Intensive Physical Therapy Mitigates Cognitive Decline in People with Parkinson's disease
Received: 02-Aug-2019 / Accepted Date: 20-Aug-2019 / Published Date: 29-Aug-2019 DOI: 10.4172/2161-0460.1000475
Abstract
Background: Mild Cognitive Impairment in Parkinson’s disease (PD-MCI) is considered a predictor for the development of dementia, a major source of eventual treatment-refractory disability. Physical activity, which has proved effective in improving motor symptom, has also been proposed as a possible non-pharmacological intervention for preventing/delaying the onset of cognitive impairment in Parkinson’s disease (PD).
Objectives: This study evaluates the effect of a 4-week rehabilitation therapy on cognitive functions in mid-stage PD-MCI patients.
Methods: 40 PD-MCI patients were randomized to receive Physical Therapy (PT) or no physical therapy (CT) according to a controlled single-blind design. Subjects in the PT group (n. 17) attended a rehabilitation program with 6 sessions/week, each lasting 60 minutes, for 4 weeks in addition to their usual pharmacological therapy; subjects in CT group (n. 22) received only pharmacological therapy. Cognitive and motor functions were assessed at baseline (T0) and at the end of the intervention period (T1) in both groups.
Results: PT group showed a significant T0 to T1 improvement in global cognitive functioning (MOCA) and in working memory tests, associated to a parallel improvement in motor performances. Cognitive and motor scores did not change in CT group. When comparing the two groups, we detected a significant improvement in global cognitive functioning (MoCA) and attention/working-memory in the PT group.
Conclusions: Though preliminary and limited to a short period of time, these findings suggest the idea that a physical training program has a positive impact on cognitive functions in PD-MCI.
Keywords: Cognitive decline; Physical therapy; Parkinson’s disease
Introduction
Mild Cognitive Impairment in Parkinson’s disease (PD-MCI) is considered a predictor for the development of dementia in PD, a complication associated with a reduction of quality of life for patients and caregivers [1]. At present, there is no fully proven pharmacological treatment for cognitive impairment in PD and the available pharmacological armamentarium consists in symptomatic drugs, often burdened by intolerable side effects [2]. Increasing evidence suggests that environmental and lifestyle factors impact on cognitive functions and brain plasticity during aging [3]. Moreover, studies on the role of cognitive reserve in modulating the clinical expression of neurodegenerative disease provide the substrate for non-pharmacologic approach [4,5]. Differences in cognitive reserve may result from innate factors, can be significantly affected by age and can also be modulated by life experiences [6]. For example, educational attainment, attendance of training courses, intellectual involvement in job tasks and participation in cognitively stimulating activities during leisure time are commonly used as proxies for cognitive reserve [4].
On this point, non-pharmacological intervention may represent adjunctive therapy to medications in order to delay the onset of the cognitive deficits or dementia. Among non-pharmacological interventions for cognitive impairment in Alzheimer’s disease and dementia, several modalities have been tested in recent years, but supporting evidence for their use is still preliminary [7]. Previous studies observed a positive effect of cognitive training on cognition both in healthy elderly people and patients in the early stage of neurodegenerative diseases such as PD-MCI [8,9-11].
Furthermore among non-pharmacological intervention, physical exercise has also shown cognitive benefits in healthy elderly subjects and in PD patients, thus supporting the potential cognition-enhancing role of exercise for PD [12,13].
To date, physical exercise has been proposed primarily to target motor symptoms in PD, but studies examining the neuroprotective effects of exercise and physical activity interventions on cognitive decline in PD are emerging. Several exercise interventions have indeed proved to be effective on cognition in PD: tango, aerobic exercises and resistance exercise training [14-16]. Mckee and colleagues found evidence of beneficial effects of adapted tango (30 hours) on visuospatial function in PD patient, while Ridgel et al. investigated the effects of a low-intensity passive cycling intervention on executive functions measured with Trail Making A and B [17,18]. Recently, some studies also demonstrated the beneficial effects of moderate aerobic exercises on executive functions in people with mild to moderate PD [19-25]. Though these emerging results are encouraging, evidence that physical activity may reduce dementia risk has not been established and issues regarding type, frequency and duration of exercises, as well as the best timing in which operate (disease stage and level of cognitive deterioration) remain unresolved.
Given the well-established irreversibility of cognitive impairment in neurodegenerative disorders, the scientific attention has shifted more and more on the identification of early interventions that, applied before the onset of deficits, may delay their full development. In this frame, the aim of the present study was to assess the effect of 4-week intensive physical training (6 session/week, 60 minutes/day) on both motor and cognitive impairments in patients with mid-stage PD-MCI.
Methods
Participants and measures
Patients with idiopathic PD were recruited from the Neurorehabilitation Unit and Parkinson and Movement Disorders Unit of IRCCS Mondino Foundation. The protocol was approved by the local Ethics Committee and all patients gave written consent before being enrolled in the study. The trial has been registered on ClinicalTrials.gov (ID: NCT04012086).
We enrolled patients with PD-MCI according to the following inclusion criteria:
• diagnosis of idiopathic PD according to UKPDBB criteria and Hoehn & Yahr scale ≤ 3 [26,27];
• presence of PD-MCI single- or multiple-domain [1];
• age between 50 and 85 years;
• educational level ≥ 5 years.
Exclusion criteria were:
• pre-existing cognitive impairment (e.g. aphasia, neglect);
• severe disturbances in consciousness;
• concomitant severe psychiatric disease or others neurological conditions (e.g. depression and behavioural disorders).
All patients were treated with dopamine agonists or L-DOPA and had been on a stable therapy schedule for at least 3 months. No variations were allowed during the training and follow-up period. All groups were sex and age-matched.
The PD-MCI diagnosis was formulated on the basis of a comprehensive neuropsychological evaluation (baseline cognitive assessment- T0) according to the guidelines (level II criteria) [1].
The following standardized tests assessing different domains were used:
• global cognitive function: Mini-Mental State Examination (MMSE) and Montreal Montreal Overall Cognitive Assessment (MoCA) [28,29];
• memory: verbal (Verbal Span, Digit Span) and spatial (Corsi’s block-tapping test- CBTT) span [30]; verbal long-term memory (Logical Memory Test immediate and delayed recall) (Rey’s 15- word test immediate and delayed recall) [31,32]; spatial longterm memory (Rey Complex Figure delayed recall- RCF-dr) [33];
• logical-executive functions: non-verbal reasoning (Raven’s Matrices 1947- RM47) [31]; categorical abstract reasoning (Weigl’s Sorting test) [30]; frontal functionality (Frontal Assessment Battery- FAB) [34]; semantic fluency (animals, fruits, car brands), phonological fluency (FAS) [31];
• attention: visual selective attention (Attentive Matrices) Carlesimo et al., simple speed processing and complex attention (Trail Making Test parts A- TMT A and part B- TMT B) [35];
• visuospatial abilities: constructive apraxia Rey Complex Figure copy- RCF-copy [30,33].
At follow-up evaluation, we used a selection of previous tests in order to selectively investigate various features of executive functions. All the test scores were corrected for age, sex, and education and compared with the values available for the Italian population.
Motor performances were also assessed by means of MDS-Unified Parkinson’s Disease Rating Scale, part III, Tinetti balance and gait score and Hauser Index both at the baseline the follow-up evaluation [36-38].
Study design and procedures
This study is a prospective controlled, parallel-group randomized study. At baseline (T0) all the PD patients recruited underwent both cognitive and motor assessments. Patients enrolled were randomized to receive Physical Therapy (PT) or no physical therapy (CT). The physical therapy program consisted of 6 individual sessions/week, each lasting 60 minutes for 4 weeks in addition to their usual pharmacological therapy; while subjects in CT group received only pharmacological therapy. Cognitive and motor performances were evaluated after 4 weeks (T1) by means of the above-mentioned tests to detect the effect of physical therapy on both motor and cognitive performances (T0 vs T1).
Our physical therapy program included a variety of different exercise modalities (aerobic exercises, treadmill training and exercise intervention program) performed under the supervision of a physiotherapist, in order to facilitate goal-directed learning through cognitive engagement (learning through verbal feedback, cues, maintaining motivation and attention, improving awareness.
Statistical analysis
The sample size was calculated on the basis of the global cognitive functioning measured by MoCA, which is more sensitive to executive dysfunction in PD. For the sample-size calculation we considered as primary outcome a basal value of MoCA equal to 24.5 ± 2.9, as reported in Uc et al. [20]. We considered as a clinical significant improvement an increase of 3 points in MoCA. Using a statistical significance threshold of 0.05 for type-1 error and a study statistical power of 80%, we obtained a sample size of 21 patients for group.
Statistical analysis was performed using “Stata” version v.13.0 (StataCorp, Texas). Groups were homogenous for gender, age and years of educations. For categorical variables, differences between groups were tested with the Fisher’s exact test.
Differences between groups (PT vs CT) were analyzed using Student’s t test for paired samples. To assess intra-group differences in the baseline and follow-up conditions, a Student’s t test for paired samples were used. Data are expressed as mean values ± SD.
Moreover, changes in the mean scores between T1 to T0 of the neuropsychological and motor assessments were evaluated in the 2 groups. The percentage change difference (delta) was defined as the difference between the mean score at follow-up (T1) and the baseline as a percentage of one of the numbers (delta = [mean score at T1- mean score at T0]/T0 x 100). Student’s t test for paired samples was used to compare the delta changes for numerical variable while categorical variables were tested with Fisher’s exact test. The results are presented as the percentage differences of the mean values from T0 and as the differences among the study groups. P-value of <0.05 was considered significant.
Our hypothesis was that PT group had a higher probability of maintaining or improving their cognitive level than CT. The scores of the neuropsychological tests were considered as outcome measures.
Results
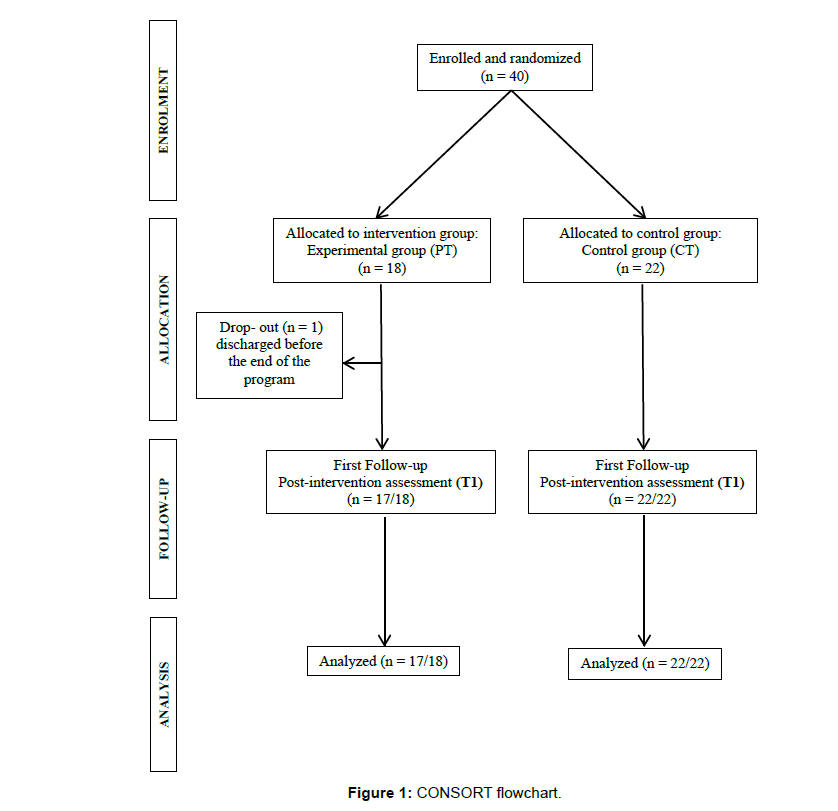
We enrolled 40 PD-MCI patients; 18 subjects were assigned to PT group and 22 to CT group. One subject in the PT group did not complete the training program. Therefore, the final dataset was formed by 17 patients in PT group and 22 in CT group (see CONSORT diagram in Figure 1). Moreover, patients with executive-single-domain PD-MCI were 13/18 in PT and 18/22 in CT respectively, the remaining patients presented multiple-domain PD-MCI.
Patients’ demographic and clinical characteristics are shown in Table 1. Groups were homogeneous for age, sex, education, disease severity stage and disease duration. The two groups were also homogeneous as regards motor scores (Table 2). The cognitive profile of the 2 groups was similar, although they differed for the score at the Digit span test that was significantly lower in the PT group (p= 0.001) (Table 3).
| Tot. | PT | CT | ||
|---|---|---|---|---|
| N. Subjects | 39 | 17 | 22 | |
| Age (year) | 72.4 ± 6.5 | 73.9 ± 7.2 | 71.2 ± 5.8 | p=0.19 |
| Sex (M/F) | 26/13 | 9/8 | 17/5 | p=0.11 |
| Disease duration (year) | 9.7 ± 4.0 | 10.2 ± 5.2 | 9.3 ± 2.9 | p=0.52 |
| Education (years) | 7.9 ± 3.4 | 6.8 ± 2.9 | 8.8 ± 3.5 | p=0.06 |
| Hoehn & Yahr | 2.3 ± 0.3 | 2.3 ± 0.3 | 2.3 ± 0.4 | p=0.33 |
Table 1:Demographic and clinical characteristics.
At T1, PT group showed a significant improvement in motor performances (UPDRS III: p=0.003; Tinetti: p=0.006; Hauser: p=0.012), associated to a parallel improvement in MoCA (p=0.018) and working memory tests (Verbal Span: p=0.003; Digit span: p=0.026).
Regarding our primary outcome (MoCA scores) at T1 11/17 subjects (64.7%) of PT group showed an improvement in MoCA scores, while in CT group the improvement was detected only in 2/22 (9.1%).
At variance, in the CT group we did not detect any significant changes in either the cognitive or the motor scores.
The intergroup analysis showed significantly better results at T1 in PT group than CT group in Tinetti and Hauser (p=0.049 and p=0.003, respectively) as well as in MoCA (p=0.006). Table 2 and Table 3 show comparison within and between groups of motor and cognitive scores, respectively.
| PT (17) | CT (22) | P (between) | |||
|---|---|---|---|---|---|
| p1 | p2 | ||||
| UPDRS-III | T0 | 36.4 ( ± 14.4) | 35.1 ( ± 3.4) | ||
| T1 | 31.2 ( ± 15.3) | 35.4 ( ± 1.8) | |||
| p (within ) | 0.003* | 0.763 | 0.702 | 0.21 | |
| Tinetti | T0 | 14.6 ( ± 6.6) | 13.7 ( ± 4.2) | ||
| T1 | 17.3 ( ± 7.6) | 13.3 ( ± 4.5) | |||
| p (within) | 0.006* | 0.743 | 0.58 | 0.049* | |
| Hauser | T0 | 4.5 ( ± 1.9) | 4.5 ( ± 1.7) | ||
| T1 | 3.3 ( ± 2.0) | 4.6 ( ± 0.9) | |||
| p (within) | 0.012* | 0.6 | 0.86 | 0.003* | |
Data are presented as mean + SD. Student’s t-test for paired sample comparing both T0 and T1 scores within each group separately and the scores between the 2 groups over time. Fisher’s exact test where used for Hauser scores. p1: PT vs CT at T0; p2: PT vs CT at T1. *Significant result.
Table 2: Motor scores in PT and CT groups: comparison within and between groups.
| PT (17) | CT (22) | p(between) | |||
|---|---|---|---|---|---|
| p1 | p2 | ||||
| MMSE | T0 | 24.5 ( ± 2.5) | 24.53 ( ± 2.48) | ||
| T1 | 25.0( ± 1.7) | 24.44 ( ± 2.4) | |||
| P (within) | 0.145 | 0.162 | 0.937 | 0.474 | |
| MoCA | T0 | 19.3 ( ± 0.88) | 17.43 ( ± 2.23) | ||
| T1 | 20.1( ± 0.88) | 17.35 ( ± 2.38) | |||
| P (within) | 0.018* | 0.473 | 0.051 | 0.006* | |
| Verbal Span | T0 | 3.40 ( ± 0.38) | 3.67 ( ± 0.80) | ||
| T1 | 5.05 ( ± 0.52) | 5.14 ( ± 2.88) | |||
| P (within) | 0.003* | 0.007* | 0.205 | 0.926 | |
| Digit Span | T0 | 3.93 ( ± 0.92) | 4.77 ( ± 0.46) | ||
| T1 | 4.41 ( ± 0.64) | 4.36 ( ± 0.52) | |||
| P (within) | 0.026* | 0.001* | <0.001* | 0.796 | |
| Corsi’s block-tapping (CBTT) | T0 | 3.69 ( ± 0.73) | 3.95 ( ± 0.89) | ||
| T1 | 3.94 ( ± 0.99) | 3.86 ( ± 0.83) | |||
| P (within) | 0.253 | 0.328 | 0.326 | 0.793 | |
| Raven’s Matrices 1947 (RM47) | T0 | 20.96( ± 4.98) | 20.5 ( ± 4.95) | ||
| T1 | 22.19 ( ± 4.19) | 20.90 ( ± 4.80) | |||
| P (within) | 0.082 | 0.125 | 0.776 | 0.385 | |
| Weigl’s Sorting test | T0 | 6.59 ( ± 1.73) | 6.46 ( ± 2.78) | ||
| T1 | 6.48 ( ± 1.83) | 6.42 ( ± 2.62) | |||
| P (within) | 0.729 | 0.674 | 0.883 | 0.938 | |
| Frontal Assessment Battery (FAB) | T0 | 11.88 ( ± 2.30) | 12.14 ( ± 2.29) | ||
| T1 | 12.57 ( ± 2.41) | 12.28 ( ± 2.28) | |||
| P (within) | 0.15 | 0.27 | 0.722 | 0.701 | |
| Attentive Matrices | T0 | 39.20 ( ± 6.53) | 40.42 ( ± 9.89) | ||
| T1 | 39.14 ( ± 5.78) | 40.10 ( ± 10.30) | |||
| P (within) | 0.96 | 0.48 | 0.671 | 0.741 | |
| Trail Making Test A (TMT A) | T0 | 143.5 ( ± 45.26) | 127.11 ( ± 36.93) | ||
| T1 | 157.31 ( ± 71.58) | 127.88 ( ± 40.14) | |||
| P (within) | 0.42 | 0.894 | 0.262 | 0.152 | |
| Trail Making Test (TMT B) | T0 | 303.43 ( ± 81.28) | 287.59 ( ± 128.4) | ||
| T1 | 346.31 ( ± 94.15) | 288.18 ( ± 120.69) | |||
| P (within) | 0.077 | 0.916 | 0.677 | 0.135 | |
| Phonological fluency (FAS) | T0 | 25.65 ( ± 8.71) | 24.39 ( ± 8.60) | ||
| T1 | 24.88 ( ± 5.94) | 23.57 ( ± 9.74) | |||
| P (within) | 0.427 | 0.491 | 0.653 | 0.627 | |
Data are presented as mean + SD. Student’s t-test for paired sample comparing both T0 and T1 scores within each group separately and the scores between the 2 groups over time. P1: PT vs CT at T0; p2: PT vs CT at T1. *Significant result.
Table 3: Cognitive scores in PT and CT groups: comparison within and between groups.
The comparison between groups of the percent changes from T0 to T1 in the mean scores at the neuropsychological and motor assessment showed significant different values at the MMSE (p=0.043), MoCA (p=0.003), Digit span (p=0.001), UPDRS-III (p=0.0004), and Hauser (p=0.002) in favour of the PT group (Table 4).
| PT | CT | p-value | |
|---|---|---|---|
| MMSE | 2.4% ± 6.0 | -0.3% ± 1.1 | 0.042* |
| MoCA | 4.6% ± 6.9 | -0.5% ± 2.9 | 0.003* |
| Verbal Span | 18.9% ± 34.9 | -8.2% ± 10.1 | 0.001* |
| DIGIT Span | 4.3% ± 16.3 | -4.3% ± 14.2 | 0.084 |
| Corsi’s block-tapping (CBTT) | 7.9% ± 24.7 | -1.6% ± 9.3 | 0.103 |
| Raven’s Matrices 1947 (RM47) | 8.2% ± 16.7 | 2.5% ± 6.3 | 0.149 |
| Weigl’s Sorting test | 0.5% ± 22.0 | -1.1% ± 3.5 | 0.818 |
| Frontal Assessment Battery (FAB) | 7.4% ± 18.5 | 1.3% ± 5.3 | 0.149 |
| Attentive Matrices | 0.9% ± 11.8 | - 0-9% ± 5.3 | 0.523 |
| Trail Making Test A (TMT A) | 13.3% ± 52.4 | 1.4% ± 13.9 | 0.37 |
| Trail Making Test B (TMT B) | 19.8% ± 39.5 | 1.0% ± 6.8 | 0.062 |
| Phonological fluency (FAS) | 1.2% ± 17.4 | -2.1% ± 21.3 | 0.601 |
| UPDRS-III | -14.4% ± 11.0 | 1.8% ± 12.1 | 0.0004* |
| Tinetti | 20.4% ± 25.5 | 4.3% ± 43.0 | 0.18 |
| Hauser | -30.2% ± 29.4 | 17.3% ± 55.5 | 0.003* |
The statistic used is the percentage difference change from baseline of two groups (PT vs CT). Data are reported as percentage mean ± standard deviation. *Significant result.
Table 4: Delta analysis in percentage mean scores of the neuropsychological and motor scores between T1 and T0, Comparison between groups (PT vs CT) at T1.
Discussion and Conclusions
Cognitive impairment is a well-established outcome of people with PD in the mid-advanced stages of disease, where it represents a major source of eventual treatment-refractory disability and negatively impacts quality of life. Despite the increasing interest and awareness of non-pharmacological therapies for cognitive impairment in neurodegenerative disease, an effective and validated treatment is still lacking.
Our study evaluated the effect of physical therapy on cognitive functions in mid-stage PD-MCI patients. Our findings showed that after our intensive program of physical rehabilitation, PD-MCI subjects improved significantly in their executive and attentive function (MoCA, Verbal Digit and Digit Span) performances. The cognitive improvement was paralleled by an improvement in motor scores (Tinetti and Hauser scores) when compared to CT group. Accordingly, the analysis of the percentage change of neuropsychological performances from T0 to T1 confirmed the effect of PT on cognition, showing a more evident change both in the global cognitive function scores and workingmemory/ attention test scores (MMSE, MoCA, and Digit Span).
Our data are in line with previous studies that showed an improvement mainly in the frontal lobe-based executive functions after physical activity in individuals with mild to moderate PD severity [18,20,23,39]. A possible explanation for the exerciseinduced improvement in cognitive impairment could be represented by a neuroprotective/neurorestorative mechanism [21,40]. On this regards, previous preclinical and clinical studies have shown different mechanisms by which physical activity may affect cognition. Some findings suggested that aerobic exercises increase the levels of serotonin and norepinephrine in brain, which may positively affect non-motor symptoms, particularly in the early stages of PD [12,41,42]. Moreover, exercise may increase the production of growth factors and promote gray and white matter changes, especially in prefrontal region, with a particular effect on executive functions, well known to be compromised in PD [43-45]. All these changes in central nervous system mediated by physical therapy could be reflected in improvement of some aspect of executive functions measured by MoCA which is the most widely used screening test in PD because of its sensitivity to investigate the logicalexecutive dysfunctions in these subjects [46,47].
Since prefrontal cognitive circuits are critically involved in early phases of motor learning, another important component of exercise in PD is cognitive engagement [21].
On this regard, in this study we used a particular rehabilitation approach that combine motor skill practice with cognitive engagement. This training may have helped to promote neuroplasticity that is the most important factor for driving motor and cognitive behavioral improvement in PD. Moreover, the social aspect of an exercise intervention (i.e., more social engagement with study staff, trainers and other participants than if the participant had remained at home) might also contribute to cognitive improvement as previously suggested [25].
On this view, the theory of cognitive reserve can offer an alternative explanation of the positive effect of physical exercise on cognition [4]. This theory states that engagement over the lifespan in cognitively and socially stimulating activities could affect cognitive trajectories in later life. This mechanism could allow elderly people with neurodegenerative disorders to endure longer the neurodegenerative brain damage, manifesting only mild symptoms.
A limitation of our study is the relatively small number of subjects, who were however carefully selected for adequate matching in the experimental groups. Furthermore, the lack of a long-term follow-up assessment of physical program on cognitive and motor performance over time prevented us from verifying the retention of these positive effects and the potential capability of PT to limit the conversion of PD-MCI to PD-dementia. Further studies focused on this topic will be necessary to test these hypotheses.
Nonetheless, our data clearly highlighted an interesting and useful interplay between motor and cognitive features in PD where actually there no valid interventions that could mitigate the progression of cognitive impairment.
Trial registration: ClinicalTrials.gov ID NCT04012086
Funding sources for study: none
Conflict of interest declaration: none
References
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraubet D, et al. (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27: 349-356.
- Orgeta V, McDonald KR, Poliakoff E, Hindle JV, Clare L, et al. (2015) Cognitive training interventions for dementia and mild cognitive impairment in Parkinson’s Disease. Cochrane Database of Systematic Reviews.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, et al. (2017) Dementia prevention, intervention, and care. Lancet 390: 2673-2734.
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, et al. (2010) Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 133: 2196-2209.
- Nucci M, Mapelli D, Mondini S (2012) Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin Exp Res 24: 218-226.
- Zucchella C, Sinforiani E, Tamburin S, Federico A, Mantovaniet E, et al. (2018) The Multidisciplinary Approach to Alzheimer’s Disease and Dementia. A Narrative Review of Non-Pharmacological Treatment. Front Neurol p: 9.
- Lampit A, Hallock H, Valenzuela M (2014) Computerized Cognitive Training in Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis of Effect Modifiers. Gandy S, ed. PLoS Med 11: e1001756.
- Leung IHK, Walton CC, Hallock H, Lewis SJG, Valenzuela M, et al. (2015) Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology 85: 1843-1851.
- Petrelli A, Kaesberg S, Barbe MT, Timmermann L, Rosen JB, et al. (2015) Cognitive training in Parkinson’s disease reduces cognitive decline in the long term. Eur J Neurol 22: 640-647.
- Bernini S, Alloni A, Panzarasa S, Picascia M, Quaglini S, et al. (2019) A computer-based cognitive training in mild cognitive impairment in Parkinson’s disease. Neurorehabilitation 44: 555-567.
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuleyet E, et al. (2004) Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci. 101: 3316-3321.
- Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A (2016) The Therapeutic Potential of Exercise to Improve Mood, Cognition, and Sleep in Parkinson’s Disease. Mov Disord 31: 23-38.
- Amara AW, Memon AA (2018) Effects of Exercise on Non-motor Symptoms in Parkinson’s Disease. Clin Ther 40: 8-15.
- Hindle JV, Petrelli A, Clare L, Kalbe E (2013) Nonpharmacological enhancement of cognitive function in Parkinson’s disease: A systematic review. Mov Disord 28: 1034-1049
- Murray DK, Sacheli MA, Eng JJ, Stoessl AJ (2014) The effects of exercise on cognition in Parkinson’s disease: A systematic review. Transl Neurodegener 3: 1-13.
- McKee KE, Hackney ME (2013) The Effects of Adapted Tango on Spatial Cognition and Disease Severity in Parkinson’s Disease. J Mot Behav. 45: 519-529.
- Ridgel AL, Kim C-H, Fickes EJ, Muller MD, Alberts JL (2011) Changes in executive function after acute bouts of passive cycling in Parkinson’s disease. J Aging Phys Act 19: 87-98.
- Goldman JG, Weintraub D (2015) Advances in the treatment of cognitive impairment in Parkinson’s disease. Mov Disord 30: 1471-1489.
- Uc EY, Doerschug KC, Magnotta V, Dawson D, Thomsenet TR, et al. (2014) Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 83: 413-425.
- Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, et al. (2013) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol 12: 716-726.
- Picelli A (2016) Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson’s disease: a pilot, single-blind, randomized controlled trial. Funct Neurol. 31: 25-31.
- Tanaka K, Quadros AC de, Santos RF, Stella F, Gobbi LTB, et al. (2009) Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn 69: 435-441.
- Frazzitta G, Maestri R, Ghilardi MF, Riboldazzi G, Perini M, et al. (2014) Intensive Rehabilitation Increases BDNF Serum Levels in Parkinsonian Patients. Neurorehabil Neural Repair 28: 163-168.
- David FJ, Robichaud JA, Leurgans SE, et al. (2015) Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov Disord 30: 1657-1663.
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, et al. (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 30: 1591-1601.
- Hoehn MM, Yahr MD (1967) Parkinsonism: Onset, progression and mortality. Neurology 17: 427-442.
- Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M (1996) Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol 3: 198-202.
- Conti S, Bonazzi S, Laiacona M, Masina M, Coralli MV (2015) Montreal Cognitive Assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol Sci 36: 209-214.
- Spinnler H (1987) Italian standardization and classification of Neuropsychological tests. The Italian Group on the Neuropsychological Study of Aging. Ital J Neurol Sci 8: 1-120.
- Carlesimo GA, Caltagirone C, Gainotti G, Fadda L, Gallassi R, et al. (1996) The Mental Deterioration Battery: Normative Data, Diagnostic Reliability and Qualitative Analyses of Cognitive Impairment. Eur Neurol. 36: 378-384.
- Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol Sci 22: 443-447.
- Laiacona M, Inzaghi MG, De Tanti A, Capitani E (2000) Wisconsin card sorting test: A new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurol Sci 21: 279-291.
- Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, et al. (2005) The Frontal Assessment Battery (FAB): Normative values in an Italian population sample. Neurol Sci 26: 108-116.
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, et al. (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 17: 305-309.
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, et al. (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 23: 2129-2170.
- Tinetti ME (1986) Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 34: 119-126.
- Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, et al. (1983) Intensive Immunosuppression in Progressive Multiple Sclerosis. A randomized, three-arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med. 308: 173-180.
- Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, et al. (2011) Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand 123: 13-19.
- Hou L, Chen W, Liu X, Qiao D, Zhou F-M (2017) Exercise-Induced Neuroprotection of the Nigrostriatal Dopamine System in Parkinson’s Disease. Front Aging Neurosci 9: 358.
- Sayal N (2015) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci 22: 3017-3022.
- Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, et al. (2013) Serotonin Is Required for Exercise-Induced Adult Hippocampal Neurogenesis. J Neurosci 33: 8270-8275
- Fontanesi C, Kvint S, Frazzitta G, Bera R, Ferrazzoli D, et al. (2016) Intensive Rehabilitation Enhances Lymphocyte BDNF-TrkB Signaling in Patients With Parkinson’s Disease. Neurorehabil Neural Repair 30: 411-418.
- Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, et al. (2014) BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci 8: 985.
- Ahlskog JE (2011) Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 77: 288-294.
- Burdick DJ, Cholerton B, Watson GS, Siderowf A, John Q, et al. (2014) People with Parkinson’s disease and normal MMSE score have a broad range of cognitive performance. Mov Disord 29: 1258-1264.
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, MacAskill MR, Livingston L, et al. (2010) The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology 75: 1717-1725.
Citation: Avenali M, Picascia M, Minafra B, Tassorelli C, Sinforiani E, et al. (2019) Intensive Physical Therapy Mitigates Cognitive Decline in People with Parkinson’s disease. J Alzheimers Dis Parkinsonism 9: 475. DOI: 10.4172/2161-0460.1000475
Copyright: © 2019 Avenali M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3986
- [From(publication date): 0-2019 - Dec 01, 2025]
- Breakdown by view type
- HTML page views: 3097
- PDF downloads: 889