Investigation of Gastroprotective Potential of Grape Seed Proanthocyanidin Exract in Experimental Models of Gastric Ulcer, in Wistar Rats
Received: 01-Mar-2018 / Accepted Date: 10-Apr-2018 / Published Date: 16-Apr-2018 DOI: 10.4172/2161-069X.1000561
Abstract
Aim and Objective: To investigate the anti-ulcer activity of grape seed proanthocyanidin extract (GSPE) in various experimental models of gastric ulcer, in wistar rats.
Materials and Methods: Rats were assigned to 5 groups in each model; Normal control, Vehicle control (5% CMC treated), GSPE (50 mg/kg, p.o. 4 days), GSPE (100 mg/kg, p.o. 4 days), Famotidine (40 mg/kg, p.o) or Omeprazole (50 mg/kg, p.o.). Three animal models used in study were (n=6) 1. Pylorus ligation model: After 4 days of pretreatment, fasted rats were anaesthized and abdomen was incised and pylorus part was ligated. Immediately after ligation drug and vehicle was administered. 4 hours later, animals were sacrificed, abdomen was opened and stomach was removed and assessed; Indomethacin treated model: In this model after 4 days of pretreatment, indomethacin (50 mg/kg) was given to the fasted rats to induce ulceration. After 5 hour rats were sacrificed and stomach was removed and assessed; Stress induced ulcer model: After 4 days of GSPE pretreatment, on day 4th fasted animals were immobilized in restrainer. The restrainer was exposed to cold environment (4-7°C) to produce gastric ulcer. At the end of 3 hour stress exposure animals were sacrificed stomach was removed for ulcer assessment.
Results: Gastric acidity and ulcer index in pylorus ligation models were significantly decreased on GSPE (100 mg/kg) drug treatment. Administration of GSPE extract led to significant decrease in MDA levels, in both 50 and 100 mg/kg treated groups. Similar results were obtained in case nitrite/nitrate and MPO levels in each model. Furthermore, the obtained results revealed that pylorus ligation; indomethacin and stress models also significantly decreased the anti-oxidant levels like catalase, SOD and GSH. Antioxidant levels were lowered in vehicle treated control rats in each model and these levels were significantly increased in GSPE treated groups in each model.
Discussion: In the present study GSPE (100 mg/kg) prevented both the increase in markers of oxidative stress and the decrease in levels of endogenous oxidants. In contrast of Famotidine and omeprazole only prevented the increase in the markers of oxidative stress.
Conclusion: Hence, the GSPE higher doses are a better therapeutic option for the treatment of gastric ulcer in terms of better therapeutic health benefits and lesser side effects.
Keywords: Gastric ulcer; Indomethacin; Gastric acidity; Oxidative stress
Abbreviations
GSPE: Grape Seed Proanthocyanidin Extract; GSH: Glutathione; SOD: Superoxide Dismutase; CAT: Catalase; PL: Pylorus Ligation; UI: Ulcer Index; TBARS: Thio Barbituric Reactive Substances
Introduction
Herbs are safer to use and has fewer side effects, than synthetic western medicines, but only with the correct usages [1]. Grape seed extract, a waste product of wine and grape juice industry, contain various chemical compounds but the active chief chemical constituents are polyphenolic flavanoid called proanthocyanidin (PAs) [2,3]. PAs are of great interest in nutrition and medicine because of their potent antioxidant capacity. In addition to their free radical scavenging and antioxidant activity, PAs have been reported to have antibacterial, antiviral, anticarcinogenic, antiallergic and vasodilatory actions [4].
GSPE, belongs to flavanoid group is obtained from the seeds of Vitis vinifera grapes (red), which contains 95% proanthocyanidins [2]. Invitro studies using chemiluminescence assay demonstrated that GSPE exhibited superior antioxidant activity as compared to vitamin C, E and betacarotene [5,6]. GSPE shows protective effect against diabetic neuropathy in relation to its antioxidant properties [7]. GSPE suppresses ROS generation in-vitro , in human umblical vein endothelial cells (HUVEC) [8]. Proanthocyanidins have been shown to exert a novel spectrum of biological, pharmacological, therapeutic and also chemoprotective properties against oxidative stress [9].
Proanthocyanidin from grape seeds dose-dependently inhibited the paw edema in rats induced by carageenan and ear edema of mice induced by croton oil, which may be due to inhibition of prostaglandins, stabilization of lysosomal membrane and decreased formation of lipoperoxidation products. Also, GSPE has been shown to produce anti-arthiritic effect in collagen-induced arthiritis in mice through a reduction in the production of type 2 collagen specific IgG2a and inflammatory cytokines such as TNF-α and IL-17. In addition, GSPE also suppressed osteoclastogenesis in mice both in-vivo and invitro [10] and shown to produce protection against ethanol-induced ulcer in rats [11]. GSPE also shows protective effect in 2, 4, 6-trinitro benzene sulfonic acid (TNBS) induced ulcerative colitis in rats [12]. Hence the present study was designed to investigate the gastroprotective potential of GSPE in detail.
Gastric ulcer is a common global disease with increasing incidence and prevalence, despite significant medicinal advances. Despite the widespread use of conventional antiulcer therapy that effectively reduces gastric acid secretion, ulcers frequently recur, with 1 year recurrence rates estimated to range from 60% to 100% [13].
Gastric ulcer is a serious gastrointestinal (GI) disorder, and occurs when the gastric mucosa gets damaged, leading to perforations of the stomach lining and even bleeding. Typically, gastric ulcer is referred to that restricted to the stomach, while ulcers of the stomach and duodenum together are known as peptic ulcers [14]. Gastric and duodenal ulcers affect thousands of people and are currently considered a global health problem [15]. It is a complex pluricausal disease and is known to develop due to imbalance between aggressive and protective factors [16,17]. Several endogenous and exogenous factors are responsible for gastric ulceration. These include H. pylori infection, increased production of gastric acids, pepsin and stomach juices, certain types of medicines, notably the non-steroidal antiinflammatory drugs (NSAIDs), and even personal factors such as consumption of tobacco, alcohol and caffeine, as well as emotional and physical stresses [18].
In traditional medicine, several plants and herbs have been used to treat gastrointestinal disorders, including gastric ulcers [19]. Herbal drugs obtained from the plant source are relatively less expensive, safe, and possess good tolerability even in higher doses [20].
Materials and Methods
Grape seed proanthocyanidin extract was purchased from Bio-gen extracts (P) Ltd Banglore, India. Indomethacin was obtained from Jagsonpal Pharmaceuticals Ltd, Rudrapur, India; Omeprazole from Ranbaxy Labs., Mumbai, India and Famotidine from Cipla Ltd, India were used. All other reagents or chemicals used were of analytical grade. Animal feed was purchased from Ashirwad Industries, Ropar, India. Animals used were wistar rats of either sex weighing 200-240 g were procured from the Animal House, ISF College of Pharmacy, Moga. The animals were kept in polypropylene cages (3 rats in each cage) at an ambient temperature 25 ± 2°C and relative humidity 55-65%. A 12-12 hour light and dark schedule was maintained in the animal house. The rats were fed over commercially available feed (Aashirwad Industries, Ropar, and Punjab) and water ad libitum. Experimental protocol was approved by Institutional Animal Ethical Committee (IAEC) and was conducted as per the CPCSEA guidelines for animal experimentation and care.
Indomethacin-induced ulcer in rats
All the rats except control rats were pre-treated with test drugs for 4 days. On day fourth, indomethacin (50 mg/kg, p.o.) suspended in 0.5% CMC was given to all the groups to induce acute gastric ulcer, after 30 min of test drug treatments. After 5 hour, the animals were sacrificed; stomach dissected out and was examined for ulcer index [21]. The fraction showing maximum efficacy in this model had been subjected to estimate its efficacy on pylorus ligation induced ulcer model in rat. Animals were divided into 5 different groups (n=6/grp). 5 groups were made in each model; normal control, Ind. Vehicle control, GSPE (50 mg/kg, p.o., 4 days), GSPE (100 mg/kg, p.o., 4 days) and Famotidine (40 mg/kg, p.o.).
Pylorus ligation induced ulcer model
Firstly, Animals were fasted for 24 hour and anesthetized with thiopental sodium (40 mg/kg, i.p.). Rats were pretreated with test drugs for 4 days. On day fourth, the abdomen was incised and pylorus ligated. Immediately after pylorus ligature, all the drugs and vehicle were administered. Four hours later, the animals were sacrificed; the abdomen was opened, and another ligature placed at the oesophageal end. The stomachs were removed, and the gastric contents collected and centrifuged at 3000 rpm [22]. In pylorus ligation induced ulcer model 5 groups were made, normal control, Ind. Vehicle control, GSPE (50 mg/kg, p.o., 4 days), GSPE (100 mg/kg, p.o., 4 days) and Omeprazole (50 mg/kg, p.o.).
Stress induced ulcer model
Rats were pretreated with test drugs for 4 days. On day fourth, 24-48 hour fasted animals were immoblized in rigid plastic devices that facilitated precise standardization of restraint volume and conditions [23]. Restrainer was exposed to cold environment (4-7°C) to produce gastric ulcers. At the end of the 3 hour stress exposure, the animals were killed by decapitation and stomachs were removed for measuring the areas of erosion [24]. The ulcer index was calculated as the sum of the length (mm) of each injury per rat. In this model, 5 groups were made; normal control, Stress Vehicle control, GSPE (50 mg/kg, p.o., 4 days), GSPE (100 mg/kg, p.o., 4 days) and Omeprazole (50 mg/kg, p.o.).
Drug administration
In pyloric ligation induced ulcer model, the grape seed proanthocyanidin extract and its fractions were suspended in 0.5% w/v carboxymethylcellulose (CMC) and administered orally with the help of gastric canula immediately after pylorus ligation.
Assessment of ulcer index and gastric acidity
Ulcer index (UI) was determined as sum of the length (mm) of all lesions for each stomach and the protection percentage was calculated from the following formula [25]:
% Protection=[(UIcontrol − UI treated) / UI control] × 100
The total acid secretion in the gastric juice in the supernatant volume was determined by titration using a 0.01 mol NaOH solution, and phenolphthalein as indicator. Acidity was calculated by using the formula:
Total Acidity=(vol of NaOH × normality of NaOH) / 0.1 × 100 mEq/L
Determination of pH and gastric volume
The pH of the gastric juice was measured using the pH meter Gastric volume was measured after centrifuging the gastric fluid, allowed to stand, decant, and poured into the measuring cylinder of graduation 0.01 ml.
Biochemical estimations
Measurement of Lipid-peroxidation: Lipid-peroxidation, as evidenced by the formation of thiobarbituric acid reactive substances (TBARS) was measured by using the thiobarbituric acid test of [26]. In brief, 0.2 ml of homogenate was added to 0.8% thiobarbituric acid, 8.1% sodium dodecyl sulfate (SDS) and acetic acid (20%) in distilled water. After heating for 60 min in a water bath at 95°C, the mixture was then cooled and extracted with a mixture of n-butanol/pyridine (15:1,v:v). The absorbance of the reaction product present in the upper organic layer separated by centrifugation was measured spectrophotometrically at 532 nm. The results were expressed in μM of MDA/mg of protein.
Estimation of antioxidant enzyme levels
Superoxide dismutase (SOD): SOD was assessed by utilizing the technique [27]. A single unit of enzyme was expressed as % inhibition of nitroblutetrazolium (NBT) reduction/min. The results were expressed in SOD units/mg of protein.
Catalase (CAT): CAT was assayed colorimetrically at 620 nm and expressed as μmoles of H2O2 consumed/min/mg protein as described by Sinha et al. [28]. In brief, a mixture (1.5 ml) contained 1.0 ml of 0.01 M phosphate buffer ph 7.0, 0.2 ml of tissue homogenate and 0.4 ml of 2 M H2O2. The reaction was stopped by addition of 0.2 ml of dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). The results were expressed in H2O2 moles/mg of protein.
Reduced Glutathione (GSH): GSH was estimated by the method of Ellman [29]. In brief, 10% TCA was added to the homogenate and the mixture was centrifuged 1.0 ml of supernatant was treated with 0.5 ml of Ellmans reagent (19.8 mg of 5,5’-dithiobisnitro benzoic acid in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2 M, pH 8.0). The absorbance was measured spectrophotometrically at 410 nm. The results were expressed in mg of GSH/g of protein.
Estimation of nitrite/nitrate: Nitrite level in tissue homogenate was estimated using Greiss reagent method. In brief, 200 μl of protein-free supernatant, 30 μl of 10% NaOH was added followed by 300 μl of tris- HCl buffer and mixed well. To this, 530 μl of Griess reagent (0.3% N-1[Napthyl-ethylene diamine-dihydrochloride] in distilled water+3% Sulphanilamide in 1 M HCl) was added and incubated in the dark for 10-15 minutes, and the absorbance was read at 540 nm (Spectrophotometer, Beckman DU 640B, and Switzerland). The results were expressed in μM of nitrite/mg of protein.
Estimation of Myeloperoxidase (MPO) activity: MPO activity, an indicator of polymorphonuclear leukocyte (PMN) accumulation was determined as described previously [30]. Firstly tissue was homogenized in a solution containing 0.5% (w/v) hexadecyltrimethylammonium bromide (Sigma-aldrich) dissolved in 10 mM potassium phosphate buffer (pH 7), and centrifuged for 30 min at 20,000 g at 4˚C. An aliquot of the supernatant was then allowed to react with a solution of tetramethylbenzidine (1.6 mM) and 0.1 mM hydrogen peroxide. The rate of change in absorbance was measured spectrophotometrically at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μmol peroxide min-1 at 37˚C and was expressed in milliunits per g wet tissue [30].
Statistical Analysis
The results were expressed as mean ± standard deviation (S.D) and analyzed using analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. P-value <0.05 was considered as statistically significant. Statistical analysis was performed using Graph Pad Instant Software, (Version 3.01).
Results
Combined results of three models (Indomethacin induced, pylorus ligation induced and stress induced model for gastric ulcer).
Effect of various doses of grape seed proanthocyanidin extract on ulcer index in 3 models of gastric ulceration
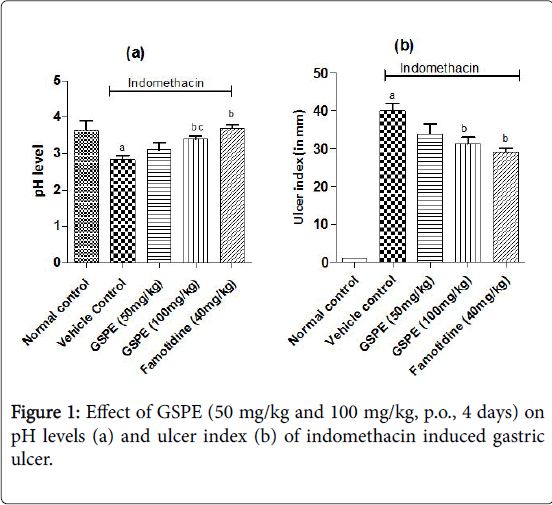
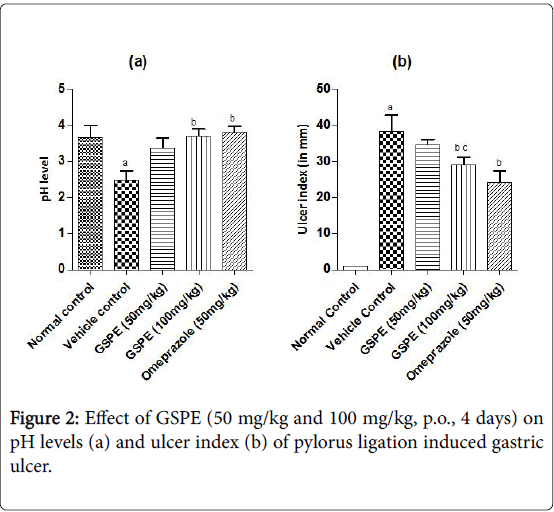
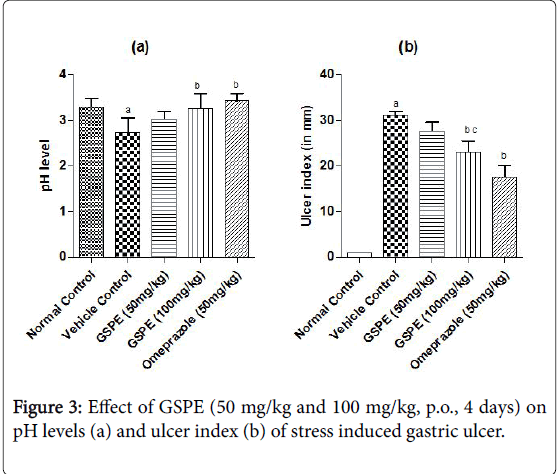
The ulcer index was increased markedly in the vehicle treated indomethacin induced (Figure 1), pylorus ligation induced (Figure 2) and stress induced gastric ulcer (Figure 3) group as compared to the respective normal control group. However, repeated administration of GSPE (50 and 100 mg/kg, p.o) for 4 days shown significant and dose dependent decrease in ulcer index. Similarly, standard drug treatment (Omeprazole 50 mg/kg or Famotidine 40 mg/kg, p.o) significantly decreased the ulcer index as compared to the vehicle control group Figures 1b, 2b and 3b.
Indomethacin induced gastric ulcer
Values are expressed as mean ± S.D; n=6; a denotes for P
Values are expressed as mean ± S.D; n=6; a denotes for P
Stress induced gastric ulcer
Values are expressed as mean ± S.D; n=6; a denotes for P
Effect of various doses of grape seed proanthocyanidin extract on pH levels
The pH levels were significantly acidic in vehicle treated indomethacin induced, pylorus ligation induced and stress induced gastric ulcer group as compared to the respective normal control group shown in Figures 1a, 2a and 3a. However, repeated administration of GSPE (100 mg/kg, p.o) for 4 days shown remarkable increase the pH levels. GSPE (50 mg/kg and 100 mg/kg, p.o) significantly increased the gastric pH level towards the normal. Standard drug treatment (Omeprazole 50 mg/kg or Famotidine 40 mg/kg, p.o) also increased the gastric pH greatly to significant levels as compared to vehicle control group.
Effect of various doses of grape seed proanthocyanidin extract on TBARS level
There was a significant increase in mucosal TBARS level (measured as MDA) was observed in vehicle control group in each model to the levels of 14.98 in indomethacin induced model, 16.09 in pylorus ligation induced and 15.57 in stress induced model for gastric ulcer. Treatment with GSPE (50 mg/kg and 100 mg/kg, p.o) produced a dose dependent decrease in the level of TBARS. GSPE treatment has produced greater decrease in TBARS level even as compared to that of standard drug treatment group (Omeprazole 50 mg/kg or Famotidine 40 mg/kg, p.o).
Effect of various doses of grape seed proanthocyanidin extract on nitrite/nitrate levels
A significant increase in nitrite/nitrate level was observed in the vehicle control rats as compared to the normal control rats. Nitrite/ nitrate levels were reduced on treatment with GSPE (50 mg/kg, p.o) in a significant and dose dependent manner. However this decrease was significant on GSPE (100 mg/kg, p.o) treatment in various ulcer models. The decrease in nitrite/nitrate levels was greater in GSPE (100 mg/kg, p.o) treatment group as compared to standard drug (Omeprazole 50 mg/kg or Famotidine 100 mg/kg, p.o) treatment group, but the difference was not statistically significant.
Effect of various doses of grape seed proanthocyanidin extract on myeloperoxidase levels
The MPO activity in mucosal tissue was significantly increased in vehicle control group as compared to the normal control group. Treatment with GSPE 50 mg/kg and 100 mg/kg significantly decreased the MPO levels as compared to the vehicle control group. MPO levels were noted 0.69 in indomethacin induced model, 0.78 in pylorus ligation model and 0.70 in stress induced model for gastric ulcer on GSPE (100 mg/kg, p.o) drug treatment. Compared to that of vehicle control group, GSPE (100 mg/kg) treatment was effective as the standard drug treatment group.
Effect of various doses of grape seed proanthocyanidin extract on SOD levels
SOD levels in various groups are shown in indomethacin induced model, pylorus ligation model and stress induced model in gastric ulcer. SOD levels were significantly decreased in vehicle control group in each model as compared to the normal control, and levels were slightly recovered on GSPE (50 mg/kg, p.o) drug treatment.
SOD levels were significantly and dose dependently increased on GSPE drug treatment as compared to vehicle control group. The increased level was significantly greater than that with standard drug treatment (Omeprazole 50 mg/kg or Famotidine 40 mg/kg, p.o).
Effect of various doses of grape seed proanthocyanidin extract on catalase levels
A significant decrease in the catalase levels was noted in the vehicle control rats. The levels of catalase in vehicle control were 12.33 in indomethacin induced model, 12.4 in pylorus ligation model and 10.7 in stress induced model for gastric ulcer and these levels were increased to the levels of 17.08, 17.18 and 19.9 in respective models on GSPE (100 mg/kg) treatment.
GSPE (100 mg/kg) treatment markedly increased the catalase levels as compared to the vehicle control group. Therefore, GSPE treatment has shown dose dependent increase in catalase levels. The maximum increase in the catalase levels was shown by GSPE (100 mg/kg) treatment group.
Effect of various doses of grape seed proanthocyanidin extract on glutathione levels
The levels of glutathione were significantly decreased in the vehicle control group as compared to the normal control group. These levels were significantly and dose dependently increased on GSPE (50 mg/kg) and GSPE (100 mg/kg) treatment group. GSPE (100 mg/kg) treatment group increased the GSH levels to a similar extent as that of standard drug treatment group. GSH levels got recovered on GSPE treatment.
Pylorus ligation model for gastric ulcer
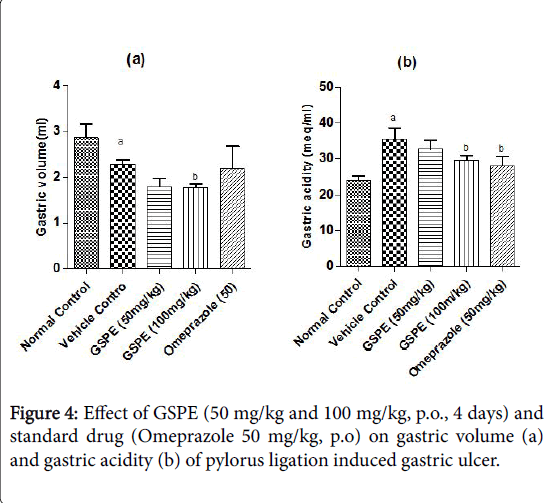
Effect of various doses of grape seed proanthocyanidin extract (GSPE) on gastric volume and gastric acid secretion in pylorus ligation model of gastric ulcer: In pylorus ligated vehicle control group gastric vol. was significantly decreased as compared to the normal control group shown in Figure 4a. Administration of GSPE (50 mg/kg and 100 mg/kg, p.o; 4 days) significantly increased the gastric volume as compared to vehicle control rats.
The effect of GSPE (100 mg/kg) was not statistically significant from that of standard drug Omeprazole (50 mg/kg, p.o) treatment. A marked increase in the levels of gastric acidity was seen in pylorus ligated vehicle control rats Figure 4b. Repeated administration of GSPE (50 mg/kg) decreased the gastric acidity, and was more significant on treatment with GSPE (100 mg/kg) dose. Further on treatment with omeprazole (50 mg/kg) significantly decreased the gastric acidity levels. Gastric acidity levels in pylorus ligation model are depicted in Figure 3b.
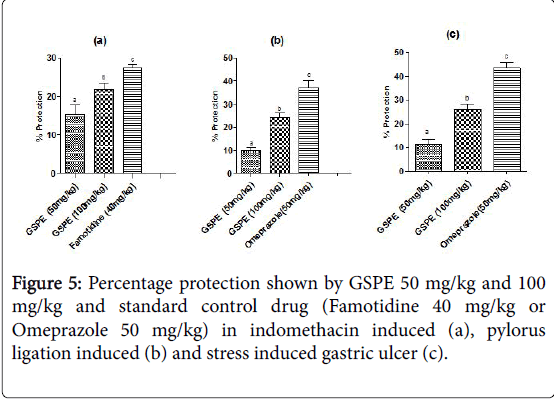
Percentage protection shown by various doses of gspe on experimental models of gastric ulcer (Figure 5): Percentage protection shown by indomethacin induced model of ulcer was 15.31 on GSPE (50 mg/kg) treatment, 21.85 on GSPE (100 mg/kg) treatment and 27.35 on Famotidine (40 mg/kg) drug treatment.
In case of pylorus ligation induced gastric ulcer % protection was 9.89 in GSPE (50 mg/kg), 24.14 in GSPE (100 mg/kg) and 37.30 in Omeprazole (50 mg/kg) treatment group. In stress induced model of gastric ulcer % protection was 11.42, 25.98 and 43.52 in the respective groups.
Values are expressed as mean ± S.D; n=6; a denotes for P
Percentage protection shown by various doses of gspe on experimental models of gastric ulcer
Values are expressed in mean ± S.D; n=6; a denotes for P
Discussion
Medicinal plants are among the most attractive sources of new drugs, and have been shown to produce promising results for the treatment of gastric ulcer [31]. Grape seed proanthocyanidin extract (GSPE) is safe for consumption as its LD50 was found to be higher than 5000 mg/kg, when administered orally to fasted male and female albino rats. In addition, 2000 mg/kg body weight was found to be a noobserved- effect-level (NOEL) for systemic toxicity [6,32]. A large number of herbal extracts are used in folk medicine to treat various types of digestive disorders [19,33]. Grape seed proanthocyanidin extract is an herbal extract containing 95% proanthocyanidin having potent anti-oxidant properties [34].
Indomethacin, a NSAID induced ulcer in rat is a widely used experimental model to assess the pathophysiology of ulcer induced by NSAIDs and screening of gastroprotective agents. It induces gastric lesions through a number of mechanisms which includes inhibition of prostaglandin synthesis, increased expression of interleukin-1 (IL-1), generation of reactive oxygen species (ROS), and induction of apoptosis [35,36]. Although various mechanisms are involved in the pathogenesis of pyloric ligation induced peptic ulcer, gastric acid secretion and accumulation are thought to be the most important factors. In addition it is well established that various antisecretory agents, such as H2 receptor antagonists and proton pump inhibitors prevent gastric lesions [37].
Stress can arise from prolonged anxiety, tension, and emotion, severe physical discomfort, haemorrhage and surgical shock, burns and trauma, thereby resulting in severe gastric ulceration. The mechanism of gastric ulceration is poorly understood. Recently research has shown that cold stress causes severe haemorrhage ulcer through derangement of the mucosal antioxidant enzyme such as super oxide, dismutase and peroxides. NSAIDs are known to induce gastric ulcer not only by denaturing mucous glycoproteins but also by free radical formation [38]. Stress causes an ischemic condition in the gastric mucosa by the activation of the parasympathetic and sympathetic nervous systems resulting in vasoconstriction, which in turn causes free radical generation.
Further, stress has also been found to inactivate mucosal prostaglandin synthase by accumulating H2O2, which in turn inhibits the synthesis of prostaglandins known to favour the generation of reactive oxygen species [39]. Cold restraint stress model is commonly used for evaluating drugs having gastroprotective activity by virtue of its anti-stress effect. Pylorus ligation method is used for evaluating drugs which are effective by decreasing acid and pepsin secretion [22]. Thus it can be assumed from above observation that the gastroprotective activity of GSPE is unique as compared to most conventional anti-ulcer drugs as it is not based mainly on acid and pepsin secretion, or acid does not play major role in stress related mucosal disorder.
The present study confirms observations in our earlier study on gastro-protective potential of grape seed proanthocyanidin extract in NSAIDs-induced gastric ulcer in wistar rats [40]. In the present study preliminary results showed that GSPE single dose treatment did not show any significant effect in pylorus ligation-induced ulcer model (data not shown). However, repeated pre-treatment for 4 days in all the models of gastric ulcer showed positive results. Administration of investigated extract led to significant decrease in MDA levels, in both GSPE 50 mg/kg and GSPE 100 mg/kg treated groups. Standard control drug treatment showed even lesser decrease in MDA units as compared to the GSPE 100 mg/kg treatment group. Similar results were obtained in case nitrite/nitrate and myeloperoxidase levels in each model.
Sulfhydryl compounds such as GSH play an important role in scavenging reactive oxygen species and have been implicated to protect the gastric mucosa [41]. The treatment of experimental animals with doses of 50 mg/kg and 100 mg/kg of Grape seed proanthocyanidin extract produced a moderate, but significant and dose-dependent increase in activity of GSH in gastric mucosa. Increase in GSH shows that extract was able to diminish the excessive production of free radicals provoked by indomethacin and pylorus ligation. Furthermore, the obtained results revealed that pylorus ligation; indomethacin and stress models also significantly decreased the anti-oxidant levels like catalase, SOD and GSH. Antioxidant levels were lowered in vehicle treated control rats in each model and these levels were significantly increased in GSPE treated groups in each model.
Several studies have demonstrated that nitrite/nitrate production is involved in the genesis of intestinal inflammation and that its inhibition can reduce inflammatory damage [42,43]. The increase in nitrite/nitrate in ulceration models was associated with an elevated expression of iNOS and NO production [44,45]. NO, could rapidly interact with oxygen free radicals to yield a variety of cytotoxic nitrogen species that induce lipid peroxidation and other cellular oxidative stress [46]. The present study showed that GSPE remarkably decrease the elevated levels of nitrite/nitrate. This may be due to GSPE induced decrease in iNOS and NO activity in gastric mucosa [47]. In similar study anti-ulcer activities of grape seed extracts GSE-1 (with low flavanol content), GSE-2 (with high flavanol content) and procyanidins at a dose of 200 mg/kg shown to inhibit the stomach mucosal injury induced by ethanol. The observed protection by free radical scavenging activity and the defence action of procyanidins covering the stomach surface by strong ability to bind protein.
It is known that a variety of cytokines such as interleukin -1β, interleukin-8 and tumour necrosis factor α are expressed in ulcerated mucosa and they exaggerated the inflammatory response. Increased H₂O₂ production has also been reported to increase the production of IL-17 and TNF-α [48,49]. GSPE reduces the production of intracellular H₂O₂ by T-cell-receptor-stimulated CD4+ splenocytes [6,50-55]. GSPE induce decrease in TNBS (2,4,6-trinitrobenzoic acid) was associated with decreased production of pro-inflammatory cytokine IL-1β in the colon in rats [7]. Similarly GSPE might supress the production of these pro-inflammatory cytokine and subsequent oxido-nitrostative stresses.
Taken as a whole, these data provide evidence that reactive oxygen species play an important role in the pathogenesis of gastric ulceration [56-58]. Thus, scavenging free radicals, reducing the depletion of endogenous anti-oxidant enzymes, and counteracting GSH deficiency could be the target for therapeutic intervention in gastric ulcer [59]. In addition, GSPE (50 mg/kg and 100 mg/kg) treatment facilitated the recovery of damaged mucosal tissue [60]. These studies have implicated the generation of oxygen-derived free radicals and lipid peroxidation as one of the most important mechanisms involved in the pathogenesis of gastric ulcer [61-63]. Therefore, the grape seed proanthocyanidin might exert its activity in one or more aforementioned assays. This provides favourable results showing gastroprotective potential of GSPE in treatment of disorders caused by oxidative stress [64,65].
Conclusion
On the basis of above data, it may be concluded that various doses of GSPE have shown anti-ulcer effect in wistar rats. Also, GSPE treatment produced significant gastro-protective effects against indomethacin-induced, pylorus ligation induced and stress induced gastric damage. Hence, the GSPE higher doses are a better therapeutic option for the treatment of gastric ulcer in terms of better therapeutic health benefits and lesser side effects.
References
- Vanden Hoek TL, Shao ZH (2003) Herbal antioxidants: Cardiovascular potential and danger. Textbook of complimentary and alternative medicine. Partenon, New York, pp: 45-58.
- Wren AF, Cleary M, Frantz C, Melton S, Norris L (2002) 90-day oral toxicity study of a grape seed extract (IH636) in rats. J Agric Food Chem 50: 2180.
- Shi J, Yu J, Pohrly JE, Kakud Y (2003) Polyphenolics in grape seed biochemistry and functionality. J Med Food 6: 291-299.
- Song X, Siriwardhana N, Rathore K, Lin D, Wang HC (2010) Grape seed proanthocyanidin suppression of breast cell carcinogenesis induced by chronic exposure to combined 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene. Mol Carcinog 49: 450-463.
- Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, et al. (1997) Oxygen    free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commn Mol Path Pharmacol 95: 179-189.
- Bagchi, D, Bagchi M, Stohs SJ, Das DK, Ray SD, et al. (2000) Free radicals and grape proanthocyanidins extract: importance in human health and disease prevention. J Toxicology 148: 187-197.
- Li XL, Cai YQ, Qin H, Wu YJ (2008) Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS- induced ulcerative colitis. Can J Physiol Pharmacol 86: 841-849.
- Zhang FL, Gao HQ, Wu JM, Ma YB, You BA, et al. (2006) Selective inhibition by grape seed proanthocyanidin extracts of cell adhesion molecule expression induced by advanced glycation end products in endothelial cells. J Cardiovasc Pharmacol 48: 47-53.
- Ye X, Krohn RL, Liu W, Joshi SS, Kuszyski CA (1999) The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultures human cancer cells. Mol Cell Biochem 196: 99-108.
- Chao CL, Chang NC, Weng CS, Lee KR, Kao ST, et al. (2011) Grape seed extract ameliorates tumor necrosis factor-α-induced inflammatory status of human umbilical vein endothelial cells. Eur J Nutr 50: 401-409.
- Saito M, Hosoyama H, Ariga T, Kataoka S, Yamaji N (1998) Antiulcer activity of grape seed extract and procyanidins. J Agric Food Chem 46: 1460-1464.
- Wang YH, Ge B, Yang XL, Zhai J, Yang LN, et al. (2011) Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int Immunopharmacol 11: 1620-1627.
- Glavin GB, Szabo S (1992) Experimental gastric mucosal injury: laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. FASEB J 6: 825-831.
- Klopell FC, Lemos M, Sousa JPB, Comunello E, Maistro EL, et al. (2002) Nerolidol, na antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Zeitschrift fur Naturforschung 62: 537-542.
- Szabo S (1981) Mechanisms of mucosal injury in the stomach and duodenum: time sequence analysis of morphologic, functional, biochemical and histochemical studies. Scan J Gastroenterol 22: 21-28.
- Koda-Kimble MA, Young LY, Alldredge BK, Corelli RL, Guglielmo BJ, et al. (2009) Applied therapeutics: The clinical use of drugs. (9th Edn) Lippincott Williams & Wilkins inc.
- Tomtitchong P, Siribumrungwong B, Vilaichone RK, Kasetsuwan P, Matsukura N, et al. (2012) Systematic review and meta-analysis: Helicobacter pylori eradication therapy after simple closure of perforated duodenal ulcer. Helicobacter 17: 148-152.
- Hiruma-Lima CA, Gracioso JS, Bighetti EJ, Grassi-Kassisse DM, Nunes DS, et al. (2002) Effect of essential oil obtained from Croton cajucara Benth. on gastric ulcer healing and protective factors of the gastric mucosa. Phytomedicine 9: 523-529.
- Sairam K, Rao CV, Goel RK (2001) Effect of Centellaasiatica Linn. on physical and chemical factors induced gastric ulceration and secretion in rats. Indian journal of Experimental Biology 39: 137-142.
- Santin JR, Lemos M, Júnior LCK, Niero R (2010) Antiulcer effects of Achyrocline satureoides (Lam.) DC (Asteraceae) (Marcela), a folk medicine plant, in different experimental models. J Ethnopharmacol 130: 334-339.
- Shay H, Komarou SA, Fells SS, Meranze D, Grueinstein M, et al. (1945) A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5: 43-61.
- Yelken B, Dorman T, Erkasap S, Dundar E, Tanriverdi B (1999) Clonidine pretreatment inhibits stress-induced gastric ulcer in rats. Anesth Analg 89: 159-162.
- Filaretova LP, Filaretov AA, Makara GB (1998) Corticosterone increase inhibits stress-induced gastric erosions in rats. Am J Physiol Gastrointest Liver Physiol 274: G1024-G1030.
- Njar VCO, Adesanwo JK, Makinde JM, Jaiwo OB (1994) Antiulcer activity of the stem bark extract of Entandrophargma angolenses. Phytotherpia Research 8: 44-48.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21: 130.
- Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47: 389-394.
- Ellman, Davies MH, Birt DF, Schnell RC (1984) Direct enzymatic assay for reduced and oxidized glutathione. J Pharmacol Methods 12: 191-194.
- Mullane KM, Kraemer R, Smith B (1985) Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 14: 157-167.
- Borrelli F, Izzo AA (2000) The plant kingdom as a source of anti-ulcer remedies. Phytotherapy Research 14: 581-591.
- Yamakoshi J, Saito M, Kataoka S, Kikuchi M (2002) Safety evaluation of proanthocyanidins rich extract from grape seeds. Food Chem Toxicol 40: 599-607.
- Scholl I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, et al. (2005) Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr 81: 154-160.
- Ozkan G, Ulusoy S, Alkanat M, Orem A, Akcan B, et al. (2012) Antiapoptotic and antioxidant effects of GSPE in preventing               cyclosporine A-induced cardiotoxicity. Ren Fail 34: 460-466.
- Miller TA (1993) Antiulcer activity of Momardica charantia. Am J Physiol 245: 601-623.
- Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, et al. (1993) Antiulcer activity of Aclipta alba. Gastentrology 34: 732-737.
- Patigaroo SA, Hashmi SF, Hasan SA, Ajmal MR, Mehfooz N (2011) Clinical manifestations and role of proton pump inhibitors in the management of laryngopharyngeal reflux. Indian J Otolaryngol Head and Neck Surg 63: 182-189.
- Desai JK, Goyal RK, Parmar NS (1997) Pathogenesis of peptic ulcer disease and current trends in therapy. Indian J Physiol Pharmacol 41: 3-15.
- Bandyopadhyay U, Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK (1999) Role of reactive oxygen species in mercaptomethylimidazole- induced gastric acid secretion and stress-induced gastric ulceration. Current Sci 76: 55-63.
- Belaiche J, Burette A, De Vos M, Louis E, Huybrechts M, et al. (2002) Observational survey of NSAID-related upper gastro-intestinal adverse events in Belgium. Acta Gastroenterol Belg 2: 65-73.
- Kimura M, Goto S, Ihara Y, Wada A, Yahiro K, et al. (2001) Impairment of glutathione metabolism in human gastric epithelial cells treated with vacuolating cytotoxin from Helicobacter pylori. Microb Pathog 31: 29-36.
- Lamine F, Fioramonti J, Bueno LF, Cauquil E, Lobysheva I, et al. (2004) Nitric oxide released by Lactobacillus farciminis improves TNBS-induced colitis in rats. Scand J Gastroenterol 39: 37-45.
- Reinders CI, Hellstorm PM, Bjork J, Weitzberg E, Lundberg JO (2004) Effect of intravenous L-NMMA on nitric oxide production in collageneous colitis. Scand J Gastroenterol 39: 32-36.
- Kubes P, McCaffetry DM (2000) Nitric oxide and intestinal inflammation. Am J Med 109: 150-158.
- Beck PL, Xavier R, Wong J, Ezedi I, Mashimo H, et al. 2004. Paradoxical roles of different nitiric oxide synthase isoforms in colonic injury. Am J Physiol 286: G137-G147.
- Rajat B, Rahul D (2018) Neurotrophic factors and Parkinson’s disease. Clin Invest (Lond.) 8: 53–62.
- Zhou YH, Yu JP (2007) Effects of ginkgo biloba extract on the antioxidation in rats with TNBS-induced colitis. Shijie Hua ren Xiaohua Zazhi 15: 1701-1705.
- Shang FJ, Zhao LY, Zheng QS, Wang JP, Xu Z, et al. (2006) Simvastatin inhibits lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiomyocytes: the role of reactive oxygen species. Biochem Biophys Res Commun 351: 947-952.
- Miyoshi N, Oubrahim H, Chock PB, Stadtman ER (2006) Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc Natl Acad Sci USA 103: 1727-1731.
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595: 01-75.
- Andrew H, Soll MD (1990) Pathogenesis of peptic ulcer and implication of therapy. N Engl J Med 322: 909-916.
- Bighetti AE, Antonio MA, Kohn LK, Rehder VLG, Foglio MA, et al. (2005) Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania laevigata Schultz Bip. Phytomedicine 12: 72-77.
- Blumenthal M (1998) Perspectives on the Safety of Herbal Medicines. F.P. Poisoning & Toxicology Compendium. Lexi-Comp Inc, Hudson, Ohio, p: 259.
- Brown LM (2000) Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol Rev 22: 283-297.
- Inagaki H, Chan JK, Ng JW, Okabe M, Yoshino T, et al. (2002) Primary thymic extranodal marginal-zone B-celllymphoma of mucosa-associated lymphoid tissue type exhibits distinctiveclinicopathological and molecular features. Am J Pathol 4: 1435-1443.
- Cuzzoocrea S, Di Paola R, Mazzon E, Genovese T, Muia C, et al. (2004) Role of endogenous and exogenous ligands for the peroxisome proliferators activated receptors alpha (PPAR-alpha) in the development of inflammatory bowel disease in mice. Lab Invest 84: 1643-1654.
- Di Carlo G, Mascolo N, Izzo AA, Capasso F (1999) Flavonoids: Old and nes aspects of a class of natural therapeutic drugs. Life Sciences 64: 337-353.
- Duran N, Justo GZ, Melo PS, De Azevedo MB, Brito AR, et al. (2003) Evaluation of the antiulcerogenic activity of violacein and its modulation by the inclusion complexation with betacyclodextrin. Can J Physiol Pharmacol 81: 387-396.
- Graham DY, Smith JL, Spjut HJ, Torres E (1998) Gastric adaptation: Studies in humans during continuous aspirin administration. Gastroenterology 95: 327-333.
- Jainu M, Devi CSS (2005) Antiulcerogenic and ulcer healing effects of Solanumnigrum (L.) on experimental ulcer models: possible mechanism for the inhibition of acid formation. J Ethnopharmacol 104: 156-163.
- Kuhn AM (1994) Antacids, Histamine H2 antagonist, antiulcer drugs in pharmacotherapeutics A nursing process. (3rd edn) Churchill Livingstone Publication, New York, pp: 1073-1075.
- Lakshimi V, Singh N, Shrivastva S, Mishra SK, Dharmani P, et al. (2009) Gedunian and photogedunin of Xilocarpus granatum show significant antisecretory effects and protect the gastric mucosa of peptic ulcer in rats. Phytomedicine 10: 16-19.
- Morise Z, Grisham MB (1998) Molecular mechanisms involved in NSAID-induced gastropathy. J Clin Gastroenterol 27: 87-90.
- Suzuki Y, Ishihara M, Segami T, Ito M (1998) Anti-ulcer effects of antioxidants quercetin alpha-tocopherol nifedipine and tetracycline in rats. Jpn J Pharmacol 27: 435-441.
- Xu L, Li B, Cheng M, Zhang W, Pan J, et al. (2008) Oral administration of grape seed proanthocyanidin extracts downregulate RAGE dependant nuclear factor- kappa BP65 expression in the hippocampus of streptozotocin induced diabetic rats. Exp Clin Endocrinol Diabetes 116: 215-224.
Citation: Bhardwaj V, Bhardwaj R, Reddy KBV, Sharma PL (2018) Investigation of Gastroprotective Potential of Grape Seed Proanthocyanidin Exract in Experimental Models of Gastric Ulcer, in Wistar Rats. J Gastrointest Dig Syst 8: 561. DOI: 10.4172/2161-069X.1000561
Copyright: © 2018 Bhardwaj V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6215
- [From(publication date): 0-2018 - Nov 08, 2025]
- Breakdown by view type
- HTML page views: 5297
- PDF downloads: 918