweitzer M, Magnuson T, Brown TT, et al. (2017) Weight regain in patients with symptoms of post-bariatric surgery hypoglycemia. Surg Obes Relat Dis 13(10):1728-34.
[Crossref] [Google Scholar] [Pubmed]
- Hepprich M, Wiedemann SJ, Schelker BL, Trinh B, Stärkle A, et al. (2020) Postprandial hypoglycemia in patients after gastric bypass surgery is mediated by glucose-induced il-1β. Cell Metab 31(4):699-709.
[Crossref] [Google Scholar] [Pubmed]
- Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, et al. (2020) Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: Cosponsored by american association of clinical endocrinologists/american college of endocrinology, The obesity society, american society for metabolic & bariatric surgery, obesity medicine association, and american society of anesthesiologists. Surg Obes Relat Dis 16(2):175-247.
[Crossref] [Google Scholar] [Pubmed]
- Gupta RA, Patel RP, Nagral S (2013) Adult onset nesidioblastosis treated by subtotal pancreatectomy. JOP 14(3):286-8.
[Crossref] [Google Scholar] [Pubmed]
- Kikuchi T, Chujo D, Takahashi K, Takahashi N, Tanno Y, et al. (2017) Insulinoma presenting with reactive hypoglycemia: Evaluating the effect of tumor resection via continuous glucose monitoring. Intern Med 56(22):3067-71.
[Crossref] [Google Scholar] [Pubmed]
- Antwi K, Fani M, Heye T, Nicolas G, Rottenburger C, et al. (2018) Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: Evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imaging. 45(13):2318-27.
[Crossref] [Google Scholar] [Pubmed]
- Luo Y, Pan Q, Yao S, Yu M, Wu W, et al. (2016) Glucagon-like peptide-1 receptor PET/CT with 68Ga-NOTA-Exendin-4 for detecting localized insulinoma: A prospective cohort study. J Nucl Med. 57(5):715-20.
[Crossref] [Google Scholar] [Pubmed]
- Wild D, Antwi K, Fani M, Christ ER (2021) Glucagon-like Peptide-1 Receptor as Emerging Target: Will It Make It to the Clinic? J Nucl Med 62:44S-50S.
[Crossref] [Google Scholar] [Pubmed]
- Boss M, Mikkola K, Buitinga M, Brom M, Wild D, et al. (2019) 68Ga-NODAGA-exendin-4 PET/CT for the localization of insulinomas. Nuklearmedizin 58(02):124.
- Christ E, Wild D, Antwi K, Waser B, Fani M, et al. (2015) Preoperative localization of adult nesidioblastosis using ⁶⁸Ga-DOTA-exendin-4-PET/CT. Endocrine 50(3):821-3.
[Crossref] [Google Scholar] [Pubmed]
- Boss M, Rottenburger C, Brenner W, Blankenstein O, Prasad V, et al. (2022) 68Ga-NODAGA-Exendin-4 PET/CT improves the detection of focal congenital hyperinsulinism. J Nucl Med 63(2):310-5.
[Crossref] [Google Scholar] [Pubmed]
- Hepprich M, Antwi K, Waser B, Reubi JC, Wild D, et al. (2020) Brunner's gland hyperplasia in a patient after Roux-Y gastric bypass: An important pitfall in GLP-1 receptor imaging. Case Rep Endocrinol 2020:4510910.
- Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, et al. (2005) Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: Evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 48(11):2236-40.
[Crossref] [Google Scholar] [Pubmed]
- Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, et al. (2005) Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353(3):249-54.
[Crossref] [Google Scholar] [Pubmed]
- Meier JJ, Butler AE, Galasso R, Butler PC (2006) Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care 29:1554-9.
[Crossref] [Google Scholar] [Pubmed]
- Liu Q, Duan J, Zheng Y, Luo J, Cai X, et al. (2018) Rare malignant insulinoma with multiple liver metastases derived from ectopic pancreas: 3-year follow-up and literature review. Onco Targets Ther 11:1813-9.
[Crossref] [Google Scholar] [Pubmed]
- Christ E, Wild D, Ederer S, Béhé M, Nicolas G, et al. (2013) Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: A prospective multicentre imaging study. Lancet Diabetes Endocrinol 1(2):115-22.
[Crossref] [Google Scholar] [Pubmed]
- Craig CM, Liu LF, Deacon CF, Holst JJ, McLaughlin TL (2017) Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia 60(3):531-40.
[Crossref] [Google Scholar] [Pubmed]
- Salehi M, Prigeon RL, D'Alessio DA (2011) Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 60(9):2308-14.
[Crossref] [Google Scholar] [Pubmed]
- Salehi M, Gastaldelli A, D'Alessio DA (2014) Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 146(3):669-80.e2.
[Crossref] [Google Scholar] [Pubmed]
- Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, et al. (2007) Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: Possible contribution to impaired incretin effects in diabetes. Diabetes 56(6):1551-8.
[Crossref] [Google Scholar] [Pubmed]
- M B, M G (2018) Beta cells in diabetes remission and hypoglycaemia after Roux-en-Y gastric bypass surgery, visualised by 68Ga-exendin-4 PET/CT. EASD Virtual Meeting. Berlin.
- Reubi JC, Perren A, Rehmann R, Waser B, Christ E, et al. (2010) Glucagon-like peptide-1 (GLP-1) receptors are not overexpressed in pancreatic islets from patients with severe hyperinsulinaemic hypoglycaemia following gastric bypass. Diabetologia 53(12):2641-5.
[Crossref] [Google Scholar] [Pubmed]
- Clancy TE, Moore FD, Zinner MJ (2006) Post-gastric bypass hyperinsulinism with nesidioblastosis: Subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg 10(8):1116-9.
[Crossref] [Google Scholar] [Pubmed]
- Mulla CM, Goldfine AB, Dreyfuss JM, Houten S, Pan H, et al. (2019) Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. Obes Surg 29(7):2092-2099.
[Crossref] [Google Scholar] [Pubmed]
- Larraufie P, Roberts GP, McGavigan AK, Kay RG, Li J, et al. (2019) Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep 26(6):1399-1408.
[Crossref] [Google Scholar] [Pubmed]
- Capristo E, Panunzi S, De Gaetano A, Spuntarelli V, Bellantone R, et al. (2018) Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: A randomized trial. J Clin Endocrinol Metab 103(6):2136-46.
[Crossref] [Google Scholar] [Pubmed]
- Sheehan A, Patti ME (2020) Hypoglycemia after upper gastrointestinal surgery: Clinical approach to assessment, diagnosis, and treatment. Diabetes Metab Syndr Obes 13:4469-82.
[Crossref] [Google Scholar] [Pubmed]
Citation: Hepprich M, Antwi K, Wiesner P, Cavelti-Weder C, Donath MY, et al. (2022) Glucagon-Like Peptide-1 Receptor Pet/Ct in Patients with and Without Post Gastric Bypass Hypoglycemia: A Prospective, Matched Case-Control Study. J Gastrointest Dig Syst.12:686. DOI: 10.4172/2161-069X.1000686
Copyright: © 2022 Hepprich M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4168
- [From(publication date): 0-2022 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 3660
- PDF downloads: 508