Mini Review Open Access
Is There Any Relationship between Apolipoprotein E Polymorphism and Idiopathic Parkinson's Disease?
Oh Dae Kwon*Department of Neurology, School of Medicine, Catholic University of Daegu, Daegu, South Korea
- *Corresponding Author:
- Oh Dae Kwon
Department of Neurology, Daegu Catholic University Medical Center
School of Medicine, Catholic University of Daegu
17- 33, Duryugongwon-ro, Namgu, Daegu
South Korea, 42472
Tel: +82 536504298
E-mail: dolbaeke@cu.ac.kr
Received date: November 22, 2016; Accepted date: December 26, 2016; Published date: January 02, 2017
Citation: Kwon OD (2016) Is There Any Relationship between Apolipoprotein E Polymorphism and Idiopathic Parkinson’s Disease? J Alzheimers Dis Parkinsonism 7:292. doi:10.4172/2161-0460.1000296
Copyright: © 2016 Kwon OD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Apolipoprotein E is a plasma protein that has an important role in transport and metabolism of lipids in serum as well as in central nervous system. Among the three common alleles, the ε2 allele has the most stable structure followed by ε3 and ε4 in order. There is evidence for a deleterious role of apolipoprotein ε4 by atherosclerosis and amyloid beta accumulation in brain and body by the differences of three-dimensional structures among the apoE isoforms. APOE ε4 also seems to be related to cognitive decline in Parkinson’s disease but not too related to the age at onset of Parkinson’s disease.
Keywords
Apolipoprotein E; Polymorphism; Parkinson’s disease; Cognition.
Introduction
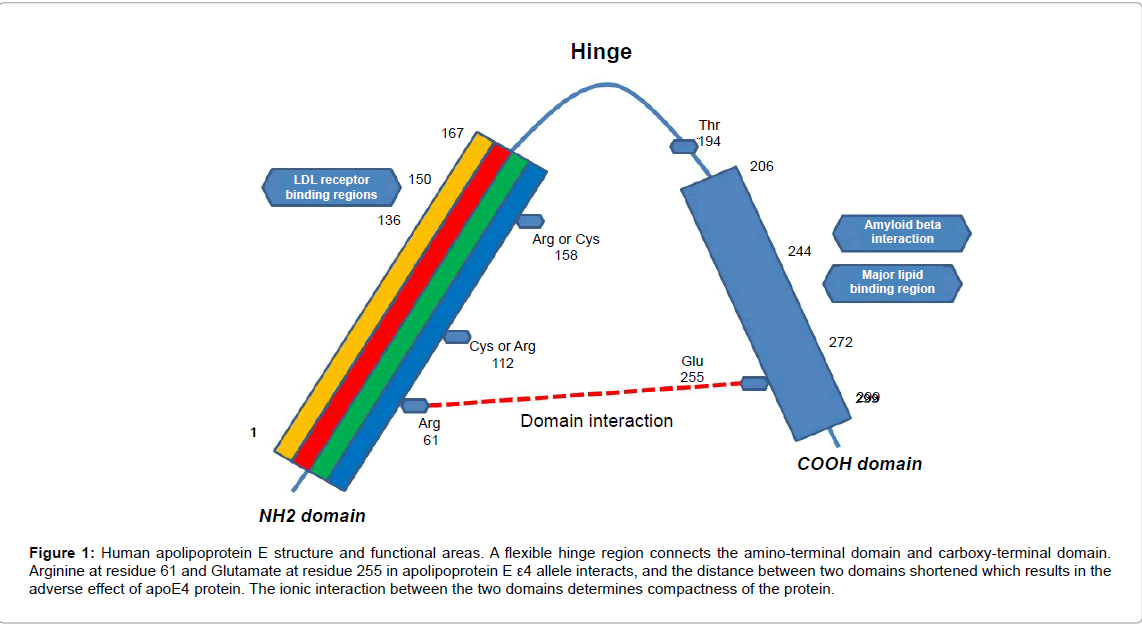
he human apolipoprotein E (apoE) protein is an important protein in plasma, cerebrospinal fluid, and interstitial fluid of central nervous system [1]. It consists of single chain lipoprotein with 299 amino acids, produced in the liver, brain, spleen, lung, etc. The amino-terminal, having four helices, binds to low-density lipoprotein and the carboxyterminal, an amphipathic alpha helix, binds to lipid. The three common wild types of APOE alleles are ε2, ε3, ε4 which located on chromosome 19q. These three alleles encode similar proteins different only in 112th residue and 158th residue, respectively [2] (Figure 1). The roles of apoE are catabolism of lipoprotein constituents, lipid transportation, and membrane repair process [3]. In the human brain, glial cells, vascular smooth muscle cells, and choroid plexus produce apoE. Intriguingly, neurons also produce apoE under neuronal injury or other stressful conditions, and the newly synthesized apoE recruit cholesterol and phospholipids from the body and central nervous system to protect neurons and to repair damaged neurons. In these process apoE4 causes a direct toxic effect on the neurons [4]. Apolipoprotein E was known as a protein constituting lipid-rich plasma lipoproteins in the early 1970s. In 1993, Clinical characteristics of APOE polymorphism was reported through genetic linkage study. APOE ε4 allele considered as harmful to human cognition because the frequency of the ε4 allele in patients with Alzheimer’s disease was more than three times higher than that of cognitively normal person. Moreover, APOE ε4 allele carriers with normal cognition develop Alzheimer’s disease earlier than people without APOE ε4 allele [5]. On the other hand, APOE ε2 allele was considered protecting cognitive function because the frequency of ε2 allele was lower in patients with Alzheimer’s disease than that of cognitively normal person. The most common APOE ε3 allele was considered to be neutral or protective effect because the biochemical characteristics is between APOE ε2 and APOE ε4 [6,7].
There are several explanations of the harmful effect of APOE ε4. A mouse model study on the apoE effect on blood-brain barrier revealed that apoE4 cause blood-brain barrier more susceptible to injury by increased cytokine and increase matrix metalloproteinase 9 pathways [1]. Domain interaction theory may explain the harmful effect of APOE ε4. In this theory, the Arg-61 of the amino domain and the Glu-255 of the carboxy-domain interacts ionically to cause a structural change which results in apoE4 becomes the more compact, meaning of the distance between the two domains becomes shorter than other alleles. When a significant amount of apoE4 is in neurons by the response to injury, it again give stress to the neurons to begin a pathological response. Compact apoE4 is recognized as abnormal in central nervous system, and neuron-specific protease cut off the carboxy terminal of apoE4. The fragmented apoE4 is neurotoxic, and it impairs neuronal mitochondrial function and also causes cytoskeletal changes. Damaged neurons by apoE4 toxicity are destroyed by proteolysis [1]. Interaction of the two domain is less efficiently occur in other alleles because Cys- 112 is firmly bound into the amino terminal [4,8] (Figure 1). The other theory is that proteolytically cleaved apoE4 can do major role in the development of Alzheimer’s disease. A fragment of apoE4 was found in the pathologies of Alzheimer’s disease which is indicative of a toxic effect of apoE4 on the disease [9]. Lastly, apoE4 may cause neurotoxicity by mitochondrial dysfunction and formation of neurofibrillary tangles supposed by an animal study [10].
Parkinson’s Disease and Apolipoprotein E
Parkinson’s disease is the second most common neurodegenerative disease that shows specific movement symptoms such as bradykinesia, resting tremor, rigidity, and postural instabilities. One fourth of this debilitating illness is seemed familiar suggesting strong genetic effect of onset [11]. The association of APOE polymorphism and Parkinson’s disease were proposed by several features of Parkinson’s disease. First, cognitive decline develops frequently in the early or late stage of the illness, and Parkinsonism often develops in Alzheimer’s disease [12,13]. Second, Deposition of abnormal protein in the nervous system is seen in both conditions [14]. Third, pathologic changes similar to Alzheimer’s disease are frequent in Parkinson’s disease. Forty-two percent of patients with Parkinson’s disease showed senile plaques and neurofibrillary tangles in the cerebral cortex [14]. Fourth, the level of cerebrospinal biomarkers such as Amyloid-beta and Tau protein is changed in both degenerative diseases and the levels have a correlation with the severity of cognitive impairment in Parkinson’s disease [15]. Pathological studies suggest two kinds of pathological changes related to cognitive decline. One is Lewy bodies, frequently seen in brainstem and cerebrum of Parkinson’s disease as well as Lewy body disease. The other is the neuritic plaque that is main pathological findings of Alzheimer’s disease [16,17]. The prevalence of Alzheimer’s disease in Parkinson’s disease is more than six times than that of the cognitively healthy population. Moreover development of Alzheimer’s disease shortens the survival period of the patients with Parkinson’s disease [14].
Figure 1: Human apolipoprotein E structure and functional areas. A flexible hinge region connects the amino-terminal domain and carboxy-terminal domain. Arginine at residue 61 and Glutamate at residue 255 in apolipoprotein E e4 allele interacts, and the distance between two domains shortened which results in the adverse effect of apoE4 protein. The ionic interaction between the two domains determines compactness of the protein.
There are several issues not conclusive on the relation between Parkinson’s disease and APOE polymorphism. The first issue is the relationship between APOE ε4 allele and the age at onset of Parkinson’s disease. Some studies of patients with Parkinson’s disease suggested a significant association between APOE ε3/ε4 and ε4/ε4 genotype with earlier age at onset of motor symptoms in Caucasians [18,19]. In contrast, studies done with Mexican Mestizo, Korean, as well as research in the United States did not show any relationship between APOE polymorphism and age at onset of Parkinson’s disease [11,20,21]. A meta-analysis showed the relationship between APOE ε4 and lower prevalence of Parkinson’s disease [22]. They suggested relatively higher cholesterol level induced by APOE ε4 was the reason for the lower occurrence of Parkinson’s disease. However, followed studies did not show any relationship between ApoE polymorphism and age at onset of Parkinson’s disease [23,24]. Intriguingly, a genomewide linkage study comparing siblings with and without Parkinson’s disease showed that APOE ε4 might be a weak risk locus for PD with unknown mechanism [25]. Overall, above studies indicate that age at onset of Parkinson’s disease is not related to the existence of APOE ε4. The reports supporting the relationship have weak evidence and larger prospective study will reveal the answer to the question. The second issue is the relationship between the existence of APOE ε4 allele and cognitive status of Parkinson’s disease. A study of Caucasians with Parkinson’s disease showed ApoE ε4 as a risk factor for dementia in patients with Parkinson’s disease [26]. The other study comparing Parkinson’s disease with family and without family history showed the frequency of APOE ε4 allele in Parkinson’s disease with dementia was twofold than without dementia (P=0.026). This result also supported APOE relationship with dementia associated with Parkinson’s disease [11]. In concordance with these study, a meta-analysis showed an association between APOE ε4 and dementia in Parkinson’s disease [27]. Though this study was challenged by its heterogeneous sources of data, it was supported by another meta-analysis with 786 non-Hispanic Caucasians, which showed a higher prevalence of dementia in patients with APOE ε4 carriers [22]. In contrast to these two meta-analyses, a longitudinal study and a cross-sectional study did not prove such relationship between APOE polymorphism and the early development of dementia in patients with Parkinson’s disease [21,23]. Overall, the relationship between APOE polymorphism and Parkinson’s disease is weaker than that of Alzheimer’s disease. However, APOE ε4 may affect the cognitive symptoms of this degenerative disease sharing a similar mechanism of neurotoxicity.
Summary and Conclusion
The human apoE protein is an essential protein in brain function including cognitive function. Among the three common APOE alleles are ε4 allele is most harmful, followed by, ε3 allele and ε2. Higher prevalence of APOE ε4 allele in Alzheimer’s disease can be an epidemiologic evidence of the detrimental effect of the allele. The structurally compact shape of APOE ε4 allele and mitochondrial dysfunction may be laboratory proof of the mechanism of APOE ε4 toxicity. There are many pieces of evidence of the association between APOE polymorphism and Parkinson’s disease suggested by cognitive decline of Parkinson’s disease, pathological similarities, and cerebrospinal fluid biomarkers. Studies searching possible correlation between the age at onset of Parkinson’s disease and APOE polymorphism was not successful. However, shreds of evidence are convincing APOE ε4 allele negatively affect the cognitive symptoms of Parkinson’s disease.
In conclusion, the most significant effect of human APOE polymorphism can be cognitive decline by the APOE ε4 allele. It is prominent in Alzheimer’s disease and less prominent in Parkinson’s disease. Therefore, genotyping APOE polymorphism may be a diagnostic test for the evaluation of risk of earlier cognitive decline in AD but adjusting this test for PD is not established until now. In future, modification of the harmful effect of APOE ε4 should be done to change the relentless cognitive decline in Parkinson’s disease and other neurodegenerative diseases.
References
- Mahley RW, Huang Y (2012) Apolipoprotein e sets the stage: Response to injury triggers neuropathology. Neuron 76: 871-885.
- Verghese PB, Castellano JM, Holtzman DM (2011) Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 10: 241-252.
- Mahley RW (1988) Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 240: 622-630.
- Mahley RW (2016) Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl) 94: 739-746.
- Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, et al. (1993) Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 342: 697-699.
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, et al. (1994) Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7: 180-184.
- Sen A, Alkon DL, Nelson TJ (2012) Apolipoprotein E3 (ApoE3) but not ApoE4 protects against synaptic loss through increased expression of protein kinase C epsilon. J Biol Chem 287: 15947-15958.
- Eberle D, Kim RY, Luk FS, de Mochel NS, Gaudreault N, et al. (2012) Apolipoprotein E4 domain interaction accelerates diet-induced atherosclerosis in hypomorphic Arg-61 APOE mice. Arterioscler Thromb Vasc Biol 32: 1116-1123.
- Rohn TT (2013) Proteolytic cleavage of apolipoprotein E4 as the keystone for the heightened risk associated with Alzheimer's disease. Int J Mol Sci 14: 14908-14922.
- Nakamura T, Watanabe A, Fujino T, Hosono T, Michikawa M (2009) Apolipoprotein E4 (1-272) fragment is associated with mitochondrial proteins and affects mitochondrial function in neuronal cells. Mol Neurodegener 4: 35.
- Parsian A, Racette B, Goldsmith LJ, Perlmutter JS (2002) Parkinson's disease and apolipoprotein E: Possible association with dementia but not age at onset. Genomics 79: 458-461.
- Marder K, Tang MX, Cote L, Stern Y, Mayeux R (1995) The frequency and associated risk factors for dementia in patients with Parkinson's disease. Arch Neurol 52: 695-701.
- Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, et al. (2004) Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord 19: 60-67.
- Boller F, Mizutani T, Roessmann U, Gambetti P (1980) Parkinson disease, dementia and Alzheimer disease: Clinicopathological correlations. Ann Neurol 7: 329-335.
- Mollenhauer B, Rochester L, Chen-Plotkin A, Brooks D (2014) What can biomarkers tell us about cognition in Parkinson's disease? Mov Disord 29: 622-633.
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61: 413-426.
- Jellinger KA, Attems J (2006) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 112: 253-260.
- Zareparsi S, Kaye J, Camicioli R, Grimslid H, Oken B, et al. (1997) Modulation of the age at onset of Parkinson's disease by apolipoprotein E genotypes. Ann Neurol 42: 655-658.
- Buchanan DD, Silburn PA, Prince JA, Mellick GD (2007) Association of APOE with Parkinson disease age-at-onset in women. Neurosci Lett 411: 185-188.
- Lopez M, Guerrero J, Yescas P, Boll MC, Familiar I, et al. (2007) Apolipoprotein E epsilon4 allele is associated with Parkinson disease risk in a Mexican Mestizo population. Mov Disord 22: 417-420.
- Ryu HG, Kwon OD (2010) Apolipoprotein E epsilon 4 allele is not associated with age at onset or MMSE of Parkinson's disease in a Korean study. Parkinsonism Relat Disord 16: 615-617.
- Gao J, Huang X, Park Y, Liu R, Hollenbeck A, et al. (2011) Apolipoprotein E genotypes and the risk of Parkinson disease. Neurobiol Aging 32: 2106 e1-6.
- Kurz MW, Dekomien G, Nilsen OB, Larsen JP, Aarsland D, et al. (2009) APOE alleles in Parkinson disease and their relationship to cognitive decline: A population-based, longitudinal study. J Geriatr Psychiatry Neurol 22: 166-170.
- Federoff M, Jimenez-Rolando B, Nalls MA, Singleton AB (2012) A large study reveals no association between APOE and Parkinson's disease. Neurobiol Dis 46: 389-392.
- Martinez M, Brice A, Vaughan JR, Zimprich A, Breteler MM, et al. (2005) Apolipoprotein E4 is probably responsible for the chromosome 19 linkage peak for Parkinson's disease. Am J Med Genet B Neuropsychiatr Genet 136B: 72-74.
- Pankratz N, Byder L, Halter C, Rudolph A, Shults CW, et al. (2006) Presence of an APOE4 allele results in significantly earlier onset of Parkinson's disease and a higher risk with dementia. Movement Disorders: Official Journal of the Movement Disorder Society 21: 45-49.
- Huang X, Chen P, Kaufer DI, Troster AI, Poole C (2006) Apolipoprotein E and dementia in Parkinson disease: A meta-analysis. Arch Neurol 63: 189-193.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4030
- [From(publication date):
January-2017 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 3110
- PDF downloads : 920