Is the Pinworm Causing Acute Suppurative Perforating Appendicitis in Children? A Case Series
Received: 06-Jun-2017 / Accepted Date: 21-Jun-2017 / Published Date: 29-Jun-2017 DOI: 10.4172/2161-0681.1000312
Abstract
Introduction: The pinworm Enterobius vermicularis is the most common parasite reported to be associated with appendicitis. It frequently occurs in children between the age of 5 to 10 years. The aim of our study is to focus on the relationship between the pinworm and acute suppurative perforating appendicitis.
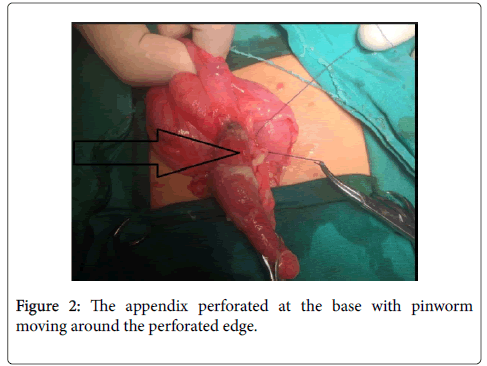
Case series: 4 children from rural areas of Upper Egypt with mean age 11 years presented to emergency department Aswan and Assiut universities hospitals during the period from March 2016 to January 2017 complained of right lower quadrant abdominal pain. The appendix in all children was perforated at the base or the middle of the appendix with mobile pinworm was noted moving around the perforated edge.
Discussion: Pinworm was associated with different pathologic changes ranging from lymphoid hyperplasia, acute appendicitis, and suppurative appendicitis to a normal appendix. It is wrangling whether pinworm can cause appendicitis or if they are an incidental finding during an appendectomy.
Conclusion: The awareness of pinworm and its preventive measures should be delivered to school children and their mothers to overcome the risk of infection and its dangerous drawback. The importance of histopathological and faecolith examinations to the resected appendix specimens to detect the correct incidence of pinworm causing appendicitis in Egypt.
Keywords: Enterobius vermicularis; Pinworms; Suppurative perforating appendicitis; Worm appendicitis in pediatrics; A case series
315888Introduction
The association of Enterobius vermicularis, with acute appendicitis, varies from 0.2 to 41.8% [1-9]. Infection is seen in all age groups and all socioeconomic classes but it is more common in children. E. vermicularis is spread by feco-oral route and is associated with close living, poor hygiene, temperate and tropical climates [10,11]. Parasites can be associated with the development of classic appendicitis. In series, there is a range of pathologic findings from nonspecific changes to frankly ruptured appendicitis [9]. The surgeon should understand that the clinical management of these cases is different from that for an ordinary appendicitis, as it requires treatment with antihelminthic drugs.
Case Series
4 children from rural areas of Upper Egypt with mean age 11 years [10-12], presented to emergency department Aswan and Assiut Universities Hospitals during the period from March 2016 to January 2017, complained of right lower quadrant abdominal pain. The pain was sharp inconsistency, sometimes crampy. All children were lived with their families and they were students. No significant medical or family histories were reported.
On examination, the children appeared pale but nontoxic. The abdominal examination gives the typical picture of acute appendicitis, the pain localized to the right iliac fossa, tenderness at MC Burney's point, +ve Rovsing sing, +ve rebound tenderness. The pain associated with nausea vomiting once or twice, preceded by diarrhea 5 days duration in two cases, no constipation. All children had a low-grade fever with temperature ranging (37.7°C-38.4°C), tachycardia pulse rate range (93-105) beats\minute; all children had elevated WBCs count range (13,000-16,000) mcL, with no significant neutrophilia or eosinophilia. Additional preoperative laboratories finding are within normal limits.
Abdominal ultrasound reviled minimal fluid collection at the pelvic in 3 cases with visible pelvic appendix. Plain erect abdominal x rays showed gaseous distension but no air fluid level or gas under the diaphragm.
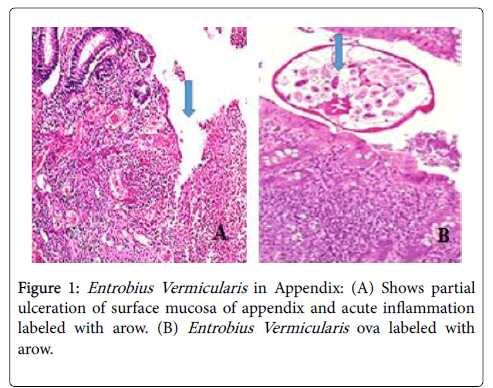
A written consent obtained from the parent for appendectomy under general endotracheal anesthesia. Experienced pediatric surgeons made appendectomy through McBurney's incision. The appendix in all children was perforated at the base or the middle of the appendix with mobile pinworms was noted moving around the perforated edge. The appendixes were pelvic in position at 3 cases and retrocecal in one case. Toilet and lavage made for the pelvic cavity to drainage the minimal amount of suppurative collection. Closure of the abdominal wall with pelvic drain did. The pathology reports revealed appendicular wall infiltrated by acute inflammatory cells and pus cells. The surface epithelium shows partial ulceration. The lumen filled with Enterobius Vermicularis ova (Figure 1A and B).
Postoperative stool analysis in two cases showed Entamoebia histolytica infection. The patients were placed on antihelminthic treatment on the 2nd postoperative days, oral dose of mebendazole repeated 1-2 weeks, also, on IV metronidazole/12 h due to suppurative appendicitis and for Entamoebia histolytica infection in the combination of gram +ve and gram –ve antibiotics (Figure 2).
The patient discharged on the 3rd postoperative days after removal of a pelvic drain.
The families were advised that all members should be treated with antihelminthic drugs and other close contacts.
Discussion
Pinworm of pediatric appendicitis continued to be a rare surgical presentation it is frequency 1.52% [12]. Pinworm was associated with different pathologic changes ranging from lymphoid hyperplasia, acute appendicitis, and suppurative appendicitis to a normal appendix [7].
It is wrangled whether pinworms can cause appendicitis or if they are an incidental finding during appendectomy [8]. In the few cases reported synchronous acute inflammation and intraluminal Enterobius vermicularis infestation, it is unclear whether pinworms obstructing the lumen caused appendicitis, the pinworms induced a hypersensitivity reaction inducing appendiceal inflammation, or if the inflammation was from another cause [8]. Studies support the hypothesis that appendiceal lumen obstruction by pinworm infestation gives rise to symptoms ambiguous from suppurative appendicitis [13]. Ova release from female parasites may be a trait of appendiceal obstruction, which consequently is followed by bacterial overgrowth and finally ending to acute appendicitis. The reversibility of these processes may be questioned [14]. As the appendix is a residual organ, it can be easily infiltrated by numerous intestinal contents such as vegetable fibers, faecolith, worms and their eggs. These foreign particles can lead to the obstruction of the appendiceal lumen. If the lumen is obstructed, the persistent mucosal secretion, generation of bacteria and/or the presence of worms may cause an increase in the intraluminal pressure. Increased pressure hinders the blood supply of the appendix wall and mucosal damage may cause bacterial transgression, inflammation, sepsis and finally necrosis [15]. Recent study reviewing 2267 cases of appendicitis showed that there was a highly significant difference in the incidence of pinworm infestation in normal and in inflamed appendices, which may indicate that the presence of pinworm in the appendix can cause symptoms of acute appendicitis [16].
In rare cases, intraperitoneal pinworm contamination may present in some patients due to infection of the peritoneum via the fallopian tube or in patients with appendiceal or intestinal rupture. In these patients, the immune system reacts by creating granulation tissue around the pinworms. Symptoms related to extraintestinal pinworm infestation may include mesenteric abscess formation, omenities, enterocutaneous fistula, urinary tract infection, salpingitis, fallopian tube infiltration, salpingooophoritis, and tubo-ovarian abscess [17-19].
Laboratory findings of these patients show extreme discrepancy including high to normal values [14]. It is important that these patients use antihelminthic treatment after operative intervention because the appendectomy treats only a sequel but not the root of the disease. An oral dose of mebendazole is the treatment of choice to E. vermicularis infestation, which is repeated in 1–2 weeks. Reinfection may be predictable because humans do not promote a preventative immunity against pinworms [14]. Finally, we believe that the pinworm may cause perforating suppurative appendicitis due to different pathological changes ranging from obstruction of the lumen to hypersensitivity reaction, further studies are necessary to deduce the relationship between Enterobius vermicularis and acute suppurative perforating appendicitis.
Conclusion
Pinworm is an uncommon cause of acute suppurative perforating appendicitis in Upper Egyptian children. Early diagnosis of pinworms infection and proper treatment might prevent probable further complications that may necessitate surgical intervention. The awareness of pinworms and its preventive measures should be included in all health education programs and should be delivered to schoolchildren and their mothers to overcome the risk of infection and its dangerous drawback. Future researches on the relation of pinworms and acute suppurative perforation appendicitis are important with a special recommendation to the significance of the appendix specimens investigation by both histopathological and faecolith examinations to detect the correct incidence of pinworms causing appendicitis in Egypt.
Acknowledgement
To my colleagues, Dr. Mohammed Elsayed Nader assistant lecturer of pediatric surgery Aswan University Hospital, for his intraoperative macroscopic photo picture, for Dr. Marwa T. Hussien pathologist at South Egypt Cancer Institute, Assiut University, Assiut, Egypt for microscopic photo picture and for my brother Basel Magdy Abdelmohsen Ain Shams University for his languish checker.
Conflict of Interests
No conflict of interest.
Sources of Funding
No funding has been used for this research.
Ethical Approval
No ethical approval has been applied for this case series study, only the written and oral consent by the relatives of the patients.
Consent
A written consent has been obtained from the patients' relatives for operative intervention and for the publication of this case series.
References
- Akbulut S, Tas M, Sogutcu N,Arikanoglu Z, Basbug M, et al. (2011) Unusual histopathological findings in appendectomy specimens: A retrospective analysis and literature review. World JGastroenterol15:1961-1970.
- Gupta SC, Gupta AK, Keswani NK, Singh PA, TripathiAK, et al. (1989) Pathology of tropical appendicitis. J Clin Pathol 42: 1169-1172.
- Yildirim S, Nursal TZ, Tarim A, Kayaselcuk F, Noyan T, et al. (2005) A rare cause of acute appendicitis: parasitic infection. Scand J Infect Dis 10: 757-759.
- Da Silva DF, Da Silva RJ, Da Silva MG, Sartorelli AC, Rodrigues MAM, et al. (2007) Parasitic infection of the appendix as a cause of acute appendicitis. Parasitology Res 102: 99-102.
- Aydin O (2007) Incidental parasitic infestations in surgically removed appendices: a retrospective analysis. Diagn Pathol 2:16.
- Goldman DA, Wilson CM (1997) Pinworm Infestation In: Hoekelman Ra, Primary Pediatric 18: 13-24.
- Akhigbe T, Smith F, Adeyemo A, Adeyanju T, Condon E, et al. (2013) Pinworm And Appendicitis In Children. The Int J Surgery.
- Panidis S, Paramythiotis D, Panagiotou D, Batsis G, Salonikidis S et al. (2011) Acute appendicitis secondary to Enterobius vermicularis infection in a middle aged man: a casereport. J Med Case Rep 5: 559.
- Marjorie JA, Robert LG, Jonathan IG, Hammond S, Caniano DA, et al. (2004) Clinical manifestation of appendiceal pinworms in children: an institutional experience and a review of the literature. Pediatr Surg Int 20: 372-375.
- Leo LX, Chi J, Upton MP, Ash LR (1995) Eosinophilic colitis associated with larvae of the pinworm Enterobius vermicularis. Lancet 346: 410-412.
- Arca MJ, Gate RL, Groner JI, Harmond S, Carniano DA, et al. (2004) Clinical manifestation of appendiceal pinworm in children: an institutional experience and review of literature. Pediatr Surg Int 20: 372-375.
- Sah SP, Bhadani PP (2006) Enterobious vermicularis causing symptoms of appendicitis in Nepal. Trop Doct 36: 160-162.
- Aydin O (2007) Incidental parasitic infestations in surgically removed appendices: a retrospective analysis. Diagn Pathol 2: 16.
- AbdellatifMZM, Abdel-Hafeez EH, Belal US, Mohamed RM, Abdelgelil NH, et al. (2015) Identification of parasitic infections in appendectomy specimens using histopathological and faecolith examinations. Parasitologists United J 8:101-106.
- WiebeBM (1991) Appendicitis and Enterobious vermicularis. Scand J Gastroenterol 26: 336-338.
- Nordstrand IA, Jayasekera LK (2004) Enterobius vermicularis and clinical appendicitis: worms in the vermiform appendix. ANZ J Surg 74: 1024-1025.
- Panidis S, Paramythiotis D, Panagiotou D, Batsis G, Salonikidis S, et al. (2011) Acute appendicitis secondary to Enterobius vermicularis infection in a middle aged man: a case report. J Med Case Rep 5: 559.
- Tandan T, Pollard AJ, Money DM, Scheifele DW (2002) Pelvic inflammatory disease associated with Enterobius vermicularis. Arch Dis Child 86: 439-440.
Citation: Abdelmohsen SM, Osman MA (2017) Is the Pinworm Causing Acute Suppurative Perforating Appendicitis in Children? A Case Series. J Clin Exp Pathol 7:312. DOI: 10.4172/2161-0681.1000312
Copyright: © 2017 Abdelmohsen SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7127
- [From(publication date): 0-2017 - Aug 24, 2025]
- Breakdown by view type
- HTML page views: 6155
- PDF downloads: 972