Individual PKR Activation in Peripheral Blood Mononuclear Cells Correlates with Cognitive Decline in Alzheimer’s Disease
Received: 13-Apr-2019 / Accepted Date: 26-Apr-2019 / Published Date: 02-May-2019 DOI: 10.4172/2161-0460.1000465
Abstract
Background: Among the pathogenic processes in Alzheimer’s Disease (AD), systemic inflammation, autophagy and metabolic alterations have been described. The double-stranded RNA-dependent protein kinase (PKR) is an ubiquitous cellular kinase involved in these several pathogenic processes, and previous studies reported its involvement in AD. PKR can be activated by phosphorylation (PT451-PKR) and cleaved in catalytic form (PKR-KD). We have previously described a correlation between PKR activation in peripheral blood mononuclear cells (PBMCs) and cognitive status in advanced AD. However, the link between PKR’s activation in PBMCs and cognitive decline in AD remains unknown.
Methods: Thirty-one patients with AD were included. Acute and chronic inflammations were considered as exclusion criteria. Cognitive status was assessed with Mini Mental State Examination (MMSE) and AD assessment scale (ADAScog) at baseline, 6, 12 and 24 months of follow-up. PKR expression and its activation were measured using western blot at baseline, 12 and 24 months.
Results: At baseline, MMSE mean score was 20.5 ± 2.6; Except for total PKR expression between baseline and M12, no longitudinal significant variation of PKR activation was found. Higher PKR-KD activation was correlated with faster cognitive decline at an individual level at M6 (r=0.33, p=0.0453), M12 (r=0.48, p=0.0033) and M24 (r=0.36, p=0.0407). Other PKR forms were correlated with cognitive decline at M12.
Conclusions: Our results suggest that PKR activation in PBMCSs can predict cognitive decline in AD at an early stage at an individual level.
Keywords: Alzheimer’s disease; Cognitive decline; Chronic inflammations; Cognitive disorders
Introduction
Alzheimer’s disease (AD) is a neurodegenerative pathology marked by cognitive disorders. Post mortem studies of AD patients show senile plaques and neurofibrillary tangles. Cause of neuronal degeneration is not completely resolved and many pathogenic mechanisms like inflammation [1], autophagy [2] or metabolic alterations [3] are involved.
The double-stranded RNA-dependent protein kinase (PKR) is an ubiquitous cellular kinase involved in several pathways like inflammation [4], apoptosis [5] and protein synthesis [6]. PKR can be activated by Protein Activator of the Interferon-Induced Protein Kinase (PACT) in a phosphorylated form (PT451-PKR) [7], full length PT451- PKR can be cleaved in a more active form (C terminal catalytic kinase domain (KD), PT451-PKR-KD) [8]. PKR is involved in inflammation via nuclear factor-kappa B (NF-κB) pathway activation through interaction with the Inhibitor of KappaB Kinase beta (IKKβ), a subunit of the IKK complex, leading to nuclear translocation of NF-κB [4]. PKR activation down-regulates translation by eIF2α phosphorylation [6]. In Peripheral Blood Mononuclear Cells (PBMCs) from AD patients, in tri-culture of neurons/astrocytes/microglia and in transgenic mice, treatment with a specific PKR inhibitor highly decreases pro-inflammatory cytokine production [9-11]. In vitro studies showed relationship between PKR and AD specific lesions. In cell culture, Aβ exposition induces PKR activation which leads to apoptosis [12]. In return, PKR seems involved in Aβ production by the regulation of BACE1 [13]. Similarly, PKR after activation by Glycogen synthase kinase 3 beta (GSK-3β) participates in Tau phosphorylation [14].
Several clinical studies suggest an involvement of PKR in the physiopathology of AD. Post mortem studies show a raise of phosphorylated PKR co-localizing with phosphorylated [15]. In Cerebrospinal Fluid (CSF), levels of PKR and P-PKR discriminate control from AD patients [16]. Furthermore in longitudinal study, high CSF phosphorylated PKR levels were associated with faster cognitive decline [17]. CSF is not routinely collected in clinical practice and blood sample is less invasive and easier to perform. Our previous results showed PKR involvement in PBMCs of AD patients with a negative correlation between the level of PKR activation and cognitive test scores [18]. The aim of this study was to determine relationship between PKR and its activation in PBMCs with the cognitive decline through a 2-years follow-up of AD patients.
Materials and Methods
Chemical
Ficoll Histopaque® 1077, newborn calf serum heat inactivated, sodium fluoride (NaF), phenylmethylsulfonyl fluoride (PMSF), protease and phosphatase inhibitor cocktails, sterile filtered dimethylsulfoxide HybriMax® (DMSO), Triton X100, dithiothreitol (DTT) and all reagent grade chemicals for buffers were obtained from Sigma (St Quentin Fallavier, France); RPMI 1640 medium, L-glutamine, 5000 units of penicillin (base) and 5000μg of streptomycin (base)/mL mixture, Quant-it protein assay, NuPAGE 4-12% Bis Tris 1.5 mm gel, NuPAGE antioxydant, MES SDS running buffer, NuPAGE iBlot™ nitrocellulose Transfer Stacks from Gibco-Invitrogen (Fisher Bioblock Scientific distributor, Illkirch, France). For western blot, primary antibodies rabbit anti-PT451PKR as custom antibodies produced by Eurogentec (Seraing - BELGIUM), rabbit anti-PKR from Cell Signalling (Ozyme, St Quentin Yvelines, France) and mouse anti-β-actine from Sigma (St Quentin Fallavier, France). Secondary antirabbit IgG antibody conjugated with Horseradish Peroxydase were purchased from Cell Signalling (Ozyme, St Quentin Yvelines, France), secondary anti-mouse IgG conjugated with Horseradish Peroxydase and ECL plus kits were purchased from GE Healthcare Europe GmbH (Velizy Villacoublay, France).
Patients
Patients were selected between November 2010 and December 2012 in the memory center of Poitiers University Hospital in an ancillary study of a national clinical research project (CYTOCOGMA). AD was diagnosed according to NINCDRDS-ADRDA criteria with a MMSE score between 16 and 25 at inclusion. Exclusion criteria were any other neurological disease than AD, ongoing inflammatory state or anti-inflammatory treatment.
Patients had a clinical and neuropsychological assessment with MMSE and Alzheimer’s disease assessment scale (ADAScog) (the higher the score the lower the performance) at diagnosis, after 6 months, one year and two years of follow-up. Biological assessment was conducted at baseline and after one and two years.
PBMCs extraction, culture and cellular lysis
Methods have been extensively used in our laboratory and previously described [9,19-21]. PBMCs were isolated by Ficoll Histopaque® density gradient centrifugation within two hours after blood test. Using a KOVA cell, cells were counted and seeded at 106 cells/well in six-well plates. After 48 hours of culture, cells were isolated from the medium by centrifugation. Cells were lysed using a lysis buffer (50 mM Trizma® base, 50mM NaCl pH 6.8, 1% Triton X100, 1mM PMSF, 50mM NaF, 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail). Proteins levels were assessed using Quant-it® protein assay. Samples were preserved in the freezer 80 degrees.
Western blot
Each sample was taken for a quantity of 30 μg of proteins per well and diluted with electrophoresis buffer contening 0.05M DTT. Each sample was boiled at 100°C during 5 minutes. Proteins were separated on NuPAGE gel with 200 V during 35 minutes. Proteins were transfered to NuPAGE iBlot™ nitrocellulose Transfer Stacks using iBlot™ Gel Transfer Device. Membranes were blocked for two hours at Room Temperature (RT) in Tris-buffered saline/Tween (TBST: 20mM Tris-HCl, 150mM NaCl, pH 7.5, 0.05% Tween 20) containing 5% BSA and 0.21% NaF. Membranes were incubated with primary antibodies in blocking buffer overnight on a shaker at 4°C. The primary antibodies used were rabbit anti-PT451PKR (1:100) which detect threonine 451 phosphorylated from both full-length (74 kDa) and catalytic fragment of PKR (35 kDa). It was demonstrated that the released KD domain of PKR is constitutively active [22], which is well supported by structure studies [23]. Moreover, the caspase-generated fragments of PKR cooperate to activate fulllength PKR and amplify the translation inhibitory signal [8]. Other primary antibodies rabbit anti-total PKR (1:500), mouse anti-β-actine (1:100,000) were also used. Membranes were washed twice with TBST during 10 minutes at RT on a shaker. Membranes were incubated with secondary antibodies conjugated with the peroxydase according to the primary antibody origin during one hour at RT. The second antibodies used were goat anti-rabbit IgG (1:1000) and sheep antimouse IgG (1:1000). Membranes were washed twice and incubated with chemiluminescence ECL plus® system, luminescent signal was captured by Gene Snap software and analyzed by Gene Tools software, both with Gbox system® (Syngene, Ozyme distributor, France). Protein expression level was reported to the actin expression level and expressed in arbitrary units. Activation of full length PKR was calculated using the ratio between PT451-PKR and total PKR. Activation of PKR-KD was calculated using the ratio between PT451-PKR-KD and total PKR.
Statitical analysis
All statistical analyses were carried out using the SAS 9.2 software package (SAS Inc., Cary, NC, USA). Quantitative variables are described with mean and Standard Deviation (SD). Biological values measured at baseline, M12 and M24 for AD patients were compared by non-parametric Friedman’s test for repeated measures followed by post-hoc Sheffé’s test if necessary. The correlations between the expression of total PKR or its phosphorylated forms, its activations (full-length PKR and PKR-KD) and cognitive scores were investigated with the Spearman correlation coefficient (rho) calculation. The level of significance was p<0.05.
Results
Patients characteristics
Thirty-seven patients were included, among which thirty-one were followed during two years. Two patients died and 4 were lost to follow-up. Mean age at baseline (D0) was 78.3 years. Table 1 shows neuropsychological characteristics at D0, after 6 months (M6), and one and two years of follow-up (M12 and M24). During the two years follow-up we observed an average decrease of 3.7 points for MMSE and an average increase of 4.2 points for ADAScog.
| Mean (SD) [Extremes] | D0 | M6 | M12 | M24 |
|---|---|---|---|---|
| n = 31 | n =28 | n =31 | n = 29 | |
| MMSE | 20.5 (2.6) [16-25] | 19.7 (3.5) [13-25] | 19.1 (3.9) [12-26] | 16.8 (4.9) [7-25] |
| ADAScog | 15.6 (5.9) [6.9-28.5] | 16.4 (7.3) [7.3-36.8] | 16.7 (7) [5.9-31] | 19.8 (10.1) [6-42.9] |
Table 1: Clinical and neuropsychological characteristics of patients.
Longitudinal PKR activation in PBMCs of AD patients
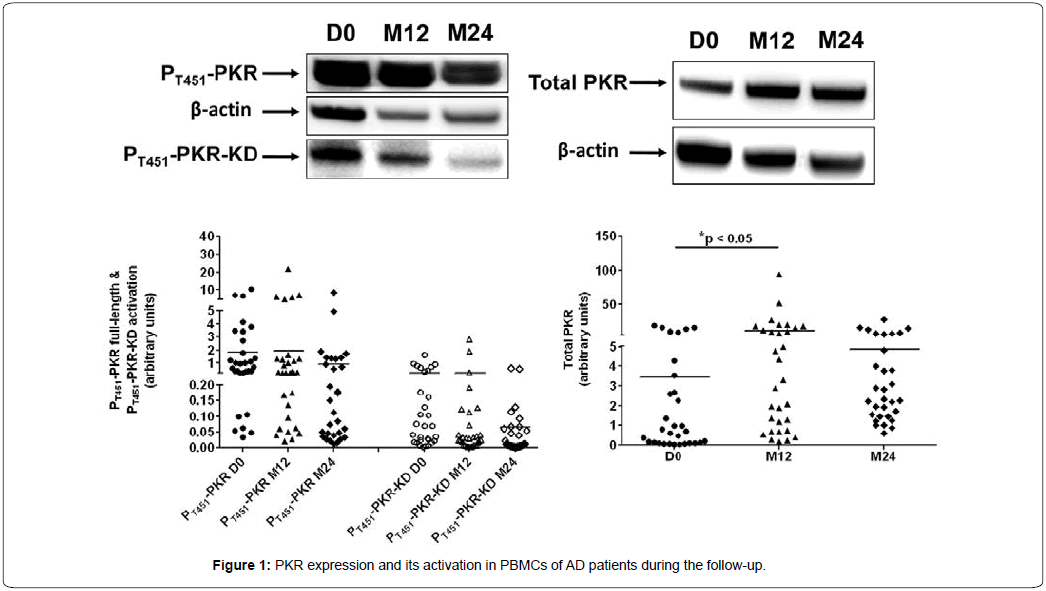
PKR consists of two functionally distinct domains: An aminoterminal regulatory domain and a carboxyl-terminal catalytic domain [24]. The regulatory domain consists of two dsRNA-binding motifs, followed by a spacer. Binding of dsRNA exposes the catalytic site and induces dimerization of PKR. Dimerization allows autophosphorylation, rendering PKR active [25]. However, dsRNA-independent protein activators of PKR, such as PACT have been identified [7]. The T451 site of PKR is one of the first active sites leading to its autophosphorylation at different sites and homodimer formation, resulting in its activation [26]. Figure 1 shows PKR expression and its activation at baseline and during the follow-up. A Friedman’s test indicated significant differences for expression of total PKR (p=0.0001), full-length PT451PKR (p=0.0247) and PT451PKR-KD activation (p=0.0053) during the followup. However, a Scheffé’s post-hoc test revealed that only the levels of total PKR expression at D0 was significant to those at M12 (increase of 215% at M12). The decrease of the PT451PKR-KD activation was no significant between D0 and M24, it reached 74.3%.
Western blots were performed to determine the expression of total PKR (74kDa) and its phosphorylated forms: full-length PT451PKR (74kDa) and PT451PKR-KD (35kDa) as described in method section. Protein expression level was reported to the actin expression level (42kDa) and expressed in arbitrary units. Activation of full-length PKR was calculated using the ratio between full-length PT451-PKR and total PKR. Activation of PKR-KD was calculated using the ratio between PT451-PKR-KD and total PKR. Representative blots showed protein expression in PBMCs of the same patient at D0, M12 and M24. A Friedman’s test showed significant differences for total PKR (p=0.0001), full-length PT451PKR (p=0.0247) and PT451PKR-KD activation (p=0.0053) during the follow-up. A Scheffé’s post-hoc test indicated p<0.05 between D0 and M12 for the levels of total PKR expression.
Correlation of PKR and activated PKR at baseline with cognitive decline
We found a correlation between total PKR expression at baseline and absolute variation of MMSE at 6 months (r=0.34, p=0.0497). We found a correlation between PKR-KD activation at baseline and cognitive decline at 6 months with absolute and relative variation of ADAScog (r=0.33, p=0.0506 and r=0.33, p=0.0453, respectively).
PKR and its activation of full PKR or PKR-KD strongly correlated with cognitive decline at one year (Table 2). No correlation was found with PT451-PKR or PT451-PKR-KD. We found a correlation between PKRKD activation and relative variation of ADAcog at two years (r=0.36, p=0.0407). Among the three parameters studied at baseline: total PKR expression, full-length PKR activation and PKR-KD activation, only the activation of PKR-KD was correlated with each assessment of cognitive score (6, 12 and 24 months). This activation could be then a good predictive biomarker of the cognitive decline in AD. No correlation was found between PKR and its activation at M12 and cognitive decline between M12 and M24 assessed by ADAScog or MMSE.
| Total PKR | Full PKR activation | PKR-KD activation | |
|---|---|---|---|
| AV ADAScog | r=- 0.4; p=0.0134 | r=0.45; p=0.0051 | r=0.40; p=0.0126 |
| RV ADAScog | r=-0.49 ; p=0.0024 | r=0.54; p=0.0009 | r=0.48; p=0.0033 |
Correlations were performed using Spearman correlation coefficient (rho) calculation, level of significance was p<0.05.
Table 2: Correlation between PKR and its activation with ADAScog Absolute Variation or Relative Variation (AV and RV) at one year.
Discussion
In this longitudinal study, results show that total PKR levels and the activation of this kinase in PBMCs are associated with individual cognitive decline at one year in newly diagnosed patients with AD at an early stage (median MMSE score at 20.5) and at two years with a lower correlation. Previous studies report a negative individual correlation between full length PKR activation and cognitive status in patients at an advanced stage with a medium MMSE score of 15.4 points [18]. Dumurgier et al. reported that a higher level of phosphorylated PKR in CSF at the T446 site was associated with a more marked decline in patients with a medium MMSE score of 20.5 at baseline [17]. We report for the first time individual correlation of PKR activation in PBMCs with individual cognitive decline. We chose to assess PKR phosphorylation at the T451 site because it is one of the first sites of phosphorylation, using custom antibodies which have shown their reliability in previous studies [9,18,27].
We assessed expression of PKR and its phosphorylated forms using western blot technique with results expressed in arbitrary units. These data were used to calculate PKR activation. Statistical analysis did not show significant differences during the two years follow-up excepted for total PKR expression which was increased at M12 compared to D0. However, a great variability of values was observed for the PKR expression by western blot, limiting other significant differences with non-parametric statistical tests. Moreover western blot quantifies only the relative expression of proteins. It would then be interesting to develop an ELISA test to determine the absolute quantity of PKR and its phosphorylated forms.
To our knowledge, this is the first longitudinal study of PKR activation in the context of AD, as previous reports were transversal. Our results show a strong correlation between total PKR expression or its activation and cognitive decline assessed by ADAScog but less correlation with MMSE. An explanation could be that ADAScog scale, which is often used as primary endpoint in randomized trials, is a more global scale with a larger range. MMSE may be less sensitive to identify light cognitive variation. The strongest correlation was found with cognitive decline after one year. Furthermore, the activation of PKRKD was highly correlated with absolute and relative ADAScog variation at all assessments M6, M12 and M24, indicating that this ratio of PT451- PKR-KD/total PKR could be a good predictive biomarker in AD. With the evolution of the disease, the severity of the cognitive degradation could no longer reflect the progression of the disease. Our results suggest an early involvement of PKR which is stable over time followed by other mechanisms like inflammation and autophagy, because PKR has many downstream targets.
Thus PKR activation can predict cognitive decline by its implication in several pathways. Beside amyloid plaques and neurofibrillary tangles, inflammatory process is involved in AD [28]. PKR plays a critical role in the control of pro-inflammatory cytokine production [29] particularly in the context of AD [9]. PKR activation may also promote neuronal death by down regulation of translation and pro apoptotic effects [30].
Our study has some limitations. First, all patients were included in the solo center of Poitiers and the number is limited. PKR and its activation need to be compared with other biomarkers such as brain atrophy or CSF markers. Similarly a comparison of PKR activation in PBMCs and in CSF should be done.
Conclusion
Our results show that PKR activation negatively correlates with cognitive decline in newly diagnosed AD patients at an early stage of the disease. The PKR-KD activation could be used as an early pronostic marker but our results need to be confirmed in a larger cohort and assessment of PKR should be performed using ELISA technique that requires to be developed directly into PBMCs without culture.
Déclarations
Ethics approval and consent to participate: All patients signed a written informed consent. This study was approved by the local ethic committee (comite de protection des personnes Ouest 3) and declared on ClinicalTrial.gov.NCT01351142.
Acknowledgements
Authors thank Claudie Ornon, Coline Bouyer for neuropsychological assessment in the memory clinic (CMRR) of Poitiers University Hospital, Pr Jean Claude. Lecron (EA 4331) for Luminex X-MAP® technology in ImageUp technical platform of University of Poitiers and Ludovic Blanchard for his help as clinical research associate.
References
- Dansokho C, Heneka MT (2018) Neuroinflammatory responses in Alzheimer's disease. J Neural Transm (Vienna) 125: 771-779.
- Zhang Y, Chen X, Zhao Y, Ponnusamy M, Liu Y (2017) The role of ubiquitin proteasomal system and autophagy-lysosome pathway in Alzheimer's disease. Rev Neurosci 28: 861-868.
- Demetrius LA, Driver J (2013) Alzheimer's as a metabolic disease. Biogerontology 14: 641-649.
- Bonnet MC, Weil R, Dam E, Hovanessian AG, Meurs EF (2000) PKR stimulates NF-kappaB irrespective of its kinase function by interacting with the IkappaB kinase complex. Mol Cell Biol 20: 4532-4542.
- Page G, Rioux Bilan A, Ingrand S, Lafay-Chebassier C, Pain S, et al. (2006) Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer's disease. Neuroscience 139: 1343-1354.
- Okumura F, Okumura AJ, Uematsu K, Hatakeyama S, Zhang DE, et al. (2013) Activation of double-stranded RNA-activated protein kinase (PKR) by interferon-stimulated gene 15 (ISG15) modification down-regulates protein translation. J Biol Chem 288: 2839-2847.
- Patel RC, Sen GC (1998) PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J 17: 4379-4390.
- Kalai M, Suin V, Festjens N, Meeus A, Bernis A, et al. (2007) The caspase-generated fragments of PKR cooperate to activate full-length PKR and inhibit translation. Cell Death Differ 14: 1050-1059.
- Couturier J, Page G, Morel M, Gontier C, Claude J, et al. (2010) Inhibition of double-stranded RNA-dependent protein kinase strongly decreases cytokine production and release in peripheral blood mononuclear cells from patients with Alzheimer's disease. J Alzheimers Dis 21: 1217-1231.
- Couturier J, Paccalin M, Lafay-Chebassier C, Chalon S, Ingrand I, et al. (2012) Pharmacological inhibition of PKR in APPswePS1dE9 mice transiently prevents inflammation at 12 months of age but increases Abeta42 levels in the late stages of the Alzheimer's disease. Curr Alzheimer Res 9: 344-360.
- Couturier J, Paccalin M, Morel M, Terro F, Milin S, et al. (2011) Prevention of the beta-amyloid peptide-induced inflammatory process by inhibition of double-stranded RNA-dependent protein kinase in primary murine mixed co-cultures. J Neuroinflammation 8: 72.
- Chang RC, Wong AK, Ng HK, Hugon J (2002) Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport 13: 2429-2432.
- Mouton-Liger F, Paquet C, Dumurgier J, Bouras C, Pradier L, et al. (2012) Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2alpha pathway. Biochim Biophys Acta 1822: 885-896.
- Bose A, Mouton-Liger F, Paquet C, Mazot P, Vigny M, et al. (2011) Modulation of tau phosphorylation by the kinase PKR: implications in Alzheimer's disease. Brain Pathol 21: 189-200.
- Peel AL, Bredesen DE (2003) Activation of the cell stress kinase PKR in Alzheimer's disease and human amyloid precursor protein transgenic mice. Neurobiol Dis 14: 52-62.
- Mouton-Liger F, Paquet C, Dumurgier J, Lapalus P, Gray F, et al. (2012) Increased cerebrospinal fluid levels of double-stranded RNA-dependant protein kinase in Alzheimer's disease. Biol Psychiatry 71: 829-835.
- Dumurgier J, Mouton-Liger F, Lapalus P, Prevot M, Laplanche JL, et al. (2013) Cerebrospinal fluid PKR level predicts cognitive decline in Alzheimer's disease. PLoS One 8: e53587.
- Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, et al. (2006) Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer's disease. Dement Geriatr Cogn Disord 22, 320-326.
- Julian A, Dugast E, Ragot S, Krolak-Salmon P, Berrut G, et al. (2015) There is no correlation between peripheral inflammation and cognitive status at diagnosis in Alzheimer's disease. Aging Clin Exp Res 27: 589-594.
- Francois A, Julian A, Ragot S, Dugast E, Blanchard L, et al. (2015) Inflammatory Stress on Autophagy in Peripheral Blood Mononuclear Cells from Patients with Alzheimer's Disease during 24 Months of Follow-Up. PLoS One 10: e0138326.
- Verite J, Janet T, Julian A, Chassaing D, Page G, et al. (2017) Peripheral Blood Mononuclear Cells of Alzheimer's Disease Patients Control CCL4 and CXCL10 Levels in a Human Blood Brain Barrier Model. Curr Alzheimer Res 14: 1215-1228.
- Saelens X, Kalai M, Vandenabeele P (2001) Translation inhibition in apoptosis: Caspase-dependent PKR activation and eIF2-alpha phosphorylation. J Biol Chem 276: 41620-41628.
- Wu S, Kaufman RJ (1997) A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem 272: 1291-1296.
- Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, et al. (1990) Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62: 379-390.
- Ung TL, Cao C, Lu J, Ozato K, Dever TE (2001) Heterologous dimerization domains functionally substitute for the double-stranded RNA binding domains of the kinase PKR. EMBO J 20: 3728-3737.
- Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, et al. (1998) Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol Cell Biol 18: 2282-2297.
- Tronel C, Page G, Bodard S, Chalon S, Antier D (2014) The specific PKR inhibitor C16 prevents apoptosis and IL-1beta production in an acute excitotoxic rat model with a neuroinflammatory component. Neurochem Int 64: 73-83.
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, et al. (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol 14: 388-405.
- Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR (2000) NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol Cell Biol 20: 1278-1290.
- Chang RC, Suen KC, Ma CH, Elyaman W, Ng HK, et al. (2002) Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2alpha in neuronal degeneration. J Neurochem 83: 1215-1225.
Citation: Julian A, Page G, Rioux Bilan A, Dugast E, Morel M, et al. (2019) Individual PKR Activation in Peripheral Blood Mononuclear Cells Correlates with Cognitive Decline in Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 9: 465. DOI: 10.4172/2161-0460.1000465
Copyright: © 2019 Julian A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4056
- [From(publication date): 0-2019 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 3192
- PDF downloads: 864