Impact of gonadal development on the proximate composition of muscle, liver and ovary of adult female rainbow trout (Oncorhynchus mykiss)
Received: 02-Jun-2022 / Manuscript No. jflp-22-63516 / Editor assigned: 06-Jun-2022 / PreQC No. jflp-22-63516 (PQ) / Reviewed: 17-Jun-2022 / QC No. jflp-22-63516 / Revised: 20-Jun-2022 / Manuscript No. jflp-22-63516 (R) / Published Date: 28-Jun-2022 DOI: 10.4172/ 2332-2608.1000350
Abstract
The present investigation was carried out to study the reproductive biology of the Oncoryhncus mykiss at Laribal hatchery, Dachigam National park, Srinagar, Jammu and Kashmir from February 2018 to March 2019. The study was carried out to identify and illustrate developmental stages of oocytes of female rainbow trout and correlate them with seasonal maturity, organo-somatic indices and with change in biochemical composition. During the present study ovary samples were taken and processed for routine histological analysis. Morphological and anatomical changes in ovaries were recorded. Different oocytes were observed in ovaries at different developmental stages. The entire reproductive phase was divided into four major divisions: - Immature phase, developing phase, spawning phase and post- spawning phase. The six different types of oocytes that were identified in this study include chromatinnucleolus oocyte, peri-nucleolus oocyte, cortical alveolus oocyte, yolk vesicle oocyte, yolk granular oocyte, and mature oocyte. The maximum level of gonadosomatic index (12.33 ± 1.04%) and reduced value of hepatosomatic index (0.82 ± 0.08%) was seen in January which signifies that December to February is the peak spawning period of rainbow trout. It was also observed that June to August is growth phase, September to November is maturation phase, and March to May is the post-spawning phase of rainbow trout. Ninety eight captive bred female rainbow trout tissues were investigated for their biochemical composition to establish their need of formulating nutrient-rich broodstock diets. The biochemical investigation of muscle, liver and ovary of captive bred female rainbow trout from Laribal hatchery, Dachigam, J&K showed that the maximum amount of moisture content (75.30%) was found both in ovaries of immature females and in muscles of spawning female (75.20%) while as significantly lowest amount of moisture was found in liver of spawning females (71.10%). The crude protein content among four phases in female rainbow trout was significantly (p < 0.05) higher (23.00%) in muscles of immature females while lowest (13.50%) in liver of spawning females. The post-hoc test revealed that the crude protein content in liver and ovary of developing females (14.00%; 14.30%) varied insignificantly. Similarly, the highest amount of lipid was recorded in liver of both developing and spawning females (9.90%; 9.12%) which was significantly (p < 0.05) higher compared to all other phases. Significantly lowest amount of lipid content (1.79%) was noticed in muscles of developing females with intermediate amount of lipid content (3.60%) in muscles of immature females. The inverse relationship between moisture and lipid was also found. It was observed that during different gonadal stages of female rainbow trout, the ovaries get its protein and lipid from other tissues mostly from liver. Therefore, it is necessary to incorporate highly assimilated feed with high protein and lipid content. The muscle of immature females thus belongs to high protein and low-fat category and can be subjugated commercially for human consumption. Hence, it was concluded that the identification and classification of various oocytes, estimation of organo-somatic indices as well as fluctuations in biochemical composition of different tissues are enormously significant in understanding the gonadal changes during different seasons of a year and thus present study is of utmost significance for preservation and management of the species studied.
Keywords: Biochemical; Protein; Lipid; Muscle; Liver; Trout; Gonads
Keywords
Biochemical; Protein; Lipid; Muscle; Liver; Trout; Gonads
Introduction
The Union territory of J&K is rich in natural water supply which produce fish and other economically important aquatic plants. Jammu and Kashmir, often known as “Paradise on Earth,” is a famous fishing location. The physio-chemical properties of J&K waters, particularly those in the Kashmir Valley, are beneficial to trout. Salmo trutta fario (Brown trout) and Oncorhynchus mykiss (rainbow trout) are two main species of trout found in J&K. Outside union territory of J&K, there seems to be a considerable market for trout fish, but output is incapable of meeting demand. This can be improved by using better hatchery management. On a theoretical level, there is a lot of potential for growing trout culture. These include the preservation of healthy brood-stock, illness control, and the development of a nutritious synthetic feed for intensive trout farming.
Breeding biology
The breeding behavior of rainbow trout has been extensively studied, although many details remain unknown. In recent years, the notion of brood-stock diets for fish has received a lot of attention. Modifications in brood- stock diet and feed have been found to significantly increase not only egg cell efficiency but also high yield. The research on broodstock feed, on the other hand, is limited and rather expensive. In light of the foregoing ideas, the primary goal of this study was to investigate the biochemical composition of various tissues of grown-up females during different gonadal stages. Because of intensive fishing and exploitation of their habitat, Kashmir’s trout species are in grave danger of extinction in a wide range of water bodies. But despite rainbow trout, being an economically significant species of Kashmir valley, there is very little information available regarding its reproductive phases with respect to histology and biochemical composition of its different tissues. As a result, it is worthwhile to investigate the functional and histological aspects of the ovary in greater depth so that useful statistics can be compiled for the proper management of this species.
Biochemical composition
Investigation of the biochemical composition of the liver, muscle, and ovary of female rainbow trout in relation to their growth and developmental phases was another goal of this study. A biochemical profile is especially important for nutritionists and researchers who are interested in producing protein-rich diets with maximum nutritional value, best flavour, quality, colour, and aroma [1]. Although there is lot of information available on the biochemical composition of many fish species, but there is a scarcity of data on the proximate composition of various tissues with respect to different gonadal stages of O. mykiss from the Kashmir valley. Biochemical composition includes estimation of moisture, protein, lipid and ash contents in different tissues of fish which are regarded to be key components of fish flesh [2], whereas the quantity of carbohydrate and non-protein components is small and is typically overlooked for regular study [3]. Biochemical compositions are a good indicator of a fish’s physiological condition. Based on the energy unit, biochemical components of the species aid in determining its nutritional and edible significance. Henceforth, accurate information about biochemical components of fish is vital for those individuals who are involved in the manufacturing of animal feed, fish feed or who are related to human nutrition, pharmaceutical manufacturers, and consumers. Keeping these facts in mind, the current study has been framed in such a way so as to collect data on nutritional profile, biological variables, and difference in biochemical composition in muscle, liver, and ovary in relation to changes in a reproductive factors in captive bred female Oncorhynchus mykiss, in order to establish it as a potentially culturable species and to implement fisheries management measures.
Material and Methods
Sample preparation
The adult female specimens of rainbow trout species were collected from the Laribal trout hatchery in Dachigam National Park, Jammu and Kashmir, based on its availability and dispersion patterns. The specimens provided by the farmers of the hatchery were all dead, and as such, no slaughtering technique was used by the researcher. Instead, the farmers slaughtered them with a manual percussive stunning method in which a hard blow was given to the fish’s head by a wooden club so that immediate loss of consciousness could be achieved. This method of slaughtering is considered a humane method for rainbow trout by the World Organization for Animal Health (OIE). From the same hatchery, dead female rainbow trout with a length of 24.0-40.0 cm and a weight of approx. 200–950g were recovered. In the second week of each month from February 2018 to March 2019, eight female rainbow trout specimens were collected. The lengths and weights of each individual fish were recorded. The samples were placed in an ice-filled box and delivered to the Cytogenetics and Molecular Biology Research Laboratory, Centre of Research and Development, University of Kashmir, India, within a few hours of collection. Various organs such as the liver, muscle, and ovaries were taken and exposed to various processes after each specimen was dissected and deguted. Each extracted sample was stored in zip-lock bags at -20°C until further analysis.
Estimation of GSI and HSI
The percentage of gonads and liver weight to total body weight was determined as the Gonado-somatic Index (GSI) and Hepato-somatic Index (HSI), respectively. The Gonado-somatic Index calculates gonadal weight as a percentage of overall body weight. It is a method for determining animal sexual maturity in relation to ovarian development [4]. The hepato-somatic index (HSI) is defined as the weight of the liver divided by the total weight of the body. It depicts the state of the animal’s energy reserve and its health condition [5].
Histological analysis Histological treatments are usually performed on dead cells or tissues. Each month, female fish ovaries were cut into small pieces of around 4-5 mm and subjected to different processes such as fixation, dehydration, embedding, sectioning, and staining in order to conduct histological examinations [6].
Proximate analysis
Muscle, liver, and ovary were excised, weighed, and put into plastic vials and frozen at -20°C. The moisture content was measured using the [7] approach of a known sample weight. To measure moisture content, all of the samples (muscle, liver, and ovary) were dried in an oven at 110˚ C for 3 hours. The moisture content of samples was determined by computing the percent moisture for each sample after measuring its mass before and after evaporation. The method for estimating crude protein content was based on a slightly modified version known as the micro-Kjeldahl’s method, given by [8]. The sample’s nitrogen content was quantified using the Kjeltec semiautomated technique, which used titration to determine nitrogen percentage, and then crude protein, was calculated by multiplying nitrogen percentage by a constant factor of 6.25 [9]. The nitrogen content was converted to crude protein content using Kjeldahl’s technique [10]. All of the tests were done in triplicate (n = 3), and the findings were represented in percentage. The fine powdered, moisture-free samples were placed in clean, pre-weighed silica crucibles and weighed again along with the other samples. The samples were then put in crucibles and heated in a muffle furnace at 650˚C for 4-6 hours, or until the residue was entirely white. After cooling in desiccators for 20–30 minutes, the samples were reweighed, and the quantity of ash was determined as the change in weight. The residue left after full ashing is the sample’s ash content. A colorimetric method was used to estimate lipid levels in tissues (muscle, liver, and ovary). To 1g of tissue sample, 9 ml of diethyl alcohol was added, and the mixture was homogenised in a homogenizer for 5 minutes before centrifugation at 7000 rpm for 10 minutes. The extract was then evaporated, and 1 ml of ethanol was added to the residue, which was then used for further analysis.
Statistical analysis
Individual sample data for various nutrients were analysed using One-way analysis of variance (ANOVA; SPSS, 16.0) versus distinct species [11-12], followed by a pair-wise comparison of means using Post Hoc Tests. In addition, the statistical programme SPSS (version 16.0) was utilised to conduct the analysis. If p < 0.05, differences were deemed statistically significant at a 5% level of significance, and if p < 0.01, statistical differences were judged significant at a 1% level of significance. The data was given in the form of a mean and standard deviation of the mean (SDM). For each determination, three samples were utilized.
Declaration of ethical principles
The use of experimental fish was done as per the animal welfare laws, guidelines, and policies approved by the “University of Kashmir” and the Government of Jammu and Kashmir Department of Fisheries” under endorsement number RWLW/Tech/17-18/1396 dated 23-10- 2017.
Results
Histological investigation of oocytes
The oocytes in this study were categorised into six developmental phases based on histological inspection, changes in oocyte parameters, and gonadosomatic index. The terminology was adopted from Yamazaki (1965), which includes chromatin-nucleolus oocyte (stage I), peri-nucleolus oocyte (stage II), cortical alveolus oocyte (stage III), yolk vesicle oocyte (stage IV), yolk granular oocyte (stage V), and mature oocyte (stage VI). Atretic oocytes were also identified in mature ovaries, but they were destroyed and reabsorbed follicles, so they were not allocated to any developmental stage.
During the various stages of reproduction, several factors, such as the gonadosomatic index, hepatosomatic index, and morphohistological features of oogenetic cells, has changed (Table 1).
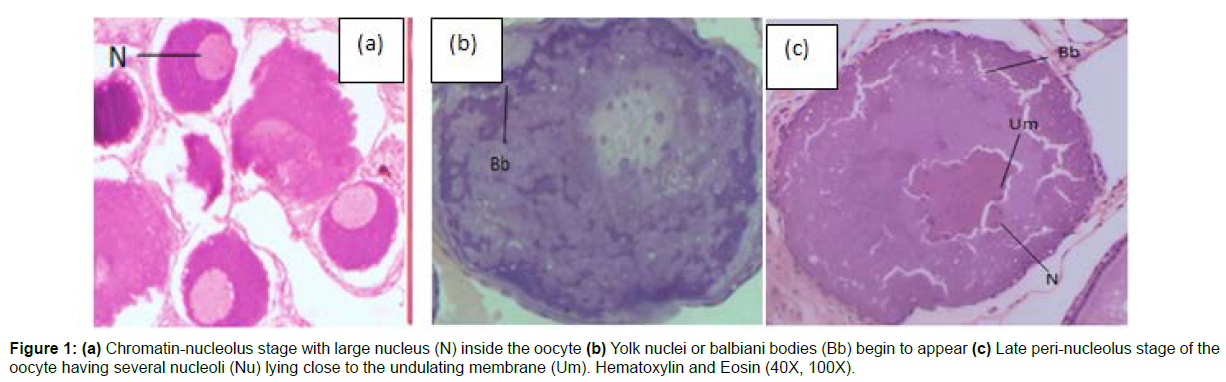
Immature Phase (June to August): During this phase, the gonads were visible as fragile, translucent thread-like filaments partially filling the abdomen, and the nucleus occupied a substantial portion of the oocyte. On histological inspection, oogonial nests and immature oocytes were found in ovigerous lamellae. The ovary contained primary oocytes, which included chromatin-nucleolus oocytes (stage I) and peri-nucleolus oocytes (stage II). The chromatin-nucleolus oocytes (Figure 1a) were characterised by a cell with very thin cytoplasm that encircled a massive basophilic nucleus, while the peri-nucleolus oocyte (Figure 1b) was characterised by the presence of yolk nuclei, also known as balbiani bodies, which originally emerged near the vicinity of the nucleus and then migrated towards the periphery of the oocyte (Figure 1). The percentage of late peri-nucleolus oocytes (stage III) with numerous nucleoli near the undulating membrane was higher at the end of this phase (Figure 1c). In this phase, the average gonadosomatic index increased to 3.22 ± 0.40% and the calculated value of the hepatosomatic index was 2.49 ± 0.20%.
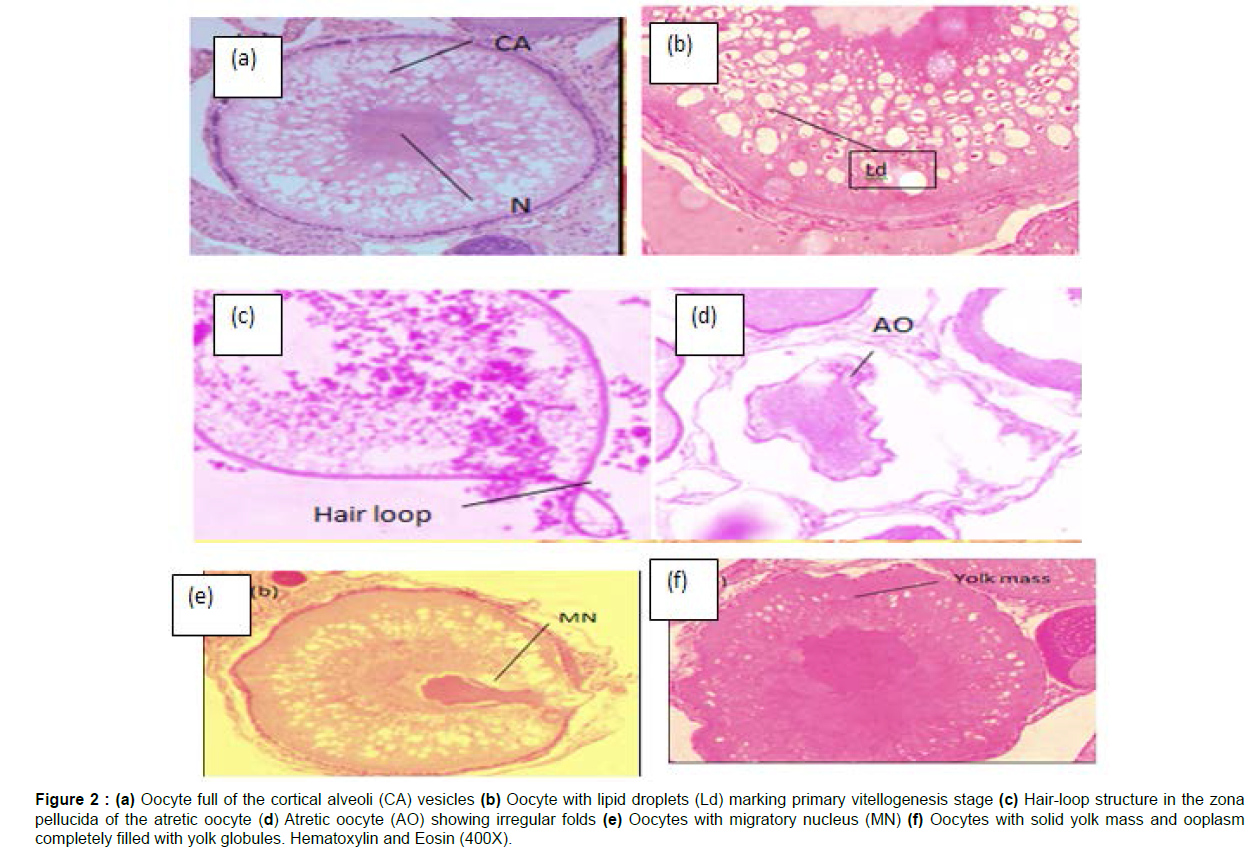
Developing Phase (September to November): The gonads were morphologically longer and amber in colour, with visible oocytes. Due to the existence of a large number of oocytes, this phase was marked by the occurrence of maximal oogenetic activity. The majority of the oocytes were in the cortical alveoli (stage IV) and yolk granular phases of development (stages V). The presence of empty vesicles filled with glycoprotein substances distinguished the cortical alveoli stage (Figure 2a) of oocytes. These vesicles were known as cortical alveoli because of their initial position in the cortical area of the cytoplasm. However, the yolk granular oocyte (Figure 2b) was characterised by the presence of yolk-filled vesicles that engulf the whole ooplasm around the nucleus.
Figure 2 : (a) Oocyte full of the cortical alveoli (CA) vesicles (b) Oocyte with lipid droplets (Ld) marking primary vitellogenesis stage (c) Hair-loop structure in the zona pellucida of the atretic oocyte (d) Atretic oocyte (AO) showing irregular folds (e) Oocytes with migratory nucleus (MN) (f) Oocytes with solid yolk mass and ooplasm completely filled with yolk globules. Hematoxylin and Eosin (400X).
A small number of immature oocytes, as well as some atretic follicles, were discovered (Figures 2c and 2d). The gonadosomatic index steadily grew from 3.22 ± 0.40 % to 8.13 ± 0.61 % throughout the development period, whereas the hepatosomatic index peaked at 1.76 ± 0.25 %.
Spawning phase (December to February): The gonads had totally expanded and filled the abdominal cavity at this point. The ovaries were filled with mature follicles (Stage V oocytes). Mature oocytes were the biggest oocytes seen in rainbow trout ovaries (Figures 2e and 2f). Condensed yolk globules with eccentric germinal vesicles were found in mature follicles, which were bigger, asymmetrical, and included condensed yolk globules with asymmetric germinal vesicles. The ovaries were full of developed follicles throughout the spawning phase, and the gonadosomatic index reached a high of 12.33 ± 1.04%. In contrast, the hepatosomatic index fell by 0.82 0.08%.
Post-spawning phase (March to May: The gonads were loose and flabby in appearance morphologically. The existence of a minimal number of mature oocytes marked this phase. Primary oocytes and early peri-nucleolar oocytes, as well as a few late peri-nucleolar oocytes, began to emerge. The yolk-filled follicles were reabsorbed at this phase. The gonadosomatic index was 2.12 ± 0.13%, while the hepatosomatic index was 1.74 ± 0.10%.
Proximate analysis of the muscle tissue
Table 1 shows the biochemical makeup of the muscle, including crude protein, moisture, ash and lipid content. Females were classified histologically into four groups: immature, developing, spawning, and post-spawning. Out of 98 samples collected, 34, 27, 18, and 19 females were discovered to be immature, developing, spawning, and post-spawning females, respectively. The findings of this study clearly demonstrate that there is a significant difference in the chief proximate components among the different developmental stages in the various tissues investigated. The muscle, liver, and ovary analysis and change in their biochemical composition of female rainbow trout revealed that the variation in biochemical composition was statistically significant (p < 0.05).
Proximate composition of the muscle in the immature phase:
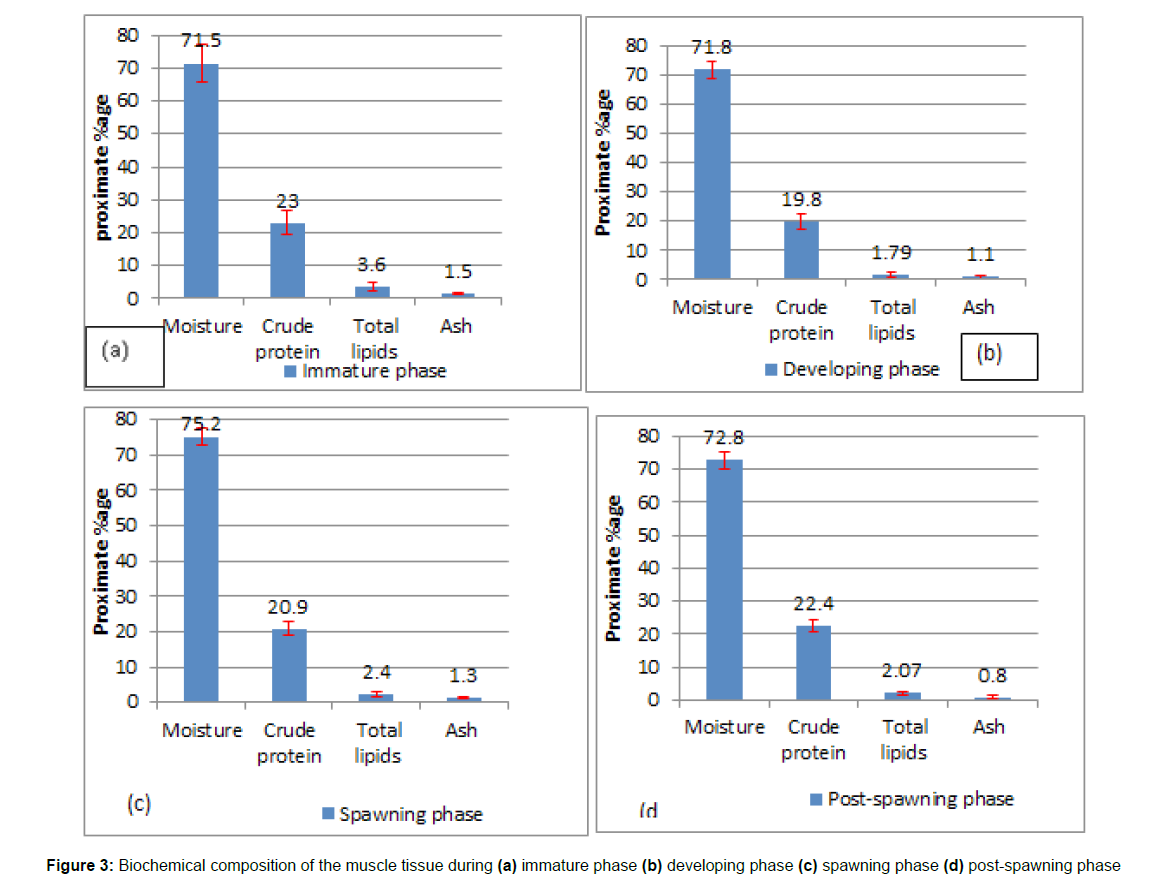
The current study analysed the biochemical composition of muscle in female rainbow trout at various gonadal phases. 98 specimens were collected, and the muscles were removed from various body parts and pooled for proximate examination (Figure 3a). The moisture content of immature females ranged from 62.5 to 89.5 %, with an average mean value of 71.5 ± 5.95 %. On a statistical note, the ANOVA test revealed that the comparison of moisture content in muscles of immature fish with respect to the other three gonadal phases was found to be statistically significant (p < 0.05). The multiple comparison test (Posthoc test) among various pairs of reproductive phases revealed that the difference between mean values of moisture content in the immature (71.5%) and developing phase (71.80%) was statistically insignificant (p = 0.7), while the comparison between immature and post-spawning (72.8%) was also observed to be statistically insignificant (p = 0.2). The pair-wise difference in the moisture content of immature muscle with respect to the spawning phase (75.20%) was found to be statistically significant (p < 0.05). The crude protein level was in the range of 14.3– 30.5%, with a mean value of 23.0 ± 3.70%. Among all the reproductive phases, the highest protein content was found in the muscles of immature fishes. On a statistical note, the ANOVA test revealed the difference in mean values between various phases differs significantly, whereas the multiple comparison test between various pairs revealed that protein content in the developing phase and spawning phase differs significantly (p < 0.01) from the immature phase but differs non-significantly from the post-spawning phase (p = 0.4). The total lipid content falls in the range of 1.30–6.30% with an average value of 3.6 ±1.32%. Statistically, the lipid content in the muscles of immature fishes differs considerably (p < 0.01) from the other three phases.
Proximate composition of the muscle in the developing phase:
The moisture content of the 27 females tested in the developing phase was found to be quantitatively similar (71.80 ± 3.12%) to that of the immature one (71.5 ± 5.95%). The moisture content was determined to be between 65.30 and 78.40%. The ANOVA test indicated that the moisture content in developing females was significant (p < 0.01). The pair-wise comparison test between moisture content in developing and spawning females was found to be statistically significant (p < 0.01), but the post-hoc test between moisture content values in developing and post-spawning females was found to be statistically insignificant (p = 0.4). The crude protein content ranged from 14.30 to 24.9%, with an average value of 19.8 ± 2.55%. The findings revealed that the mean crude protein content in developing females was significantly lower than in the other three reproductive stages. The crude protein content in growing female muscles was found to be statistically non-significant (p = 0.2) in relation to the spawning phase. The variance in crude lipid in developing females was 0.30–3.9 %, with an average mean value of 1.79 ± 0.9 % and the levels were found to be substantially lower than in other stages (Figure 3b).
Proximate composition of the muscle in the spawning phase: After histological examination, only 18 of the 98 females chosen were found to be ripe and allocated to the spawning phase. The moisture content was found to be in the 70.3–79.90% range, with a mean value of 75.2 ± 2.48% (Figure 3c). The moisture content in the muscles of ripe females reached a high (75.2 ± 2.48 %) and was statistically significant (p < 0.05) compared to the values obtained in the other three phases. An intermediate average value of crude protein (20.9 ± 2.0%) was found in females of the spawning phase. The post hoc test revealed that protein content in spawning and developing females differed non-significantly (p = 0.2) from each other but significantly (p < 0.01) from immature fishes. The crude lipid content of all females in their spawning phase was estimated to fall within the range of 1.00% to 4.2 %, with a mean average value of 2.4 ± 0.89 %. A statistically significant (p < 0.05) intermediate lipid value was obtained in females during their spawning period, followed by values of lipid content in the muscles of postspawning fish. Moisture and lipid levels were shown to be inversely proportional in fish muscles; muscles with substantially (p < 0.05) high moisture content had low lipid values. The post-hoc test indicated that the lipid content in spawning and post-spawning females differed statistically but not significantly (p = 0.3).
Proximate composition of the muscle in the post- spawning phase: The molecular makeup of muscles in 19 females classified as post-spawning is depicted visually (Fig.3d). Females that had already spawned exhibited an intermediate degree of moisture content (68.3 ± 77.3%). The crude protein content in the muscles of spent females was higher (22.4 ± 1.73%) than that of spawning females (20.9 ± 2.0%). In terms of statistics, the crude protein content in post-spawning changes non-significantly (p > 0.05) from the spawning and immature phases. The quantity of lipid detected in post-spawning females ranged from 1.0 to 3.23 percent, with an average value of 2.07 ± 0.65%. The lipid levels in the post-spawning phase differ substantially (p < 0.01) from those in the immature phase but not significantly (p > 0.05) from those in the developing and spawning phases.
Proximate analysis of the liver tissue
Table 1 shows the biochemical makeup of the liver. The findings of this study clearly demonstrate that there is a significant difference in the key components among the different developmental stages in the different tissues investigated. The differences in biochemical composition of the liver at various stages of gonad development of rainbow trout were statistically significant (p < 0.05)
| PHASE | MONTH | SAMPLE SIZE (n) |
WEIGHT (g)±S.D | LENGTH (cm)±S.D |
OVARY WEIGHT (g)±S.D |
GSI± S.D(%) |
LIVER WEIGHT (g)±S.D | HSI±S.D (%) |
|---|---|---|---|---|---|---|---|---|
| IMMATURE PHASE | JUN-AUG | 34 | 337.37a±12.67 | 28.69a±0.48 | 10.32a±1.17 | 3.22a±0.40 | 8.31a±0.70 | 2.49a±0.20 |
| DEVELOPING PHASE | SEP-NOV | 27 | 363.51a±10.39 | 31.06b±0.38 | 30.09b±2.78 | 8.13b±0.61 | 6.30b±0.88 | 1.76b±0.25 |
| SPAWNING PHASE | DEC-FEB | 18 | 547.92b±26.40 | 35.15c±0.84 | 68.25c±6.60 | 12.33c±1.04 | 4.56cb±0.58 | 0.82c±0.08 |
| POST- SPAWNING | MAR-MAY | 19 | 468.15c±40.55 | 33.20d±0.68 | 9.53a±0.72 | 2.12a±0.133 | 7.90ab±0.81 | 1.74b±0.10 |
| The values presented are the mean and standard deviation of three replicates. The superscript a, b, c, d on mean values of different phases signifies that the mean values differ significantly (p < 0.05) while as same alphabetical superscript signifies that the mean values of two phases are non-significant (p >0.05) to each other. | ||||||||
Table 1: Comparison of liver and gonadal parameters of female rainbow trout during the reproductive phase.
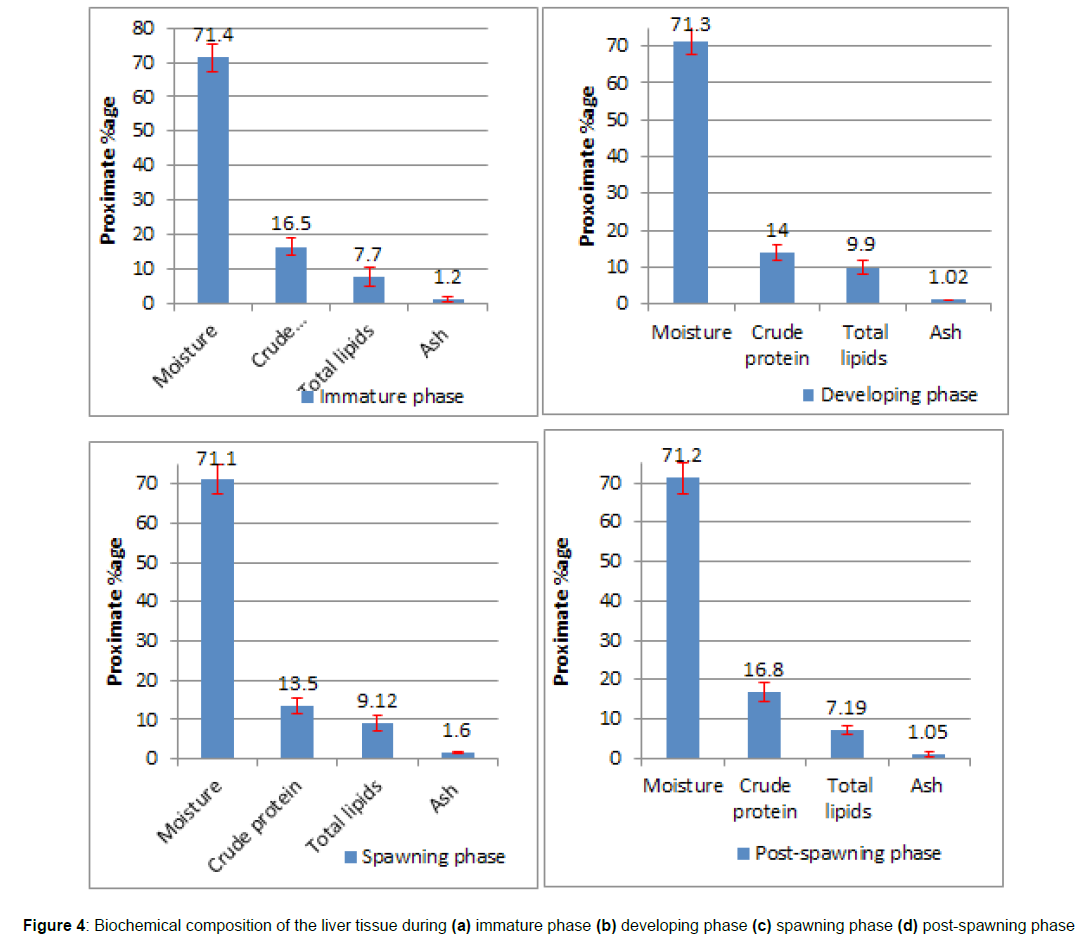
Biochemical composition of the liver in the immature phase: The present study looked at the biochemical components of the liver with respect to its maturity stages (Figure 4a). Thirty-four immature females were studied, and their livers were removed for biochemical testing. The crude protein content of rainbow trout females ranged from 10.8 to 21.6%, with an average value of 16.5 ± 2.67%. In terms of statistics, the ANOVA test indicated a significant difference (p < 0.05) in protein content across different stages of reproductive development. The posthoc test indicated that the protein content value in juvenile fishes changed substantially (p < 0.05) during the developing and spawning phases but not during the post-spawning phase (p = 0.6). The moisture content ranged from 62.5 to 79.5 percent, with a mean of 71.4 ± 3.93%. Statistically, the moisture content levels in immature fishes do not vary that much from the other three stages (p > 0.05). The lipid content ranged from 2.1 to 12.6%, with a mean of 7.7 ± 2.55%. Statistically, the post-hoc comparison test of lipid content between immature and post-spawning stages indicated that the results were non-significant (p > 0.05) to each other.
Proximate composition of the liver in the developing phase: Based on proximate analysis, the crude protein content of the liver in 27 females allocated to this phase was determined to be in the range of 10.6–18.6%. On a statistical note (p < 0.05), the mean protein content of the developing phase liver was considerably lower than that of the immature phase, although it varied significantly (p < 0.05) with regard to the post-spawning phase but not significantly (p = 0.4) with respect to the spawning phase (Figure 4b). The moisture content ranged from 64.3 to 79.3%. On a statistical note (p < 0.05), the post-hoc multiple comparison test between the developing phase in relation to the other three phases indicated that the moisture content differed insignificantly (p > 0.05) from each other. The crude lipid value varied between 6.50 and 14.50%. Statistically (p > 0.05), the difference in mean lipid values varied insignificantly (p = 0.2) with the spawning phase but significantly (p < 0.01) with the other two phases. The ash content ranged from 0.36 to 1.60% and was determined to be statistically insignificant (p > 0.05) in comparison to the immature and post-spawning mean values.
Proximate composition of the liver in the spawning phase: There were 18 fish samples allocated to this stage. Figure 4c depicts the fluctuation in the mean values of the biochemical composition of the liver at this stage. The moisture content ranged from 62.80 to 77.30%. Statistically, the change in mean moisture content values throughout the spawning phase is negligible (p > 0.05). The crude protein concentration varied from 8.00 to 16.30%. The pair-wise comparison by post-hoc test indicated that the mean values of crude protein in the spawning phase varied non-significantly (p = 0.4) from the developing phase but varied significantly (p < 0.01) in relation to the other two phases. However, crude protein levels in the liver were at their lowest during this gonadal phase. The lipid content ranged between 6.10 and 13.50 %, whereas the ash level was between 1.30 and 2.00 %. The liver of the spawning phase had an intermediate level of lipid content, and the mean values differed substantially (p < 0.05) from the immature and post-spawning phases but insignificantly (p = 0.2) from the developing phase.
Proximate composition of the liver in the post-spawning phase: The mean proximate values of liver with crude protein concentrations ranging from 12.60 to 20.81% were depicted in (Figure 4d).The ANOVA test indicated that the comparison of protein content in the liver of post-spawning fishes with regard to developing and spawning phases was statistically significant (p < 0.05), but the relationship with respect to the immature phase was non-significant (p = 0.6). The moisture content varied from 64.80 to 78.20%. Multiple comparison tests (Posthoc tests) among different pairings of gonadal stages indicate that the difference in mean moisture values in the liver was determined to be statistically non-significant (p > 0.05). The crude lipid ranged from 5.20 to 9.40% and the ash content ranged from 0.30 to 1.90%. The level of significance for the difference in lipid content of the post-spawning phase with respect to the liver of the immature phase was found to be non-significant (p = 0.4). Conversely, the post-hoc test confirmed that the lipid content in the developing and spawning phases was significant (p < 0.05).
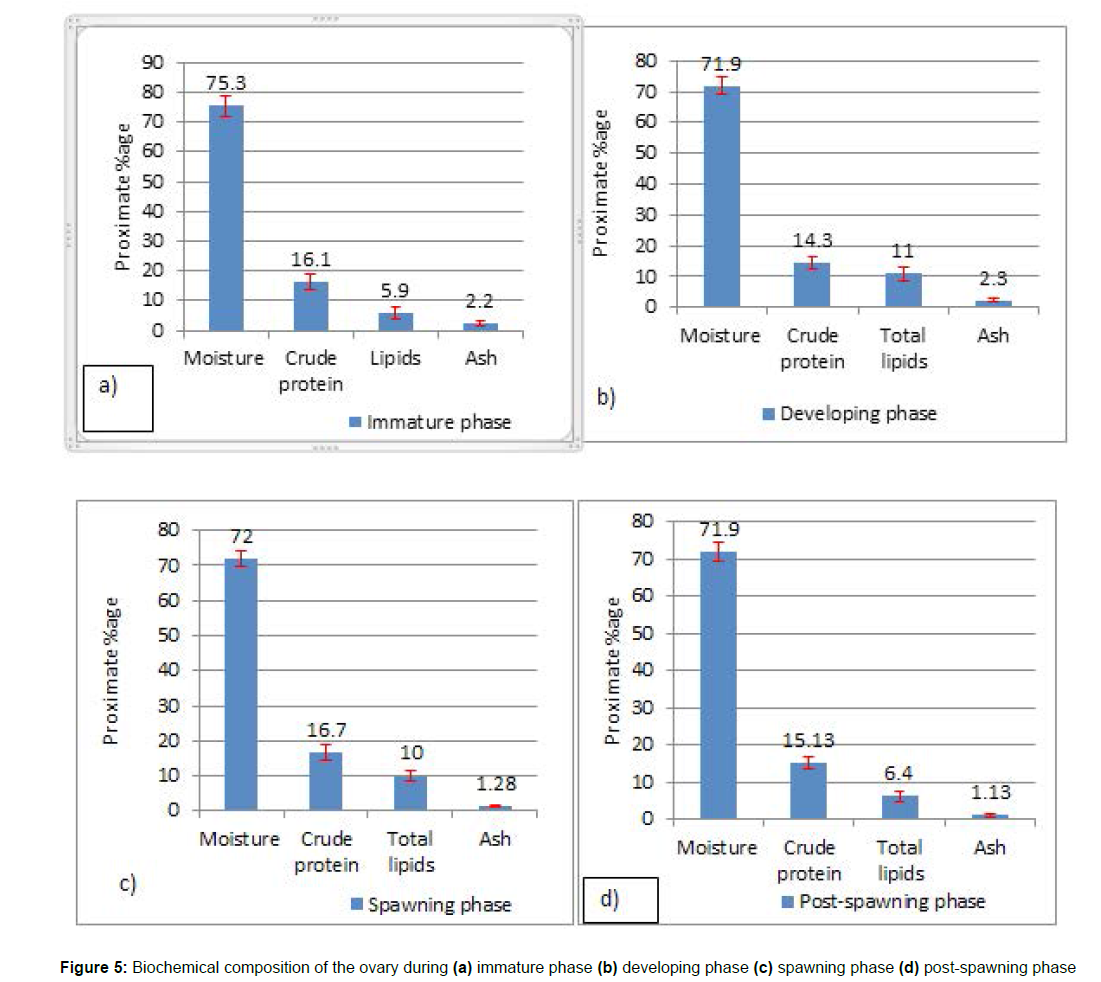
Proximate analysis of the ovary
Table 1 shows the biochemical composition of the ovary. The findings of this study clearly demonstrate that the primary biochemical components of the ovary vary greatly between maturity stages. The ovarian study of variations in biochemical composition of rainbow trout from the Laribal Srinagar hatchery in Kashmir revealed that the difference in biochemical composition was statistically significant (p < 0.05).
Proximate composition of the ovary in the immature phase: Figure 5a depicted the mean proximate composition values of moisture content ranging from 68.90% to 79.90%. The ANOVA test revealed that comparing moisture content in immature fishes to the other three gonadal phases was statistically significant (p < 0.05). The crude protein concentration varied from 8.60% to 21.60% (Figure 5a). Multiple comparison tests (Post-hoc test) among various pairs of gonadal stages reveal that the difference between mean values of crude protein in the immature and developing phases is statistically significant (p < 0.01) while the difference between the immature and spawning phases is statistically non-significant (p = 0.2). The crude lipid concentration varied from 2.60 to 9.50%, whereas the ash level ranged from 0.94 to 3.89%. The level of significance for the difference in lipid content between immature and subsequent gonadal stages was found to be significant (p < 0.01) except for the pair-wise comparison between immature and post-spawning, where the results were determined to be statistically insignificant (p = 0.3).
Proximate composition of the ovary in the developing phase: A total of 27 fish samples were allocated to this stage. (Figure 5b) depicts the fluctuation in the mean values of the biochemical composition of the ovary at this stage. The moisture level of the samples ranged from 67.30 to 78.40%. Statistically, the difference in mean moisture values in the developing phase differs substantially from the immature phase (p < 0.05), but not from spawning (p = 0.8) and post-spawning (p = 0.9). The crude protein content varied from 10.90% to 19.20%. The mean crude protein levels in the developing phase compared to the immature phase varied substantially (p < 0.01), and the crude protein values in the ovaries were lowest in this gonadal phase, according to the pairwise comparison by post-hoc test. The lipid content was between 4.40 and 13.90%, whereas the ash content was between 1.30 and 3.60%. The ovaries of the developing phase had the highest lipid content, and the mean values differed significantly ( p < 0.01) from those of the immature and post-spawning phases, but not significantly (p > 0.05) from those of the spawning phase. In terms of the ash content, the ovary of the developing phase had the highest mean value (2.30± 0.60), followed by the ovary of the immature phase.
Proximate composition of the ovary in the spawning phase: The moisture content of all 18 females was determined to be between 68.40 and 76.80%. The mean moisture values from the ovaries of the spawning and developing phases were significantly lower than those of the immature phase (p < 0.05), but they did not differ significantly from one another (p > 0.05). The protein content ranged from 12.40 to 19.80%. On a statistical note (p < 0.05), the greatest mean value of crude protein values was observed in the ovaries of their spawning phase (16.70 ± 2.13), and the differences with the immature phase were non-significant (p = 0.2) but significant with the developing and post-spawning phases (p < 0.05). The lipid content values were found to be between 7.50 and 12.50%. Statistically, the difference in mean lipid content values changes insignificantly with the developing phase (p > 0.05), but significantly (p < 0.05) with the other two phases. In the fish ovaries, moisture and lipid content were found to be inversely proportional: ovaries with significantly (p < 0.05) high moisture content had low lipid value. The ash content ranged from 0.89 to 1.80% and was found to be significant (p < 0.01) when compared to the mean values of the developing and immature phases. (Figure 5c) depicts the biochemical makeup of the ovary during the spawning stage.
Proximate composition of the ovary in the post-spawning phase: (Figure 5d) depicts the average biochemical makeup of the ovary at the post-spawning stage. Moisture levels varied from 68.4% to 77.10%. The average moisture content in the post-spawning phase differed substantially (p < 0.05) from the mean moisture content values in the immature phase, but not significantly (p = 0.9) from the developing phase and spawning phase (p = 0.8). The amount of crude protein in the samples ranged from 11.3 to 17.30%. In the post-spawning phase, an intermediate quantity of crude protein content (15.13%) was detected in the ovaries. The lipid content varied from 4.2 to 8.7%. According to the post-hoc test, the mean lipid content in the post-spawning phase differed significantly (p < 0.05) from the mean values in the spawning and developing phases but not significantly (p > 0.05) from the immature phase. The amount of ash in the samples ranged from 0.5 to 1.9 %. Numerically, at all stages of ovarian development, the mean values of ash content were the same, but on a statistical note, the mean values of ash content in the post-spawning phase differed significantly (p < 0.01) from those in the immature and developing phases, but the difference between the mean values of ash content in spawning and post-spawning was found to be non-significant (p > 0.05). However, the maximum ash content was observed in the developing phase (2.3 ± 0.60%), while the lowest amount was calculated in the post-spawning phase (1.13 ± 0.40%).
Discussion
Variations in proximate composition in the muscles, liver, and ovary were influenced by different stages of gonadal development. Different researchers have attributed different biochemical compositions to the same species, and these changes may be correlated to external and internal variables such as food regime, season, spawning and migratory effects, and habitat mode [13]. The gonadosomatic index (GSI) and hepato-somatic index (HSI) were used to estimate the reproductive condition of rainbow trout. According to [14], the changes in the gonadosomatic index can help to determine the reproductive season, spawning frequency and spawning period. In the present study, low gonadosomatic indices were found in rainbow trout both throughout the immature phase (June to August 2018) and the post-spawning period (March to May 2019). The expulsion or reabsorption of mature oocytes caused a substantial drop in the gonado-somatic index from March onwards. The GSI grew significantly from November 2018 to February 2019, indicating the spawning phase, and the highest GSI value was recorded in January 2019, suggesting maximal gonadal growth. The maximum spawning period corresponds to the maximum GSI value. As a result, it confirms a close relationship between the two (spawning period and GSI). The present study noticed that during the growth phase, the hepatosomatic index rose progressively until it spiked at the start of the development phase (September). The synthesis of vitellogenin enhances hepatic metabolism, which leads to a rise in the hepatosomatic index. This rise could possibly be due to enlargement in dimensions of liver [15]. It was also observed that during early spawning period (December 2018), the lowest hepatosomatic index value was recorded and the same could be due to transportation and storage of various nutrients and the yolk precursor (vitellogenin) from the liver to mature oocytes [16]. Many researchers believe the study of the hepatosomatic index is essential because the liver generates vitellogenin (yolk precursor), which is crucial for the maturation of oocytes and gives critical data on hepatic energy reserves and metabolic activity [17]. However, numerous comparisons using post-hoc testing indicate significant mean GSI levels (p < 0.05) across different phases, but insignificant mean GSI values (p = 0.18) between the immature and post-spawning stages. Similarly, post-hoc analysis on mean HSI values reveal significant values (p < 0.05) between various stages, but insignificant mean HSI values (p = 0.95) when comparing the developing and post-spawning phases. Moreover, this study verifies the inverse connection between GSI and HSI, indicating that the peak value of gonadosomatic index corresponds to the lowest value hepatosomatic index. This circumstance led to the hypothesis that during gonadal maturation, vitellogenin mobilization and increased gonadal weight is due to transport of vitellogenin from the liver that causes hepatic weight loss [18]. The ANOVA test demonstrated that during the four phases of reproduction, ovary and liver weight changed significantly (p < 0.05) in female rainbow trout.
Estimation of biochemical composition in muscle at different gonadal stages
The crude protein content in the muscles of captive female rainbow trout attained a peak value in the immature phase and then dropped in succeeding phases of gonadal development, with the lowest value observed in the developing phase. It is assumed that in immature muscles, proteins get stored and may be used during the maturation of females. But the current assumption contradicts the results of [19], who revealed that the protein content of fish rises as the size of the fish grows. However, the current study found that young fish had higher protein levels in their muscles and that there was no correlation between fish size and protein levels. The correlation between moisture and crude protein was also observed in the present study. The amplification of protein content in muscles causes a simultaneous reduction in moisture content during all four gonadal stages. Similarly, a direct connection between crude protein and a lipid was found. It was observed that an increase in protein content brings about an increase in lipid content as well. However, the current findings contradict those of [20], who discovered an inverse connection between protein and lipid. Moreover, among different tissues studied, muscles had a higher mean protein percentage (21.5%) than the ovary (15.5%) and liver (15.2%). The same finding has been endorsed by the findings of [21], who reported that the muscles are enriched with protein as they are mostly consumed by humans. Thus, it can be concluded that the rainbow trout’s edible muscles are enriched with protein content (15–22%) but low in lipid content (5%), so it may be considered a proteinaceous fish. The lipid content of female rainbow trout muscles differed according to gonadal stage. Females in their immature phase had the highest lipid content, followed by the muscles of spawning females. The significant drop in lipid content was seen throughout the developing phase, which may be explained by the fact that lipid content from muscles is transferred to the ovaries for gonadal maturation. The substantial (p < 0.05) rise in crude lipid content in immature fish muscles coincides with a reduction in moisture content, indicating that the two have an inverse connection. Many researchers have agreed with this conclusion. However, according to , this connection is applicable exclusively to fatty fishes, and that moisture is negatively related to protein in lean fishes. In female rainbow trout, the mean total lipid content in muscles of all gonadal stages was 2.4%, which was significantly less than that reported by [22], who found 4.46% of total lipid in Oncorhynchus mykiss, and this fluctuation could be due to sex, season, muscle location, feed, environment, age, and maturity stage [23]. Cell membranes and their components are mostly made up of phospholipids, and so are the muscles in which lipids are the chief energy reserves [24]. In certain fishes, it also serves as a key indicator of reproductive success. According to [25], diverse fish species are divided into four different classes on the basis of their total lipid content: lean fish with a lipid content of less than 2%, low fat fish with a total lipid within the range of 2–4%, medium fish with a total lipid within the range of 4–8%, and high fat fish with a total lipid value of more than 8%. As a result, according to the above mentioned chart, the lipid content found in the muscles of rainbow trout female fish can be categorised as a low-fat fish as its lipid content value falls within the range of 2–4%. The lipid contents of rainbow trout female muscles during different gonadal stages were not significantly different, indicating less mobilisation of total lipid from muscles to other tissues, as lipids are used to make cell membranes, which remain intact and make transportation difficult [26]. As a result, there is no evidence that muscle lipids have any role in the growth and development of gonads in female trout. The lipid values in the muscles in the present study were comparable to those published for rainbow trout (3.71%) by [27], although they were lower than those reported by [28], who found greater lipid content (6.55%) in the muscles of rainbow trout. This variation in lipid content among the same species could be due to various factors, which include rate of fat metabolism, maturity stage, ambient temperature, food availability, stress, and other variables, as reported by [23].
In the present study, rainbow trout female muscles had a greater moisture content (72.8%) than their liver (71.2%), while the ovaries had a similar moisture level (72.7%) as that of the muscles. According to the post hoc test, the muscles of immature females had the highest protein and fat content, but the lowest moisture content. This reduction in moisture content indicates the qualitative condition of the fish. The muscles of spawning females, on the other hand, exhibited increased moisture content and reduced lipid and protein content, which might be related to protein and lipid consumption for various metabolic activities. When it comes to spawning, the substantial increase in moisture content and concomitant reduction in protein and fat content might be attributed to oocyte development. The average moisture content in muscles (72.8%) of captive raised rainbow trout in the present investigation was somewhat lower than that revealed by [29], who found that escaped Oncorhynchus mykiss muscles had the maximum moisture content (74.23%). This variation might be related to different types of food and their availability, dietary components, and less movement of captive-bred fish [30]. The present study also depicts a converse correlation between moisture and lipid values in muscles during different gonadal stages, and the same study endorses findings made by various researchers regarding an inverse relationship between the two [31].
Estimation of biochemical composition in the liver at different gonadal stages
The moisture content of the liver remains nearly constant throughout the gonadal phases, and the values were not significantly different. Variations in biochemical composition result in changes in liver weight that have an impact on the hepato-somatic index. In the present study, the crude protein composition of the liver changes significantly during distinct gonadal phases. The highest levels of crude protein were recorded in the livers of immature and post-spawning females, whereas the lowest levels were found during the spawning period. Because of the pressure of releasing eggs and non-feeding courting activity, the spawning period contains the lowest quantity of crude protein content. The transfer of protein from the liver towards the gonads to aid reproductive development might explain the substantial decrease in protein concentration during the spawning phase. [32] Both reported on the same investigation prior to spawning in distinct species. The liver is regarded as an essential energy reserve organ because it feeds nutrients to other organs throughout various reproductive processes. As a result, the hepato-somatic index of the liver fluctuates. The lipid content of the liver grows considerably throughout the development phase (9.9%), but then drops dramatically during spawning (9.1%) and the post-spawning period (7.1%). As far as the present study is concerned, the lipid content in muscles showed no significant change during different gonadal stages, but a substantial drop in lipid content was observed in the liver at the time of the spawning phase, which might be due to lipid transportation from the liver to gonads.
This observation is consistent with the fact that lipid mobilisation takes place between various organs. Thus, the current study endorses the findings of [33], who found that lipid is transported from the liver into the gonads for the latter’s appropriate growth and development. The findings of the current work are in contrast to those of [34], who found non-significant variations in the lipid content in the liver of the White Sea bream, Diplodus sargus, during its several gonadal stages. The same observation may be due to different functioning of the liver, in which the liver might be associated with the processing and modification of lipids rather than as an energy storage organ. Thus, in the case of rainbow trout, there is no need to include dietary lipids during the spawning phase as the liver has sufficient reserve to provide the same. The current research illustrated that the average quantity of lipid in the liver during all four gonadal stages falls within the range of 7.1% to 9.9 %, which was greater than that reported by [35] in wild Catla catla (3.32–5.91%) and in farmed Catla catla (4.72– 7.43%). During the present study, in female rainbow trout species, the maximum quantity of lipid was identified in the liver followed by the ovaries, which contradicts the findings of other researchers who reported more lipids in muscles than in other tissues. The livers of spawning females contain a considerable quantity of total lipid during distinct gonadal phases, but subsequently exhibit a rapid drop in the post-spawning period. It’s possible that this is due to energy being set aside for various reproductive activities, such as mating and egg release. As a result, it may be inferred that the liver of rainbow trout females provides nutrients to numerous organs and hence plays an important role in gonadal development.
Estimation of biochemical composition in the ovary at different gonadal stages
The moisture content in the ovaries of rainbow trout studied in relation to different gonadal phases showed wide variation among each other. The moisture content values ranged from 71.0% to 75.30%. The moisture level of ovaries in relation to different gonadal stages varies significantly, with the lowest moisture content found in both developing and post-spawning ovaries and the highest moisture content found in immature ovaries. It was noted that the increase in moisture content in the ovaries of immature females brought about a simultaneous decrease in their lipid content. The juvenile ovaries of rainbow trout were found to have the maximum amount of moisture of all the organs studied. A maximum moisture level of 72.0–79.9% was observed by [36] in the ovaries of Ammodytes hexapterus. It was discovered that the quantity of total lipid in fish ovaries rises at the expense of moisture content, implying that the two have a negative connection [37]. The protein content of female rainbow trout ovaries was determined to be between 14.3 and 16.7%. The spawning ovaries had the greatest protein content (16.7%), which was substantially greater than all other reproductive phases, whereas the females that were in the developing phase had the lowest protein content (14.3%) in their ovaries. Protein content was found to have intermediate values in both immature (16.10%) and post-spawning (15.13%) ovaries. The increased protein content in the ovaries (16.7 ± 2.13%) during the spawning phase might be attributed to protein transportation from other tissues into the ovaries [38], since the quantity of protein has been found to vary with eating and breeding capacities [39]. The crude protein levels found in this research were comparable to those observed in female Epinephelus diacanthus [40]. The change in fat content in the ovaries between reproductive stages was considerably greater in this research than the variance in protein content. The fat content of the ovaries fluctuated significantly as well, with levels ranging from 5.9 to 11.0% during different reproductive stages. As the lipid content in ovaries was found to be the chief biomolecule, the highest amount of lipid was found in the developing phase (11.00 ± 2.23%), followed by the ovaries of the spawning phase (10.00 ± 1.43%), whereas the significantly lowest fat content values were found in immature ovaries (5.9 ± 1.95%). The ovaries tend to acquire lipid content during the maturation of gonads. Therefore, dietary fats should be included in appropriate quantities throughout this time. The fish consume extra feed so as to compensate for the energy that was lost during the spawning season [41].
The highest amount of lipid content was found in the ovaries of developing females, which could be attributed to the fact that the relocation of lipid content from other tissues towards the gonads might be the cause of the amplification in lipid value throughout the developing phase. The same hypothesis was reported by [33]. Therefore, it is recommended to include a high-lipid and protein-rich diet for female rainbow trout during their developing phase for their proper growth and development. The ash content in the ovaries of rainbow trout females was 0.5–3.8% and represents the maximum amount in the ovaries as compared to liver and muscle. However, in the ovaries of wild female E. diacanthus, the same findings of ash concentration were recorded by [40]. As a result, the findings of this study could be used to improve rainbow trout captive breeding and fishing management in their natural environment, as well as the development of strategies to increase the reproduction of other salmonid fish.
Conclusion
The current study provides basic information on the different types of oocytes found in the ovaries, different phases of gonadal development, fluctuations in organo-somatic indices, and the changes in the biochemical composition of the liver, muscle, and ovary of female rainbow trout. This information is useful for establishing the nutritional profile, spawning season, and brood-stock diet design. Nutritionists, dieticians, and consumers will be able to interpret the consumption of key elements in fish food using the nutritional profile. It also gives data on different development and reproductive characteristics, which aids in the correct management and conservation of captivity-bred trout species. This research potentially serves as a foundation for future investigation into the design of nutrient-rich diets, as well as for comparative comparison with wild rainbow trout in their native environment and with other species.
Contribution Statement
Azra Bashir and Md. Naimat Ali conceived and designed the experiments; Azra Bashir performed the experiments; Masood Balkhi analysed and interpreted the data; Md. Naimat Ali and Masood Balkhi contributed reagents, materials, analysis tools, or data; and Azra Bashir wrote the paper.
Declaration of Competing Interest
All authors declare that they have no conflict of interest.
Acknowledgement
The authors acknowledge and express sincere thanks and a deep sense of appreciation to all faculty members of the Centre of Research and Development, University of Kashmir, and to all my colleagues for their constant support and cheerful company. The departmental fellowship granted to the PhD programme of the first author is gratefully acknowledged.
References
- Mohamed H A Elagba, Al-Maqbaly R, Mohamed Mansour H (2010) Proximate composition, amino acid and mineral contents of five commercial Nile fishes in Sudan African. J Food Sci 4: 650-654.
- Dempson J B, Schwarz C J, Shears M, Furey G (2004) Comparative proximate body composition of Atlantic salmon with emphasis on parr from fluvial and lacustrine habitats. Journal of Fish Biology, 64: 1257-1271.
- Kendall, Smith G C (2003) Effects of acid adaptation on inactivation of salmonella during drying and storage of beef jerk treated with marinades. Int J Microbiol89: 51-65.
- Afonso-Dias I, Reis C, Andrade JP (2005) Reproductive aspects of Microchirus azevia(Risso, 1810) (Pisces: Soleidae) from the south coast of Portugal. Sci 69: 275-283.
- Rajaguru A (1992) Biology of two co-occurring tongue fishes, Cynoglossus arel and C. lida (Pleuronectiformes: Cynoglossidae), from Indian waters. Fish Bull90: 328-367.
- Allen T.C. (1992) AFIP Laboratory methods in histotechnology Armed Forces Institute of Pathology. Am J Pathol 53–58.
- A.O.A.C. (1990) Official Methods of Analysis. 15th Edition. Association of Official Analytical Chemist, Washington DC.
- Jafri A K , Qasim S Z (1965) Studies on the biochemical composition of some freshwater fishes. Liver Fish Tech 2:163-169.
- A.O.A.C. (1984) Official Methods of Analysis. Association of Official Analytical Chemists. 14th Edition,Arlington.
- A.O.A.C. (2005) Official Methods of Analysis, Arlington, VA, USA: Association of Official Analytical Chemists
- Snedecor G W (1967) One-way classifications: Analysis of variance. Statistical methods, 215-237.
- Sokal R. R., Rohlf F J (1981) Two-way ANOVA. Biometry, ed, 2, 299-337.
- Abdullahi SA, Abolude DS, EgaRA (2001) Nutrient quality of four oven dried freshwater catfish fish species in northern Nigeria. J Trop Biosciences, 1: 70-76.
- Jan M, Jan U, Shah G M (2014) Studies on fecundity and gonadosomatic index of Schizothorax plagiostomus (Cypriniformes: Cyprinidae). J Threat Taxa 6:5375-5379.
- Christensen L J, Korsgaard B, Bjerregaard P (1999) The effect of 4-nonylphenol on the synthesis of vitellogenin in the flounder Platichthys flesus. Aquat Toxicol 46:211–219.
- Feidantsis K, Ntokou A, Michaelidis B (2020) Effect of seasonality on oocyte growth, oocyte maturity stages, and reproductive capacity in the gilthead sea bream (Sparus aurata) in relation with depth. Acta Vet Eurasia46:87-98.
- Lenhardt M, Jaric I, Cakic P, Cvijanovic G, Gacic Z et al. (2009) Seasonal changes in condition, hepatosomatic index and parasitism in sterlet (Acipenser ruthenus L). Turkish J Vet Anim Sci, 33: 209-214.
- Zin T, Than A A, Naing T T (2011) Fecundity (F), gonadosomatic Index (GSI), hepatosomatic index (HSI), condition factor (K) and length weight relationship (LWR) in Channa orientalis Bloch and Schneide, 1801. Uni. Res. J. 4:47-62.
- Grikorakis K, Alexis M N, Taylor K D A, Hole M (2002) Comparison of wild and cultured gilthead sea bream (Sparus aurata); composition, appearance and seasonal variation. Int J Food Sci Technol. 37:477–484.
- Nurnadia A A, Azrina A, Amin I (2011) proximate composition and energetic value of selected marine fish and shellfish from the West coast of Peninsular Malaysia. J Food Sci 18: 16-20.
- Dabhade V F, Pathan T S, Shinde S E, Bhandare R Y, Sonawane D L (2009) Seasonal variations of protein in the ovary of fish Channa gachua. Recent res sci technol1:216-218.
- Sabetian M, Delshad S T , Moini S, Islami H R., Motalebi A (2012) Identification of fatty acid content, amino acid profile and proximate composition in rainbow trout (Oncorhynchus mykiss). J Am Sci8:670-677.
- Sikorski Z E (1990) Seafood: resources, nutritional composition, and preservation (Vol. 248). Boca Raton, FL: CRC press.
- Tocher D R (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107-184.
- Stansby M E (1962) Proximate composition of fish. Fish in Nutrition. Fishing News (books) Ltd., London.
- Gonçalves C I (2013) Seasonal and starvation-induced changes on gonads and lipid reserves of the digestive gland of Nucella lapillus (Caenogastropoda). J Mar Biolog Assoc U.K 93:817-824.
- Özden Ö (2005) Changes in amino acid and fatty acid composition during shelf‐life of marinated fish.J Sci Food Agric 85: 2015-2020.
- Gonzalez-Fandos E, Garcıa-Linares M C, Villarino-Rodrıguez A, Garcıa-Arias M T, Garcıa-Fernandez M C (2004) Evaluation of the microbiological safety and sensory quality of rainbow trout (Oncorhynchus mykiss) processed by the sous vide method. Food Microbiol21:193-201.
- Taşbozan O, Gökçe M A, Erbaş C (2016) The effect of different growing conditions to proximate composition and fatty acid profiles of rainbow trout (Oncorhynchus mykiss). J Appl Anim Res 44:442-445.
- Fuentes A, Fernández‐Segovia I, Barat J M, Serra J A (2010) Physicochemical characterization of some smoked and marinated fish products. J Food Process Preserv 34:83-103.
- Shamsan E F, Ansari Z A (2010) Biochemical composition and caloric content in sand whiting Sillago sihama (Forsskal), from Zuari estuary, Goa. Indian J Fish57: 61- 64.
- Jørgensen S E, De Bernardi R (1997) The application of a model with dynamic structure to simulate the effect of mass fish mortality on zooplankton structure in Lago di Annone.Hydrobiologia 356:87-96.
- Almansa E, Martian MV, Cejas JR, Badi P, Jerez S et al. (2001) Lipid and fatty acid composition of female gilthead seabream during their reproductive cycle: effects of a diet lacking n‐3 HUFA. J Fish Biol59:267-286.
- Pérez F, Granger B E (2007) I Python: a system for interactive scientific computing. Comput Sci Eng9: 21-29.
- Hassan M, Chatha S A S, Tahira I, Hussain B (2010) Total lipids and fatty acid profile in the liver of wild and farmed catla catla fish. Grasas Y Aceites 61:52-57.
- Robards M D, Anthony J A, Rose G A, Piatt J F (1999) Changes in proximate composition and somatic energy content for Pacific sand lance (Ammodytes hexapterus) from Kachemak Bay, Alaska relative to maturity and season. J Exp Mar Biol Ecol242 : 245-258.
- Henderson R J, Almatar S M (1989) Seasonal changes in the lipid composition of herring (Clupea harengus) in relation to gonad maturation. J Mar Biolog Assoc U.K 69 :323-334.
- Finn R N, Fyhn H J (2010) Requirement for amino acids in ontogeny of fish. Aquac Res41:684–716.
- Islam M N, Joadder M A R (2005) Seasonal variation of the proximate composition of freshwater Gobi, Glossogobius giuris (Hamilton) from the River Pamuscle tissuea. J Biol Sci 8: 532–536.
- Rao A C, Krishnan L (2011) Biochemical composition and changes in biological indices associated with maturation of the ovary in the spiny cheek grouper Epinephelus diacanthus (Valenciennes, 1828) Indian J Fish 58 : 45-52.
- Ahmed I,Sheikh Z A(2017)Study on the seasonal variation in the chemical composition, hematological profile, gonado-somatic index and hepato-somatic index of snow trout, Schizothorax niger from the freshwater Dal Lake, Kashmir. Am J Food Technol 12:1-13.
Indexed at, Google Scholar, Crossref
Indexed at Google Scholar
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Bashir A, Ali MN, Balkhi MH (2022) Impact of Gonadal Development on The Proximate Composition of Muscle, Liver and Ovary of Adult Female Rainbow Trout (Oncorhynchus mykiss). J Fisheries Livest Prod 10: 350. DOI: 10.4172/ 2332-2608.1000350
Copyright: © 2022 Bashir A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3361
- [From(publication date): 0-2022 - Nov 10, 2025]
- Breakdown by view type
- HTML page views: 2842
- PDF downloads: 519