Research Article Open Access
Heterogeneous Ganglioside Standards in LC-MS/MS: Sensitive Method for Quantifying the Major Molecular Components in Mono-Sialo Ganglioside Standards
Ashta Lakshmi Prasad Gobburi1, Renliang Zhang2, Belinda Willard2, Denise Inman3 and David J Anderson1*1Department of Chemistry, Cleveland State University, Cleveland, Ohio, USA
2Research Core Services, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA
3Department of Pharmaceutical Sciences, Northeast Ohio Medical University, Rootstown, Ohio, USA
- *Corresponding Author:
- David J Anderson
Department of Chemistry
Cleveland State University
2121 Euclid Avenue, Cleveland
Ohio 44115, USA
Tel: 001-216-687-2453
Fax: 001-216-687-9298
E-mail: d.anderson@csuohio.edu
Received date: November 04, 2015 Accepted date: November 21, 2015 Published date: November 28, 2015
Citation: Gobburi ALP, Zhang R, Willard B, Inman D, Anderson DJ (2015) Heterogeneous Ganglioside Standards in LC-MS/MS: Sensitive Method for Quantifying the Major Molecular Components in Mono-Sialo Ganglioside Standards. J Anal Bioanal Tech S13:009. doi:10.4172/2155-9872.S13-009
Copyright: © 2015 Gobburi ALP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A commercially-available mono-sialo (GM1) ganglioside standard consists of three major components with different ceramide structures: C18:0 fatty acid/C18-sphingosine, C18:0 fatty acid/C20-sphingosine and C20:0 fatty acid/C18-sphingosine. The usual multiple reaction monitoring (MRM) liquid chromatography-tandem mass spectrometry (LC-MS/MS) of gangliosides, which monitors the dehydrated sialic acid fragment (m/z 290), cannot differentiate between the individual iso-molecular weight components C18:0 fatty acid/C20-sphingosine and C20:0 fatty acid/C18-sphingosine. Present characterization of ganglioside standards quantifies only the fatty acid content by gas chromatography-flame ionization detection (GC-FID) analysis of the ganglioside mixture and does not parse out the percentages of the individual mono-sialo ganglioside components. In the present work analyzing a heterogeneous GM1 standard, results from a dehydrated sialic acid daughter ion MRM LC-MS/MS determination employing hydrophilic interaction liquid chromatography were combined with results of the fatty acid content determined by GC-FID analysis to sensitively quantify the three predominant individual molecular GM1 components in standards at concentrations as low as 50 ng/mL (Method 1). These dehydrated sialic acid MRM results (Method 1) were confirmed by a less sensitive fatty acid daughter ion MRM LC-MS/MS technique (Method 2) which could only determine molecular GM1 components in standards at high concentrations (1 μg/mL-10 μg/mL). Method 2, however, has the advantage of directly quantifying the three predominant individual molecular GM1 components for comparison with Method 1 results. Equations are derived which incorporate the combined data (Method 1) to calculate percentages of individual mono-sialo gangliosides in the standard. Percentages for the individual mono-sialo gangliosides in the standard differed by at most 2% (absolute difference in the percentages) in comparing the results obtained by the two methods.
Keywords
GM1 ganglioside; C18-sphingosine; C20-sphingosine; Long chain base; Heterogeneous calibrator; HILIC
Abbreviations
GM: Mono-sialo Ganglioside; C18:0FA: C18:0 Fatty Acid; C20:0FA: C20:0 Fatty Acid; C18S: 18 Carbon Sphingosine; C20S: 20 Carbon Sphingosine; ESI: Electrospray Ionization; HILIC: Hydrophilic Interaction Liquid Chromatography; LC-MS/ MS: Liquid Chromatography-Tandem Mass Spectrometry; MRM: Multiple Reaction Monitoring; GC-FID: Gas Chromatography-Flame Ionization Detector
Introduction
An essential component of most analytical techniques is the use of standards (calibrators) to generate a calibration curve for determining the concentration or amount of analyte in a sample [1,2]. Standards are usually prepared from pure analytes or analytes with an established purity. Additionally, standards can be prepared from reference materials in which the concentration of analyte is directly measured or traceable, to a reference technique and a primary calibrator. Although pure analyte or certified reference materials are available for many analytes, they are not available for all analytes, particularly biological compounds with inherent heterogeneity.
This heterogeneity issue is particularly prominent in the analysis of gangliosides. One study identified 137 different ganglioside and asialoganglioside components in human fetal brain tissue [3]. Gangliosides have a backbone structure of sphingosine, which is a long chain carbon molecule with a hydroxyl group (at C3) and a double bond (at C4-C5) and, prior to being incorporated into a ganglioside, have another hydroxyl group (at C1) and an amino group (at C2). Neuronal gangliosides consist almost entirely of a C18 and C20 carbon length sphingosine. It should be noted, however, that a small percentage of neuronal gangliosides (less than 5%) have a C18- or C20-sphinganine backbone, which is a saturated carbon chain not containing a double bond [4-6]. Moieties added to sphingosine (or sphinganine) to form gangliosides are an oligosaccharide bonded via a glycosidic linkage at C1 and a fatty acid bonded via an amide linkage at C2 [7]. Heterogeneity in the gangliosides results from heterogeneity in the oligosaccharide, fatty acid and/or sphingosine, giving rise to the vast number of ganglioside variants.
Commercially-available ganglioside standards are also heterogeneous in composition, which is problematic in LC-MS/MS, as well as for other analytical techniques. For example, a commercially-available GM1 ganglioside standard has three major gangliosides and is only quantified in terms of fatty acid content and not according to the individual ganglioside molecular components. This is an issue in ganglioside analysis. Current analytical techniques do not determine intact molecular components but rather are based solely on fatty acid content. Basing ganglioside analysis on fatty acid content has substantial limitations, as a particular fatty acid is usually a part in multiple ganglioside molecules. In particular, a technique which can differentiate among individual iso-molecular weight components of ganglioside needs to be developed. The present work is the first report addressing this issue.
GM1 is a subclass of gangliosides homogeneous in a particular oligosaccharide (containing one sialic acid group) but varying in the ceramide (sphingosine plus bonded fatty acid) composition. The three major ganglioside components in the particular commerciallyavailable GM1 standard characterized in the present work has the following different ceramide structures: C18:0FA-C18S, and two isomolecular components, C18:0FA-C20S and C20:0FA-C18S, where FA and S indicate fatty acid and sphingosine, respectively, the 18 and 20 indicate the number of carbons, and the :0 indicates that there are no carbon-carbon double bonds in the fatty acid.
The standard multiple reaction monitoring (MRM) LC-MS/MS techniques used in the determination of gangliosides monitors the m/z 290 dehydrated sialic acid daughter ion of the oligosaccharide. The disadvantage of this MRM technique is that the iso-molecular weight components C18:0FA-C20S and C20:0FA-C18S cannot be differentiated. Present characterization of the gangliosides in the standard is usually limited to a gas chromatography-flame ionization detection (GC-FID) analysis of the hydrolyzed/methylated fatty acids. This analysis only determines the percentage of all gangliosides containing the C18 saturated fatty acid (in this case, the combined amounts of C18:0FA-C18S, C18:0FA-C20S and the C18:0FAspinganine components), the C20:0 saturated fatty acid [in this case only C20:0FA-C18S and the C20:0FA-spinganine components, as there was no noted signal for C20:0FA-C20S (m/z 1601)] and other fatty acids. However it is unable to parse out the percentages of the individual GM1 components.
The importance of determining the individual molecular gangliosides in a standard to be used in analysis of biological samples is underscored by noted physiologic differences of the gangliosides differing in their sphingosine/sphinganine carbon length, commonly referred to as long chain bases. Physiologic differences have been noted for C18- and C20-sphingosine/sphinganine gangliosides. Gangliosides in human brain consist entirely of C18-sphingosine at birth, changing to approximately equal proportions of C18- and C20-sphingosine gangliosides in adulthood [5,8,9]. Other examples of physiologic significance of the C18-/C20-sphingosine portion of the ganglioside are as follows. It has been suggested that membrane characteristics such as fluidity, thickness and microdomain properties are related to the C18-/ C20-sphingosine content [9]. A study reported a considerable effect of increasing the C20-sphingosine ganglioside proportion of gangliosides on micelle size and aggregation characteristics, with implications for neuronal membrane formation and characteristics [10]. Differences have been noted in the rate of association of the particular ganglioside with HeLa cells and intracellular accumulation of cyclic AMP in HeLa cells depending on the length and saturation/unsaturation status of C18- and C20-sphingosine/sphinganine GM1 gangliosides [11]. In another study, different association and metabolism effects were noted for C18 compared to C20 long chain bases in rat cerebellar granule cells [12]. Thus determining individual molecular gangliosides according to their long chain base identity is important.
In the present work, data from a sensitive MRM LC-MS/MS method for gangliosides, monitoring the m/z 290 dehydrated sialic acid daughter ion, combined with ganglioside fatty acid content data determined by GC-FID was used to determine the individual gangliosides in a heterogeneous GM1 standard. This report derives equations that combine the two data sets to sensitively quantify the three major individual molecular components in a GM1 standard. The accuracy was then assessed by employing a less sensitive but specific MRM technique, monitoring the fatty acid daughter ion of the individual GM1 molecular components.
Materials and Methods
Materials
GM1 ganglioside standard from bovine brain (Cat. No. 1061) was obtained from Matreya LLC (Pleasant Gap, PA, USA). Acetonitrile and methanol were Optima LC/MS grade from Fisher Scientific (Fair Lawn, NJ, USA). Ammonium acetate (≥ 99.99%) was from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade water was from a Barnstead Nanopure water purification system from Thermo Scientific (West Palm Beach, FL, USA).
Preparation of standard solutions
A stock solution of the GM1 standard 1 mg/ml with 83% methanol was prepared. All the working GM1 standard solutions in the range 0.05-10 μg/mL were prepared from the serial dilution of the stock solution with 83% methanol. All the stock solutions and working solutions were stored at -20°C.
HPLC conditions
HPLC analysis was performed on a Waters Alliance 2695 quaternary pump system (Milford, MA, USA). The chromatographic separation was performed on a hydrophilic interaction liquid chromatography (HILIC) column (amino-propyl ligand, 50 × 1 mm, 3 μm particle size) with a guard column (amino-propyl ligand, 5 × 1 mm, 3 μm particle size) from IMTAKT USA (Portland, OR, USA). A volume of 20 μl of the working standard was injected with an autosampler at 4°C. A multiple linear gradient of mobile phases (A: 83% acetonitrile, B: 83% acetonitrile and 5 mM ammonium acetate, C: 50% acetonitrile and 50 mM ammonium acetate, D: 50% acetonitrile) was used. Sample was injected into 100% mobile phase A run for 1 min, followed in succession by a linear gradient for 1 min to 100% mobile phase B, 100% mobile phase B for 4 min, a linear gradient for 6 min to 100% mobile phase C and finally 100% mobile phase C for 8 min. Mobile phase was directed to the mass spectrometer after 4 min of the run. The column was re-equilibrated as follows: 50% acetonitrile was run for 12 min followed by 83% acetonitrile for 8 min. Flow rate was 0.1 mL/min. Mobile phases were filtered through 0.45 μm membrane filters from Millipore (Billerica, MA, USA).
Mass spectrometry conditions
The eluent from the chromatographic system was introduced into a triple quadrupole Waters Micromass Quattro Ultima instrument (Milford, MA, USA) with an electrospray ionization (ESI) source. The ESI source in a negative ionization mode was optimized as follows: capillary potential (-3 KV), cone potential (-40 V), source temperature (150°C), desolvation temperature (300°C), cone gas flow (144 L/hr) and desolvation gas flow (756 L/hr). The triple quadrupole analyzer was optimized as follows: ion energy 1 and 2 were 1.0 and 3.0 eV respectively, collision energy was 70 eV, entrance and exit potential were 120 V and multiplier potential was -650 V. The multiple reaction monitoring (MRM) transitions were m/z 1545.3 → 290.3 and m/z 1573.3 → 290.3 monitoring the dehydrated sialic acid fragment of the GM1 components and m/z 1545.3 → 283.1 (C18:0 fatty acid), m/z 1573.3 → 283.1 (C18:0 fatty acid), m/z 1573.3 → 311.3 (C20:0 fatty acid) monitoring the respective fatty acids of the GM1 components.
Fatty acid analysis
Fatty acids of gangliosides were esterified with acidic methanol heating to 100°C for 4 hrs. followed by extraction with hexane and quantified with respect to fatty acid methyl ester standards using gas chromatography with flame ionization detection employing a polar phase GC column [13,14]. The results provided by the manufacturer for the GM1 standard is 90%, 3% and 7% for C18:0, C20:0 and other fatty acid content in the GM1 standard, respectively. No C18:1 or C20:1 fatty acids were present as determined by this method.
Results and Discussion
The GM1 standard consists almost entirely of gangliosides with two sphingosine backbones: C18S and C20S, as has been established for gangliosides from animal brain [4-6]. As established by the fatty acid analysis studies, the two predominant fatty acids attached to these sphingosine backbones via amide bonds were C18:0-FA (stearic acid, 90%) and C20:0-FA (arachidic acid, 3%). The remainder 7% is other gangliosides containing a variety of other length fatty acids. In Method 1 these data were combined with sensitive m/z 290 daughter ion MRM LC-MS/MS peak area data to determine the amounts of the three predominant GM1 gangliosides: C18:0FA-C18S, C18:0FA-C20S and C20:0FA-C18S. The accuracy of this method was assessed by comparing these results with the results of Method 2, a fatty acid daughter ion MRM LC-MS/MS technique, which is less sensitive but does directly determine each of the three predominant GM1 ganglioside components. These methods assume that the MRM response factors are the same for the three molecular ganglioside components, compared between the m/z 290 MRM peaks or among the fatty acid daughter ion MRM peaks. This is a reasonable assumption given that the gangliosides have very similar structures that vary by only 2 carbon units.
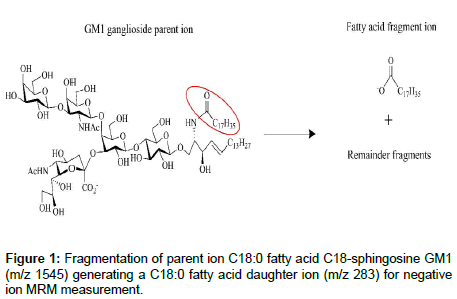
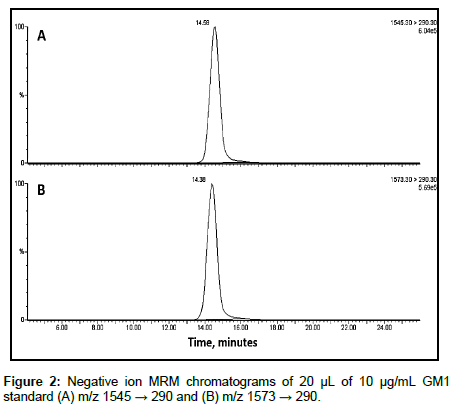
The fragmentation generating the negative fatty acid daughter ion is shown in Figure 1 [15]. The fragmentation mechanism generating the m/z 290 negative ion from the sialic acid has been reported to be cleavage of the glycosidic bond linking the sialic acid group to the oligosaccharide chain, followed by the elimination of water from the sialic acid [16]. Given in Figure 2 is the MRM chromatogram monitoring the 290 m/z dehydrated sialic acid fragment of the GM1 gangliosides for the C18:0FA-C18S (m/z 1545 → 290) and for the total C20:0FA-C18S and C18:0FA-C20S content (m/z 1573 → 290). The MRM chromatograms monitoring the fatty acid daughter ions are given in Figure 3.
Method 1: Using GC-FID fatty acid and GM1 ganglioside → 290 m/z daughter ion MRM data
Given below are Equations 1-6 in which peak area data from MRM method monitoring the m/z 290 dehydrated sialic acid daughter ion of the ganglioside and fatty acid data from GC-FID analysis of methylated fatty acids hydrolyzed from the gangliosides are inserted into the appropriate equations to solve for the percentages of the three predominant molecular GM1 ganglioside components.
Equation 1: Percentage of the different fatty acids in the total GM1 ganglioside standard: Equation 1 sums the percentages of the fatty acid distribution among the gangliosides in the standard, which is set equal to 100%.
%C18:0FA+%C20:0FA+%(other) FA=100% Eq. 1
where %C18:0FA, % C20:0FA and % (other) FA were the percentages of gangliosides having: C18:0 fatty acid (stearic acid), C20:0 fatty acid (arachidic acid) and all the other fatty acids, respectively.
The percent of each fatty acid was determined to be 90% (C18:0FA), 3% (C20:0FA) and 7% [(other) FA] by the GC-FID experiments performed by the manufacturer.
Equation 2: Percentage of the individual GM1 ganglioside components in the standard: Equation 2 is a summation expression of the percentage of three predominant molecular GM1components and a term for the combined percentage of all the other minor GM1 components, set equal to 100%. This is the base equation characterizing the percentage of individual ganglioside components in the sample.
%(C18:0FA-C18S)+%(C18:0FA-C20S)+%(C20:0FAC18S)+%( other gangliosides)=100% (2)
where %(C18:0FA-C18S), %(C18:0FA-C20S), %(C20:0FA-C18S) and %(other gangliosides) are the percentages of the various molecular GM1 components with the particular fatty acid (FA) and carbon length of the sphingosine (S) given.
Equations 3-6: Equations for calculating each term in Equation 2: Each term in Equation 2 is calculated from experimental data, either the m/z 290 daughter ion MRM peak areas or the GC-FID fatty acid results, or both, inserted into one of the Equations 3-6, as given below.
The first term in Equation 2 is determined by Equation 3:
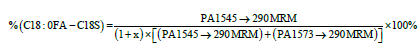 (3)
(3)
where the PA terms are the peak areas for the indicated MRM transitions and x=%(other)FA)/[%C18:0FA+%C20:0FA] (from fatty acid analysis, see Equation 1 for term definitions). In the GM1 standard used in this work the factor x=0.075. This is calculated from the values obtained from the GC-FID experiments determining the fatty acid percentage, which yielded %C18:0FA being 90%, %C20:0FA being 3% and %(other)FA being 7%. The factor x in the denominator takes into account the other gangliosides in the sample (accounting for gangliosides with other fatty acids besides C18:0FA and C20:0FA), which is inserted as a factor in the denominator, so that the denominator includes a “calculated” peak area for the rest of the GM1 gangliosides in the standard, in addition to the peak areas for the predominant GM1 components in the standard.
The second term in Equation 2 was determined by Equation 4:
%(C18:0FA-C20S)=%C18:0FA-%(C18:0FA-C18S)=90%- %(C18:0FA-C18S) (4)
The value for %C18:0FA for this particular GM1 standard was determined by the GC-FID experiments to be 90% and the value for % (C18:0FA-C18S) was determined by Eq. 3. These values were then inserted in Eq. 4 to solve for % (C18:0FA-C20S).
The third term in Equation 2 was determined by Equation 5.
%(C20:0FA-C18S)=%C20:0FA=3% (5)
It was experimentally confirmed by MS analysis that there was no C20:0FA-C20S (m/z 1601) in the GM1 standard and thus only the C20:0FA-C18 ganglioside had a C20:0 fatty acid. Therefore the %(C20:0FA-C18S) is equal to the percentage of the fatty acids that are C20:0 fatty acid in the GM1 standard. The value for the C20:0 fatty acid percentage for this particular GM1 standard was determined by the GC-FID experiments to be 3%.
The final term in Equation 2 was determined by Equation 6.
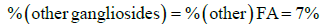 (6)
(6)
The value for % (other gangliosides) for this particular GM1 standard was determined by the GC-FID experiments to be 7%.
In summary, each term in Equation 2 for the individual GM1 components was determined from Equations 3-6. The experimental results employing these equations to determine the individual GM1 ganglioside components in the GM1 standard are discussed in Section 3.2 below. It should be noted that Equations 1 -6 do not account for any C18 or C-20 sphinganine components that may be present in the GM1 standard. Sphinganine content has been measured to be less than 5% of the total gangliosides present in animal brain samples [4-6], which introduces a small accuracy error for the Method 1 results.
Method 1 results
Duplicate injections of GM1 standards at concentrations 50, 100, 250, 500, 1000, 2000 and 10000 ng/ml on the LC-MS/MS system were done, monitoring the MRM m/z 290 dehydrated sialic acid daughter ion. The peak area data were processed according to the Method 1 utilizing Equations 3-6 to determine the percentage of each of the three predominant GM1 gangliosides in the standard, with the average of the results and associated standard deviation given in Table 1. Example calculations for determining the percentages of the three major ganglioside components in the GM1 standard according to Method 1 are given in the Supplementary Information section.
| % C18:0FA-C18S (± SD) |
% C18:0FA-C20S (± SD) |
% C20:0FA-C18S | % Other Gangliosides | ||
|---|---|---|---|---|---|
| 100% C18- and C20- Sphingosine Long Chain Bases | |||||
| Method 1a | 48.3 (± 0.7) | 41.7 (± 0.7) | 3 | 7 | |
| Method 2b | 47.8 (± 0.4) | 43.6 (± 0.4) | 1.6 | 7 | |
| 95% C18- and C20-Sphingosine and 5% C18- and C20-Sphinganine Long Chain Bases | |||||
| Method 1a | 45.7 (± 0.7) | 39.3 (± 0.7) | 3 | 12 | |
| Method 2b | 45.3 (± 0.4) | 41.3 (± 0.5) | 1.5 | 12 | |
| % Error comparing 100% and 95% Long Chain Base Sphingosine Content Results | |||||
| Method 1 | +5.4 % error | +5.8 % error | 0.0 % error | ||
| Method 2 | +5.2 % error | +5.3 % error | +6.3 % error | ||
aResults for the C18:0FA-C18S and the C18:0FA-C20S for Method 1 are based on averaged duplicate runs of 8 standards varying in concentration from 50 ng/mL to 10,000 ng/mL of the GM1 standard. The numbers for the % C20:0FA-C18S and the % other gangliosides is from the fatty acid analysis performed by the manufacturer, for which
no SD data are available.
bResults for Method 2 are based on the duplicate runs of the highest standard 10,000 ng/mL, as only this standard gave an MRM peak for % C20:0FA-C18S. SDs are
calculated for C18:0FA-C18S and the C18:0FA-C20S based on MRM peaks for averaged duplicate runs for 4 standards varying in concentration from 1000 ng/mL to 10,000
ng/mL. No SDs are calculated for the others as there were only 2 runs for C20:0FA-C18S and the other gangliosides was determined by fatty acid analysis, for which no
standard deviation data are available.
Table 1: Molecular Ganglioside Content of the Matreya Bovine Brain GM1 Standard.
A plot of the ratio of the Method 1 determined values for C18:0FAC18S to C18:0FA-C20S versus standard GM1 concentration is shown in Figure 4 to assess if there were any concentration effects or trends. The slope of the regression line (given Figure 4) is close to zero, indicating no concentration trend in the data. However the data at lower GM1 ganglioside concentration does show some variability.
Figure 4: Ratio of the percentage of the two prominent GM1 gangliosides C18:0FA-C18S/C18:0FA-C20S determined by Method 1 (triangles, dotted linear regression line) and by Method 2 (circles, solid linear regression line) versus concentration of GM1 standard injected. The regression equation of Method 1 and 2 was y=-0.0000038x+1.171 (with S.D. of slope= ± 0.0000037, S.D. of intercept= ± 0.015) and y=-0.0000044x+1.146 (with S.D. of slope=± 1.00025E-20, S.D. of intercept= ± 5.7E-17) respectively.
Method 2: Using GM1 ganglioside → fatty acid daughter ion MRM data
The following MRM transitions monitoring fatty acid daughter ion fragments are not normally used in ganglioside MS analysis because of low sensitivity:
C18:0FA-C18S, m/z 1545 → 283; C18:0FA-C20S, m/z 1573 → 283; and C20:0FA-C18S, m/z 1573 → 311
However these MRM parameters were monitored in the present work to determine the percentages of the three predominant GM1 gangliosides to compare with that obtained by the more sensitive Method 1 results. The fatty acid daughter ion MRM directly measures each individual GM1 component and thus calculations using fatty acid daughter ion data yield accurate values for the percentages of the individual GM1 ganglioside components, to which Method 1values can be compared.
The percentages of these three predominant GM1 gangliosides can be directly calculated from the ratio of each ganglioside to the total peak area as given in Equations 7-9.
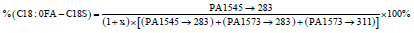 (7)
(7)
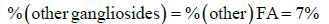 (8)
(8)
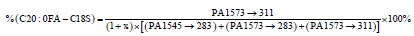 (9)
(9)
where the parameters are previously defined, with PA being the peak area of the indicated MRM peaks. For the GM1 standard used in the present work the factor x in the denominator was 0.075, the same as for Method 1. Equations 7 -9 do not account for any presence of C-18 and C-20 sphinganines, which will lead to small accuracy errors as discussed in section 3.5.
Comparison of Method 1 with Method 2 results
Duplicate injections of GM1 standard at concentrations 1000, 2000, 5000 and 10000ng/ml on the LC-MS/MS system were done monitoring the MRM fatty acid daughter ion (Method 2) to assess the accuracy of Method 1. Note, only higher concentrations could be determined for this MRM daughter ion monitoring, a less sensitive transition. The peak area data were processed according to Method 2 utilizing Equations 7-9, with the results given in Table 1. Comparing the results for Method 1 and Method 2 in Table 1 for the predominant GM1 gangliosides shows the absolute % values do not differ by more than 2% for any of the three predominant molecular GM1 gangliosides. Statistical assessment shows no significant difference for the percent values for the C18:0FA-C18S GM1 ganglioside in comparing the two methods. However the percent values for the C18:0FA-C20S GM1ganglioside is statistically lower for Method 1 (m/z 290 daughter ion MRM technique) than Method 2 (fatty acid daughter ion MRM technique), although differing by only 2% absolute percent. Example calculations for determining the percentages of the three major ganglioside components in the GM1 standard according to Method 2 are given in the Supplementary Information section.
A plot of the ratio of the Method 2 determined values for C18:0FAC18S to C18:0FA-C20S versus standard GM1 concentration is also shown in Figure 4. The regression line slope is close to zero (Figure 4) and thus no significant concentration trend is noted, as was seen for Method 1. The average of ratios of C18:0FA-C18S and C18:0FA-C20S at different concentrations by Method 1 and Method 2 were 1.163 (SD ± 0.035) and 1.125 (SD ± 0.018) respectively. These ratio means are not statistically different (p value=0.07).
Effect of sphinganine presence in GM1 standard
Since the GM1 standard most likely contains a small amount of sphinganine (up to 5% of the gangliosides found in brain samples [4-6]) calculations were done assuming 5% sphinganine content (see Supplementary Information for example calculations) and compared with the previous calculated results to determine the magnitude of the error resulting from the unmeasured presence of the sphinganine. The sphinganine gangliosides can be considered to having exclusively C18 fatty acid (being 90% of the fatty acids present) since this is the only component that can affect the sphingosine ganglioside results. With 5% sphinganine content the factor x in Equations 3, 7-9 increases from 0.075 to 0.136, the % (other) FA in Equations 1 and 6 increases from 7% to 12% and the %C18FA bonded to the sphingosine ganglioside in Equation 4 is decreased from 90% to 85%. The results assuming 5% sphinganine content are given in Table 1. There is a relative error of approximately +5-6% in the sphingosine ganglioside if an assumed 5% sphinganine content in the standard is not taken into account.
It should be noted that the sphingosine gangliosides (m/z 1545 and 1573) can be differentiated from sphinganine gangliosides (m/z 1547 and m/z 1575) because the selection of the parent ion is specific (m/z ± 0.5). However, the sphinganine gangliosides cannot be determined in this mass spectrometric experiment because the +2 isotope of the sphingosine gangliosides of 1547 and 1575 ions masks the monoisotopic sphinganine gangliosides. Thus the results need to be corrected with assumed sphinganine content or viewed as having up to 6% relative error.
Conclusion
A simple procedure for quantitatively determining the ganglioside composition of a commercially- available heterogeneous GM1 standard (extracted from a natural source, bovine) has been reported for the first time and the results have been confirmed by a comparative method. The significance of the present study is that the analysis utilizes intact ganglioside molecules data using a sensitive MRM measuring the dehydrated sialic acid fragment to determine individual molecular weight components of gangliosides, combining this with fatty acid content data. Peak area data as low as 50 ng/mL was established, which is relevant for measuring GM1 gangliosides at physiological concentrations [17]. The present work was done to confirm the validity of the method employing standard GM1 solutions. Future studies will involve assessing the method for determining these individual molecular GM1 ganglioside components in physiological samples.
Acknowledgements
This project was conducted with support from Faculty Research Development Program and the Dissertation Research Award program at Cleveland State University.
References
- De Bièvre P, Dybkaer R, Fajgelj A, Hibbert DB (2011) Metrological traceability of measurement results in chemistry. Concepts and implementation (IUPAC Technical Report). Pure Appl Chem 83:1873-1935.
- Cuadros-Rodríquez L, Bagur-González MG, Sánchez-Viñas M, González-Casado A, Gómez-Sáez AM (2007) Principles of analytical calibration/quantification for the separation sciences. J Chromatogr A 1158: 33-46.
- Flangea C, Serb A, Sisu E, Zamfir AD (2011) Chip-based nanoelectrospray mass spectrometry of brain gangliosides. Biochim Biophys Acta 1811: 513-535.
- Palestini P, Masserini M, Sonnino S, Giuliani A, Tettamanti G (1990) Changes in the ceramide composition of rat forebrain gangliosides with age. J Neurochem 54: 230-235.
- Valsecchi M, Palestini P, Chigorno V, Sonnino S (1996) Age-related changes of the ganglioside long-chain base composition in rat cerebellum. Neurochem Int 28: 183-187.
- Cantù L, Corti M, Sonnino S, Tettamanti G (1986) Light scattering measurements on gangliosides: dependence of micellar properties on molecular structure and temperature. Chem Phys Lipids 41: 315-328.
- Yu RK, Tsai YT, Ariga T, Yanagisawa M (2011) Structures, biosynthesis, and functions of gangliosides--an overview. J Oleo Sci 60: 537-544.
- Rosenberg A, Stern N (1966) Changes in sphingosine and fatty acid components of the gangliosides in developing rat and human brain. J Lipid Res 7: 122-131.
- Sonnino S, Chigorno V (2000) Ganglioside molecular species containing C18- and C20-sphingosine in mammalian nervous tissues and neuronal cell cultures. Biochim Biophys Acta 1469: 63-77.
- Yohe HC, Roark DE, Rosenberg A (1976) C20-sphingosine as a determining factor in aggregation of gangliosides. J Biol Chem 251: 7083-7087.
- Masserini M, Palestini P, Pitto M, Chigorno V, Tomasi M, et al. (1990) Cyclic AMP accumulation in HeLa cells induced by cholera toxin. Involvement of the ceramide moiety of GM1 ganglioside. Biochem J 271: 107-111.
- Valsecchi M, Chigorno V, Sonnino S, Tettamanti G (1992) Rat cerebellar granule cells in culture associate and metabolize differently exogenous GM1 ganglioside molecular species containing a C18 or C20 long chain base. Chem Phys Lipids 60: 247-252.
- Bode L, Beermann C, Mank M, Kohn G, Boehm G (2004) human and bovine milk gangliosides differ in their fatty acid composition. J Nutr 134: 3016-3020.
- Hara A, Taketomi T (1975) Long chain base and fatty acid compositions of equine kidney sphingolipids. J Biochem 78: 527-536.
- Serb A, Schiopu C, Flangea C, Sisu E, Zamfir AD (2009) Top-down glycolipidomics: fragmentation analysis of ganglioside oligosaccharide core and ceramide moiety by chip-nanoelectrospray collision-induced dissociation MS2-MS6. J Mass Spectrom 44: 1434-1442.
- Sørensen LK (2006) A liquid chromatography/tandem mass spectrometric approach for the determination of gangliosides GD3 and GM3 in bovine milk and infant formulae. Rapid Commun Mass Spectrom 20: 3625-3633.
- Garcia AD, Chavez JL, Mechref Y (2014) Rapid and sensitive LC-ESI-MS of gangliosides. J Chromatogr B Analyt Technol Biomed Life Sci 947-948: 1-7.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12351
- [From(publication date):
specialissue-2015 - Aug 23, 2025] - Breakdown by view type
- HTML page views : 11284
- PDF downloads : 1067