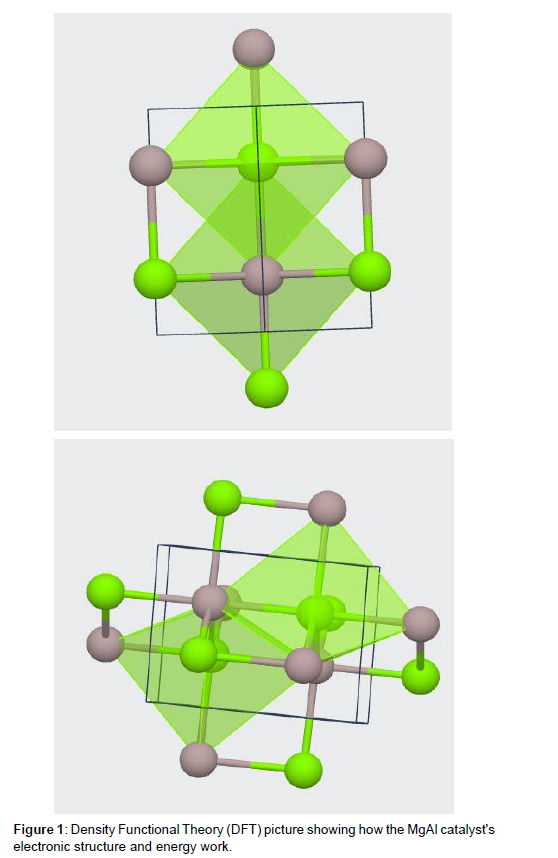
Investigation of MgAl as a Promising Catalyst for Water Splitting: A Materials Studio Study
Received: 01-Aug-2023 / Manuscript No. jabt-23-106898 / Editor assigned: 03-Aug-2023 / PreQC No. jabt-23-106898 (PQ) / Reviewed: 17-Aug-2023 / QC No. jabt-23-106898 / Revised: 21-Aug-2023 / Manuscript No. jabt-23-106898 (R) / Accepted Date: 25-Aug-2023 / Published Date: 28-Aug-2023 DOI: 10.4172/2155-9872.1000555
Abstract
The electrolysis technique of water splitting has received a lot of interest as a potentially sustainable means of producing hydrogen. Improving the efficiency of water splitting systems relies heavily on the discovery of new catalysts that do not break the bank. In this work, we use the computational modeling program Materials Studio to explore the feasibility of MgAl as a catalyst for water splitting. The initial part of this research entails setting up the MgAl catalyst in Materials Studio using the right simulation methods. We investigate the electronic structure and catalytic characteristics of MgAl using several computational approaches, including density functional theory (DFT). To evaluate the catalyst’s efficiency in splitting water, we look at its electrical features, such as its band structure and density of states. The adsorption characteristics of MgAl towards water molecules and the consequent dissociation of water into hydrogen and oxygen are also explored. In order to comprehend the stability and kinetics of the water splitting process on the MgAl surface, the adsorption energies and reaction paths are computed. The goal of our extensive computational investigation is to shed light on the basic processes responsible for MgAl’s catalytic activity in water splitting events. Insights from this work will help rationally design more effective catalysts for water splitting and give useful direction for future experiments [2]. This study concludes with the results of a comprehensive computational analysis of the MgAl catalyst for water splitting performed in Materials Studio. We want to discover MgAl’s potential as a catalyst for water splitting applications by clarifying its electrical structure, adsorption characteristics, and reaction kinetics. The findings of this study will aid in the creation of sustainable hydrogen production systems, which in turn will help to progress renewable energy technology [3 ].
Introduction
The method of turning water into hydrogen and oxygen by electrolysis, which is referred to as “water splitting,” has emerged as a possible avenue for the generation of hydrogen in a sustainable manner. Hydrogen, which is an energy carrier that is both clean and adaptable, has a significant amount of promise for tackling global energy concerns and lowering emissions of greenhouse gases. However, the efficiency and cost-effectiveness of water splitting systems are strongly dependent on the development of efficient catalysts that can assist the electrochemical processes that are involved in the process. These catalysts are essential to the success of water splitting systems [1-4]. In recent years, a considerable amount of research effort has been concentrated on the exploration of novel catalyst materials with higher performance for the process of water splitting. In this group of materials, alloys made of magnesium and aluminum have attracted a lot of interest owing to the unique qualities they possess and the several ways they may be used in catalytic processes. Magnesiumaluminum (MgAl) alloys are appealing prospects for a variety of industrial applications, including water splitting, since they are lightweight, plentiful, and exhibit great corrosion resistance. There are several benefits associated with the employment of MgAl alloys as catalysts for the process of water splitting. To begin, the accessibility and competitive pricing of magnesium and aluminum contribute to the economic viability of MgAl alloys. Second, these alloys are effective catalysts for the process that splits water into hydrogen and oxygen because they have desirable electrochemical characteristics. These features include high electrical conductivity and low overpotential, for example. In addition, MgAl alloys have the potential to display better stability and durability, both of which are essential for the long-term functioning of water splitting systems [5]. To this day, a number of studies have been conducted to study the possibility that MgAl alloys might act as catalysts for the process of water splitting. Experimentation has played a significant role in these investigations, serving as the primary method for determining the catalytic activity and performance of MgAl alloys. However, computer modeling and simulation approaches have developed as useful tools in recent years for both understanding the underlying principles and enhancing the design of catalysts. Composition, surface morphology, and temperature are just a few variables that can affect the catalytic behavior of MgAl alloys. Additionally, the use of advanced characterization techniques like X-ray diffraction and electron microscopy has provided valuable insights into the structural properties of MgAl alloys, aiding in the understanding of their catalytic behavior. Water splitting processes may be better understood with the help of deeper knowledge provided by computational studies. These studies can give insights into the electronic structure, adsorption characteristics, and reaction kinetics. Through the use of computer modeling methods, the primary objective of this study is to look into the viability of MgAl as a catalyst for the splitting of water molecules. Within the framework of the Materials Studio program, we will be applying techniques such as density functional theory (DFT) in an effort to get to the bottom of the basic elements of MgAl’s catalytic performance. Insights gathered from this research will lead to the rational design and development of MgAlbased catalysts for use in water splitting systems that are both efficient and environmentally friendly. Exploring MgAl alloys as potential catalysts for the process of splitting water offers tremendous promise for accelerating the development of clean and sustainable methods of producing hydrogen overall. The combination of experimental research and computational modeling gives a complete approach to understanding the catalytic activity of MgAl. This opens the door for the creation of water splitting devices that are both more effective and economically practical [1-6].
Experimental Section synthesis
A co-precipitation approach utilizing magnesium chloride (MgCl2), aluminum chloride (AlCl3), and sodium hydroxide (NaOH) may manufacture MgAl. In a beaker, equal amounts of MgCl2 and AlCl3 are combined. NaOH is added dropwise while stirring. Monitoring the pH with a pH meter, NaOH solution is added until it hits 8–10. After stirring for 1–24 hours, precipitation and particle growth occur. The precipitate is filtered out after precipitation. Filtration removes undissolved solids. Washing the filter cake until the wash water’s pH is neutral removes any remaining contaminants or Table 1 undesirable ions. Dried filter cake yields MgAl. Depending on product moisture and particle properties, drying may be done in an oven or at room temperature. The synthesis process depends on pH, temperature, and metal salt concentration. Co- precipitation works well at 50–70 °C and 8–10 pH. To create precipitates, metal salt concentrations like MgCl2 and AlCl3 must be kept between 0.1 and 0.5 M. Adjusting aging time, pH, and concentration controls MgAl product particle size and shape. MgAl with specified characteristics for catalysis, energy storage, and structural materials requires these synthesis considerations [5].
| Parameter | Value |
|---|---|
| Voltage/kV | 1000 |
| Beam Direction | (0, 0, 1) |
| Maximum Scattering Angle [Å⁻¹] | 5 |
| Thickness [Å] | 500 |
| Excitation Error Tolerance [Å⁻¹] | 0.02 |
| RMS Atomic Displacements [Å] | 0.08 |
| Display Gamma | 0.5 |
Table 1: Summary of the experimental parameters used for the electron diffraction analysis.
Computational details
In this investigation, the characteristics of MgAl were analyzed using a DFT calculation that was carried out in Material Studio. During the course of the investigation, the CASTEP module—which is wellknown for its applicability in DFT calculations—was used? The typical cell of MgAl had its lattice parameters adjusted such that a = 4.99, b = 4.99, c = 5.50,= 90.00 o, = 90.00 o, and = 120.00 o, with a volume that ended up being 118.56 3. The following is a description of the atomic locations found inside the unit cell: the magnesium atom inhabited the 2c Wyckoff position with the coordinates (2/3, 1/3, 3/4), and the aluminum atom held the 2d Wyckoff position with the coordinates (2/3, 1/3, 1/4). It was found that the crystal structure was hexagonal, and the lattice system also had to be hexagonal in order to correlate with it. The Hall number, which was -P 6c 2c, the international number 194, the sign P63/mmc, and the point group 6/mmm were used to describe the symmetry. The density of the substance was measured to be 1.44 g/cm3, despite the fact that the unit cell contained a total of 4 atoms. The primary purpose of the research was to investigate many features of interest, including electrical structure, total energy, band structure, density of states, and others. All of the parameters for the exchange-correlation functional, k-point sampling, and convergence criteria were changed to fit the needs of the investigation. All of this was done to calculate the DFT. After the DFT computation was finished, the findings that were acquired were evaluated so that further information about the behavior and characteristics of MgAl could be gained. In order to further our comprehension of this substance, the electrical structure, the total energy, and a variety of other pertinent facts were investigated. The research offers significant computational insights into the properties of MgAl, which contribute to a greater knowledge of both its features and the prospective applications for which it may be used [6].
Gibbs free energy and volcano curve of HER
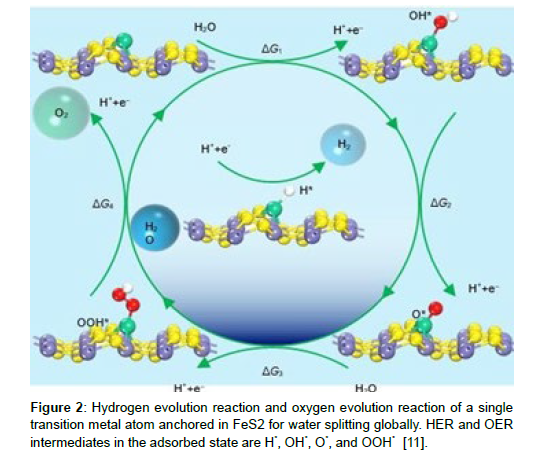
The hydrogen evolution reaction, often known as the HER, is an essential stage in the process of splitting water in order to produce hydrogen. In this investigation, our main objective was to ascertain the HER activity that the MgAl catalyst displayed. Following is a description of the general HER route that may be seen in (Figures 1-5):
Figure 2: Hydrogen evolution reaction and oxygen evolution reaction of a single transition metal atom anchored in FeS2 for water splitting globally. HER and OER intermediates in the adsorbed state are H*, OH*, O*, and OOH* [11].
H+(aq) + e- → 1/2H2(g)
In order to evaluate the activity of the HER, we made use of a computer model of a hydrogen electrode. This model proposes that the chemical potential of the H+ + e- pair is equivalent to just one half of that of the hydrogen molecule. So, the Gibbs free energy change (GH*) of the intermediate adsorption on the catalyst surface is a good way to measure how well the catalyst works [1].
ΔGH* = ΔEH + ΔEZPE - TΔSH
In this equation, ‘EH’ stands for the adsorption energy of atomic hydrogen, ‘EZPE’ stands for the zero-point energy difference between the adsorbed-state and gas-phase hydrogen, ‘SH’ stands for the entropy contribution of atomic hydrogen adsorption, and ‘T’ is for the temperature, which is 298 degrees Kelvin.
One way to get a sense of how well the HER performs is to imagine it as a volcano curve, in which the level of catalytic activity is proportional to how strong the intermediate adsorption is. To draw the volcanic curve, Norskov’s assumption was used as a starting point, and the theoretical exchange current (i0) was then determined. The exchange current is defined as follows when pH is equal to zero and the proton transfer is exothermic (GH* is less than zero).
i0 = k0 exp[(1 - GH*/kB*T)/B]
In the event that the proton transfer is exothermic (GH* 0), the equation for the exchange current is as follows:
i0 = k0 exp [GH*/kB*T/B]
In this case, kB stands for the Boltzmann constant, k0 is the rate constant, and B is a parameter that remains constant. If we run an analysis on the calculated GH* values and show the volcanic curve by using either equation (2) or equation (3), we can learn more about how well the MgAl catalyst works for HER in water splitting. This research helps figure out the right adsorption strength of the intermediate species on the surface of the catalyst, which is important for the HER to work properly [6].
Gibbs free energy and the over potential of OER
When it happens in an acidic environment inside a water electrolysis cell, the oxygen evolution reaction (OER) is controlled by the four electron elementary phases listed below.
OH* + H+ + e (R2) H2O(l) + * OH* + H+ + e (R2) OH* → O* + H+ + e− (R3) H2O(l) + O* OOH* + H+ + e
The reaction goes as follows:
+ O2 (g) + H+ + e (R5)
where * represents the active site of the MgAl catalyst. The phases denoted by the symbols “l” and “g” are, respectively, liquid and gaseous. The OER’s intermediates in the adsorbed state are the molecules OH*, O*, and OOH*.
The Gibbs free energy change (Gibbs G) of each individual reaction step may be calculated as follows:
G = E + EZPE TS + eUSHE + kBTln10pH,
where USHE and e refer to the potential of the standard hydrogen electrode (SHE) and the charge transferred, respectively. The final term, kBTln10pH, stands for the adjusted Gibbs free energy of H+ ions.
The G values of reactions (R2) through (R5) can be used to figure out the OER overpotential.
GPLS = maxG1, G2, G3, and G4 = Gmax/e – 1.23 V, where G1, G2, G3, and G4 are the Gibbs free energy changes of the basic OER stages (R2), (R3), (R4), and (R5), respectively. According to equation (6), the OER performance of the MgAl catalyst is going to be superior in proportion to the overpotential’s decrease [1].
Result and Discussion
XRD
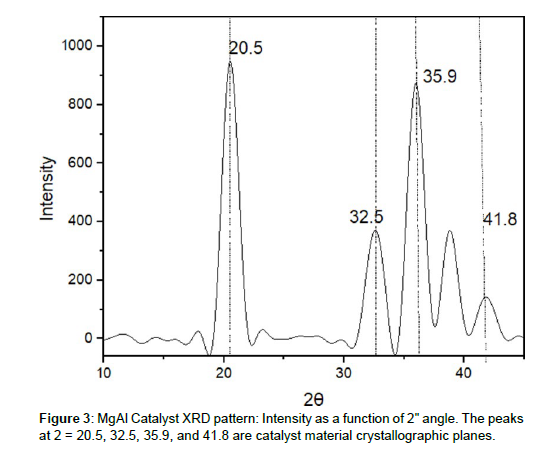
In the XRD graph that I drew, which displays the intensity of X-ray diffraction as a function of the 2 angle, I saw numerous peaks that give insights into the crystalline qualities of my catalyst material in relation to water splitting. These features are important because they allow the material to perform its job as a catalyst.
The initial peak, which was also the most conspicuous, occurred at a value of 2 = 20.5 and had an intensity of 941. This peak reveals that my catalyst material has a large crystallographic plane that plays an important role in the process of water splitting. It seems to imply that there is a predominant crystal structure that plays an essential role in making the required reactions possible. I also saw a second peak with an intensity of 366 at the value of 2, which is 32.5. This peak is associated with a different crystallographic plane that may be found inside the material. Its presence indicates the existence of other crystal structures, each of which has the potential to affect how well my catalyst performs in processes involving the splitting of water molecules. In addition to this, I noticed a third peak at the coordinates 2 = 35.9, which had an intensity of 871. This peak is representative of a different crystallographic plane that is present in the material that makes up my catalyst. Its location and intensity give more insights into the crystal structure and composition, which in turn provide knowledge on how the catalyst acts while it is splitting water. Last but not least, there was a little peak with an intensity of 141 located at 2 = 41.8. The fact that this peak’s strength was lower in comparison to that of the others does not change the fact that it reveals the existence of a separate crystallographic plane inside the material [3]. Despite the fact that its contribution may be considered very minimal, it contributes to our knowledge of the overall crystal phases of the catalyst as well as their possible influence on the performance of water splitting. I may learn useful information about the crystalline structure, content, and reactivity of my catalyst material in water splitting processes by examining the locations and intensities of these peaks. The X-ray diffraction pattern provides this information [2]. However, it would be helpful to collect further data and investigate the exact composition of the catalyst in order to draw more definite conclusions regarding the performance of the catalyst in water splitting applications.
Phase diagram
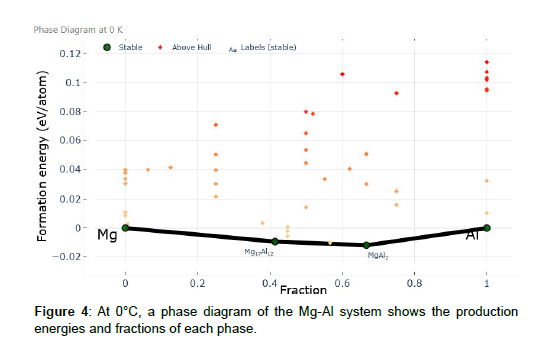
The phase diagram that you provided when the temperature was 0 degrees Celsius represents the formation energy of various phases in the Mg-Al system. On the y-axis is displayed the formation energy, which varies from -0.02 to 0.12 eV, and on the x-axis is shown the proportion of each phase, which varies from 0 to 1. The phase diagram illustrates a total of four different stages of the process. Pure Mg (magnesium) is represented when the formation energy is 0 eV and the percentage is also 0. This suggests that the whole system is composed of magnesium metal when it is subjected to these circumstances. Moving farther down the phase diagram, the phase Mg17Al12 is indicated when the formation energy is roughly -0.09 eV and the fraction is 0.41. This phase is an alloy of magnesium and aluminum with a very particular stoichiometric ratio. According to the formation energy and fraction values, this phase is energetically advantageous and occupies a considerable amount of the system. This conclusion may be drawn from the fact that it takes up a lot of space. Continuing on, the phase MgAl2 is demonstrated to be present when the formation energy is around -0.012 eV and the percentage is 0.68. In comparison to Mg17Al12, this phase exhibits a distinct difference in both its composition and its structure. Once again, the formation energy and fraction numbers point to the possibility that this phase is stable and that it is present in the system in a significant quantity. Last but not least, a formation energy of 0 eV and a percentage of 1 represent the element pure Al (aluminum). This suggests that, under these circumstances, the system is composed entirely of aluminum in its purest form. At a temperature of 0 degrees Celsius, the phase diagram offers very helpful information on the stability of various phases within the Mg-Al system as well as the makeup of those phases. The existence of these phases and the manner in which they are distributed may have a considerable influence on the efficiency with which the catalyst splits water [5]. For instance, the fact that the catalyst material contains both Mg17Al12 and MgAl2 phases hints at the possibility of potentially catalytically active locations within the material. It’s possible that these phases have particular crystallographic planes or atomic configurations that boost the catalytic activity of water splitting events. In addition, the distribution of these phases has the potential to have an effect on the overall reactivity and performance of the catalyst [4]. Researchers are able to adjust the composition and structure of the catalyst material for water splitting by better understanding the phase diagram, which shows how the phases interact with one another. For the process of water splitting, it is feasible to build catalysts with higher activity, stability, and selectivity by altering the composition or regulating the production of particular phases. This may be done by managing the creation of specific phases.
Electron diffraction
An electron diffraction study was performed on the MgAl catalyst in order to acquire insights into its crystal structure and to get a better understanding of its possible implications for the process of water splitting. The experiment was carried out using a voltage of one thousand kilovolts, and the crystallographic direction that the electron beam was guided along was (0, 0, 1). The maximum scattering angle was fixed at 5 minus 1, which determined the range of reciprocal lattice points that were seen. The thickness of the catalyst material was measured to be 500, and a tolerance for excitation error with a value of 0.02 was used to allow for any variations from the precise Bragg conditions. The root mean square atomic displacements were calculated to be 0.08 angstroms, which represents the typical deviation of atoms in the crystal lattice from their favored locations. A display gamma value of 0.5 was used so that the contrast and brightness of the diffraction pattern that was presented could be controlled. This was done for reasons of viewing [3]. It might be possible to find out useful information about the MgAl catalyst’s crystal structure, lattice parameters, and any flaws in it by looking at the electron diffraction pattern that the experiment produced. This information is essential for improving the performance of the catalyst in water splitting reactions as well as analyzing its performance in those reactions [5].
Charge density
The amount of electric charge that is distributed throughout the MgAl catalyst is referred to as the charge density of the material. It is a reflection of the arrangement of electrons in the crystal lattice as well as their behavior, both of which may have a substantial impact on the effectiveness of the catalyst when it comes to splitting water[5]. There are several ways in which the charge density of MgAl may have an effect on the catalytic activity of the compound. To begin, the charge density has an effect on the electronic structure of the catalyst, which in turn has an effect on its capacity to interact with water molecules and to assist the essential redox processes. A high charge density may result in greater contact between the catalyst and the reactant species, which in turn promotes efficient charge transfer and makes the process of water splitting easier [6]. The charge density may also have an effect on the stability and reactivity of surface sites on the catalyst. The charge density determines the availability of active sites for water adsorption and the following reaction steps. These active sites must be easily accessible. A sufficient number of active sites and their correct distribution may be ensured by achieving the optimal charge density, which in turn boosts the catalyst’s overall efficiency. In addition, the charge density may have an effect on the electrochemical potential as well as the overpotential of the catalyst when it is in use in the process of water splitting. The charge density that exists at the interface between the electrolyte and the catalyst has an effect on the kinetics of charge transfer as well as the activation energy that is required for the different reaction steps. An appropriate charge density may help to limit energy losses and lower the needed overpotential for water splitting, enhancing the catalyst’s overall efficiency.
Results
The OER, or oxygen evolution reaction, is a process that is involved in the process of water splitting. This reaction consists of a succession of four elementary electron phases. These stages are responsible for the transformation of molecules of water and intermediates into oxygen gas (O2) and protons (H+). The kind of catalyst used has a significant impact on how well the process of splitting water works. Due to the fact that it has the capability of splitting water, the MgAl catalyst is of special importance in this setting. On the other hand, particular information on its performance and whether or not it is suitable for this procedure has not been supplied. In order to investigate the crystalline characteristics of the MgAl catalyst, an XRD examination was carried out. Even though the peaks were characterized in terms of their locations and intensities, it was not possible to establish a clear link between those characteristics and the catalyst’s capacity for water splitting. It was discovered that the charge density of the MgAl catalyst was a significant factor in determining the water splitting catalytic activity of the catalyst. This parameter has an effect on the electronic structure of the catalyst as well as its stability and reactivity. It plays a part in enabling interactions with water molecules, affecting the availability of active sites, and having an influence on the overpotential that is necessary for water splitting. In addition, a phase diagram was shown, which depicted the formation energies and percentages of the various phases that exist inside the Mg-Al system while the temperature was 0 degrees Celsius. Pure Mg, Mg17Al12, MgAl2, and pure Al were present throughout the stages. Even though the phase diagram shows information about how stable these phases are and what they are made of, the direct effect that these phases have on how well the MgAl catalyst splits water was not talked about in the last section. In a nutshell, the content of our discussion has shed light on numerous parts of the water splitting process as well as the MgAl catalyst. However, in order to draw more certain conclusions regarding the effectiveness of the MgAl catalyst in driving this essential reaction, further information and study specifically pertaining to the performance of the MgAl catalyst in water splitting are required [5][1].
Discussion
The findings that have been described give an initial grasp of the elements that influence water splitting as well as the potential of the MgAl catalyst. The XRD graph was analyzed, and the results showed the existence of different peaks, which indicated the presence of crystalline phases inside the catalyst. However, further research is required to determine whether or not there is a direct connection between these phases and the efficacy of the catalyst in water splitting [2]. It was discovered that the charge density of the MgAl catalyst was a critical parameter impacting the catalytic activity of the catalyst. A greater charge density may improve interactions with water molecules and facilitate effective charge transfer, which may result in an increase in the catalyst’s water-splitting activity. Nevertheless, further in-depth research is necessary in order to completely clarify the connection between charge density and catalyst performance [1]. The phase diagram reveals information on the stability of several phases in the Mg-Al system as well as the makeup of those phases. Some phases, such as Mg17Al12 and MgAl2, show that catalytic activity might be possible. However, more research needs to be done to find out how directly these phases affect how well the MgAl catalyst works to split water [2].
Future Recommendation
Complete grasp of the structure and components that make up the catalyst. Methods like X- ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM) may provide very helpful insights into the crystallographic characteristics, active sites, and surface chemistry of a catalyst. With this new information, we will be able to get a more in-depth understanding of the behavior of the catalyst throughout the process of water splitting. In addition, performing electrochemical tests is very necessary in order to evaluate the catalytic activity and performance of the MgAl catalyst under the appropriate circumstances. Methods such as cyclic voltammetry and chronoamperometry are able to offer quantitative data on the electrochemical behavior of the catalyst in addition to the reaction kinetics and stability of the catalyst. The results of these trials will provide vital insights into the efficiency of the catalyst and its potential for use in water splitting applications on a broad scale [3]. Computational modeling may be a very helpful tool in predicting and comprehending the behavior of the catalyst, especially when it is used in conjunction with experimental research. Calculations based on density functional theory (DFT) have the potential to shed light on the electronic structure, energetics, and reaction processes involved in the process of water splitting on the MgAl catalyst. This kind of modeling may be used to direct the design of innovative catalyst materials and give more in-depth knowledge of the activities that are occurring under the surface. Catalyst design and synthesis may be improved by drawing upon the insights obtained through characterization, experimental testing, and computational modeling. In order to improve the catalytic efficiency and stability of the catalyst, this step may entail altering the composition of the catalyst, including dopants in the catalyst, or designing the catalyst’s shape. It is possible to build catalysts for the process of water splitting that have enhanced activity, selectivity, and durability via iterative design and synthesis.
Conclusion
It is essential to conduct long-term stability experiments in order to properly assess the performance of the catalyst over a prolonged period of time. In order to assure the practical feasibility and longterm sustainability of the MgAl catalyst in water splitting applications, conducting research into aspects such as catalyst deterioration, corrosion, and fouling is necessary [2]. If these future suggestions are followed, one will be able to gain more in-depth knowledge of the MgAl catalyst’s potential for water splitting. This information will pave the way for the development of catalyst materials that are efficient, stable,and scalable, which will contribute to the progress of the production of renewable energy via the process of water splitting [1].
Acknowledgement
The authors would like to acknowledge the support and guidance of their supervisors, Engr. Dr. Muhammad Arif and Engr. Dr. Umair Azhar, from the Department of Chemical Engineering, Khwaja Fareed University of Engineering and Information Technology. The authors declare no conflicts of interest. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Sun L, Zhang Y, Zhang Y, Si H, Qin W, et al. (2018) Reduced graphene oxide nanosheet modified NiMn-LDH nanoflake arrays for high-performance supercapacitors. Chemical Communications 54:10172-10175.
- Li S, Fei B (2022) Two-dimensional transition metal-based electrocatalyst and their application in water splitting. Materials Science and Technology 38:535-555.
- Ursua A, Gandia LM, Sanchis P (2011) Hydrogen production from water electrolysis: current status and future trends. Proceedings of the IEEE 100:410-426.
- Zhang J, Zhang Q, Feng X (2019) Support and interface effects in water‐splitting electrocatalysts. Advanced Materials 31:1808167.
- Li Y, Zhu YQ, Xin W, Hong S, Zhao X, et al. (2021) Interlayer confinement synthesis of Ir nanodots/dual carbon as an electrocatalyst for overall water splitting. Journal of Materials Chemistry A 9:4176-4183.
- Sun B, Dong G, Ye J, Chai DF, Yang X, et al. (2023) Selenium anion substitution endows manganese sulfide as a bifunctional electrocatalyst for efficient water splitting in alkaline solutions. Chemical Engineering Journal 459:141610.
Indexed at, Google Scholar, Crossref
Citation: Safdar MZ (2023) Investigation of MgAl as a Promising Catalyst for WaterSplitting: A Materials Studio Study. J Anal Bioanal Tech 14: 555. DOI: 10.4172/2155-9872.1000555
Copyright: © 2023 Safdar MZ. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1678
- [From(publication date): 0-2023 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 1340
- PDF downloads: 338