Malignant Mixed Mullerian Tumor with an Endometrioid Adenocarcinoma with a Component of Giant Cell Carcinoma: A Case Report and Literature Review
Received: 10-Feb-2015 / Accepted Date: 23-Feb-2015 / Published Date: 28-Feb-2015 DOI: 10.4172/2161-0681.1000212
Abstract
A 70 year old female underwent a hysterectomy due to a diagnosis of high grade endometrioid adenocarcinoma. Pathological examination revealed a Malignant Mixed Mullerian Tumor (MMMT) along with a conventional endometrioid adenocarcinoma with a component of giant cell carcinoma. Giant cell carcinoma of the endometrium is a rare tumor consisting of sheets of discohesive appearing cells with numerous anaplastic looking giant cells. Only 13 cases of giant cell carcinoma have been described and the majority present as a component of endometrioid carcinoma. The giant cells are often positive for keratin markers and are negative for CD68 and HCG which can help distinguish them from other tumors with multinucleate giant cells. Malignant Mixed Mullerian Tumors (MMMT) were once thought to originate from two separate neoplasms that underwent collision. But recently studies have found that these malignancies appear to arise from a malignant epithelial type cell that undergoes Epithelial to Mesenchymal Transition (EMT). Studies suggest that mutations in AKT2 and p53 result in increased levels of SLUG and ZEB, which represses E-cadherin. It is the loss of e-cadherin that is believed to be a central player in EMT. It is widely believed that tumors frequently display intra-tumoral heterogeneity that can result in neoplasms with striking variations in their characteristics. This case can make a nice example of how tumors can develop strikingly different characteristics.
Keywords: Malignant Mixed Mullerian Tumor; Giant cell carcinoma; Epithial to mesenchymal transition
312207Introduction
We report a case of a 70-year-old woman with a very interesting endometrial cancer. This tumor is a conventional endometrioid carcinoma with a component of a giant cell carcinoma, which is a recently described entity of which only 13 cases have been reported. In addition, a Malignant Mixed Mullerian Tumor (MMMT) was also present that, according to recent studies, appears to originate from epithelial cells that undergo transdifferentiation resulting in the sarcoma component. This appears to involve the loss of e-cadherin. This case provides the opportunity to discuss the unique characteristics of the giant cell carcinoma, as well as the evidence supporting the epithelial cell origin of the MMMT, which in the setting of intratumoral heterogeneity, can provide some insight as to how this patient can present with such an interesting tumor.
Clinical history
A 70-year-old woman with a family history of breast cancer, presented to her ob-gyn with a complaint of postmenopausal bleeding for the last several months. She had a transvaginal ultrasound, which showed an endometrial stripe of 28.6 millimeters. A biopsy was performed and a diagnosis of a high-grade endometrial carcinoma was rendered. A PET scan showed increased FDG uptake in the uterus, but no areas of possible metastasis was identified. She then proceeded to undergo a robotic total hysterectomy and bilateral salpingooophorectomy; however, due to her extensive peritoneal adipose tissue, lymph node sampling was not performed.
Pathologic findings
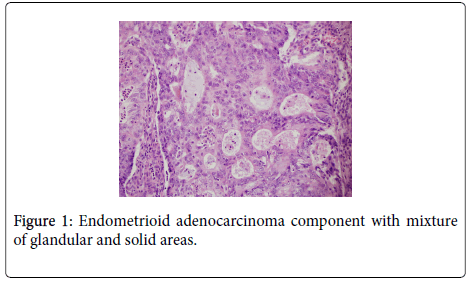
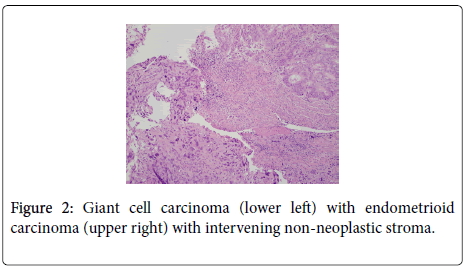
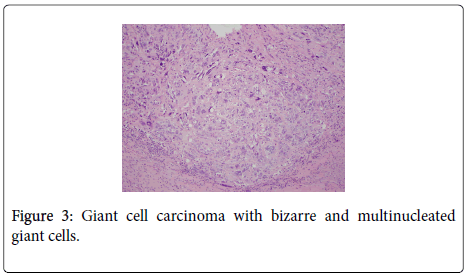
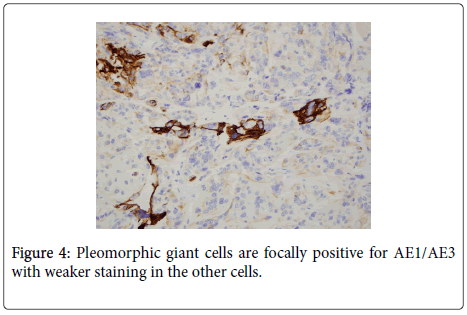
The uterus contained a 6.0 cm mass that appeared to originate from the anterior endometrium extending onto the posterior endometrium. Sectioning of the uterus revealed that the mass grossly did not appear to invade greater than 50% of the myometrium. Also present in the container was a separate 6.5 cm tan/white piece of tissue. Histological examination of the endometrial mass showed a conventional endometrioid adenocarcinoma with solid and glandular areas consistent with a FIGO grade 2 tumor (Figure 1). Areas of atypical complex hyperplasia were also identified. In addition, focal welldemarcated nodules that contained more pleomorphic appearing cells along with scattered bizarre, multinucleated giant cells were also present within this mass. (Figures 2 and 3) These cells were focally positive for cytokeratin, AE1/AE3 (Figure 4) and Epithelial Membrane Antigen (EMA); while negative for HCG and CD68. No malignant stromal component was identified in these areas. These features were most consistent with an endometrioid adenocarcinoma with a giant cell component. The tumor had only superficial myometrial invasion and focal lymphovascular invasion was identified.
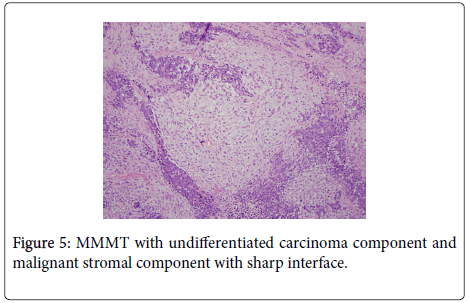
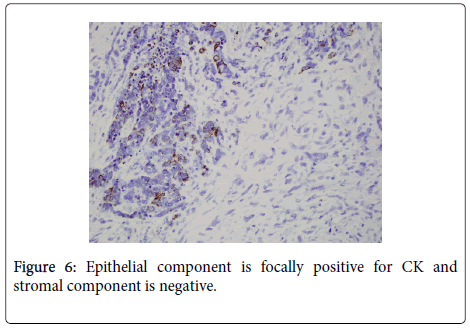
Histologic examination of the separate piece of tissue revealed a malignant biphasic neoplasm with one component consisting of welldefined nests consisting of groups of epithelial type cells with high nuclear to cytoplasmic ratio. Frequent mitotic figures and single cell necrosis was identified. The other component consisted of malignant spindle cells with pleomorphic nuclei and numerous mitoses. (Figure 5) Immunoperoxidase stain for cytokeratin was focally positive in the epithelial component, but was negative in the spindle cell component. (Figure 6) These features were most consistent with a Malignant Mixed Mullerian Tumor (MMMT) with the epithelial component, based on the morphology and pattern of cytokeratin stain was most consistent with an undifferentiated carcinoma.
Overall, this tumor had very interesting features. If the epithelial component of the MMMT were a giant cell and/or endometrioid carcinoma, this would best be called a MMMT; however, since the epithelial component in this case was an undifferentiated carcinoma, a diagnosis of MMMT with an endometrioid carcinoma with a giant cell component seemed the most appropriate choice. No disease was identified in the ovaries or fallopian tubes. The tumor was staged as FIGO 1A.
Clinical follow-up
The patient’s post-operative course was uneventful and she was discharged the following day. Two weeks later she was started on chemotherapy consisting of carboplatin and taxol.
Discussion
This is a unique case of a MMMT with a co-existing endometrioid carcinoma with a giant cell component. Giant cell carcinoma of the endometrium is a rare neoplasm composed of large, bizarre, multinucleated giant cells that is often discohesive and grows in sheets. This type of malignancy was first described in 1991 [1]. To date, 13 cases have been described. In the cases reported, patients range in age from 43 to 83 years with an average age of 65. As was described in the current case, these tumors most frequently occur in conjunction with an endometrioid carcinoma, although pure giant cell carcinomas have been described as well as one case of a clear cell and one of a serous carcinoma. In nine cases, the tumor was stage 1 and of the cases with available follow-up, six had no evidence of disease after an average of 54 months (range 15-168 months) [2-4]. These tumors are composed of pleomorphic and relatively discohesive cells with malignant multinucleate giant cells intermixed. Lymphoplasmacytic inflammation is frequently present with some cells showing emperipolesis. The giant cells are focally positive for cytokeratin and EMA while negative for CD68 and HCG [2,5]. This is not to be confused with conventional endometrial carcinomas with osteoclast like giant cells. In these cases, the giant cells have monomorphic nuclei and are positive for CD68 and negative for cytokeratin indicating a histiocytic origin [5]. Endometrial carcinomas have also been known to have choriocarcinomatous differentiation with syncytiotrophoblastic giant cells. These neoplasms typically have foci of hemorrhage and the giant cells are positive for HCG [6]. In our case, the malignant appearance of the giant cells lead to a high degree of suspicion that this was a giant cell carcinoma and the immunoprofile confirmed it. Mesenchymal neoplasms with giant cells have also been described in the uterus, including endometrial stromal tumors, smooth muscle tumors, rhabdomyosarcomas and undifferentiated sarcomas [7,8]. Although these neoplasms can present diagnostic challenges, thorough sampling of the tumor will reveal features consistent with these types of neoplasms, and immunoperoxidase studies for cytokeratin along with smooth and skeletal muscle markers can help delineate the tumors true identity. To our knowledge, no molecular studies have been undertaken in giant cell carcinomas due to their rarity in the endometrium. However, one study conducted on giant cell carcinomas of the lung found an association of these tumors to a microdeletion in transforming growth factor receptor [9]. Whether this association can be found in giant cell tumors of the endometrium remains to be seen.
Malignant Mixed Mullerian tumor (MMMT) is a biphasic neoplasm composed of a malignant epithelial component and a malignant stromal component. MMMT’s are rare tumors, which account for up to 5% of uterine malignancies [10]. The epithelial component is typically a high-grade carcinoma of endometrioid or nonendometrioid type. Studies have yielded mixed results as to which epithelial component is more common, but it appears that the majority are endometrioid and serous types over clear cell and undifferentiated types, although our case had the morphology and immunoprofile most suggestive of an undifferentiated type [11]. The stromal component can be divided into two groups. Homologous elements are sarcomas having morphologic features of mesenchymal elements normally present in the uterus. Examples are endometrial stromal sarcoma, leiomyosarcoma, and fibrosarcoma. The other type is heterologous elements. These are sarcomatous elements normally not found in the uterus, which include rhabdomyosarcoma, chondrosarcoma, liposarcoma or osteosarcoma. The presence of malignant appearing cartilage in our MMMT is an example of having heterologous elements. As was seen in this case, these tumors occur predominantly in postmenopausal women and generally are associated with a poor prognosis: 5-year survival rates reported between 30% and 45.8% in FIGO 1 and 2 tumors and 0% and 10% for FIGO 3 and 4 [12].
The distinctive morphological features of MMMT have prompted two main theories on its origin. One was that the tumor arose from a separate carcinoma and sarcoma that collided and the other was of a single cell origin. In a study of epithelial and mesenchymal clones of a MMMT cell line, it was discovered that the epithelial cells were capable of producing both morphologies, while the mesenchymal cells could not [13]. It is now thought that MMMT’s originate from a malignant epithelial cell, which, in the process of its evolution, undergoes transdifferentiation of some of its epithelial cells to malignant mesenchymal cells by losing their cell polarity and cell-to-cell contacts while acquiring mesenchymal characteristics. This process is known as Epithelial to Mesenchymal Transition (EMT). It is thought that three types of EMT exist. Type 1 occurs in embryogenesis when cells of the neural crest migrate and generates the different tissues in the embryo. Type 2 is associated with wound healing, and type 3 occurs in epithelial cancer during invasion and metastasis [11]. It appears that Ecadherin, a protein involved in cell-to-cell adhesion, plays an important role in EMT. MMMT’s have been found to frequently harbor mutations in AKT2 and p53. Studies have suggested that amplification of AKT2 can cause increased phosphorylation of glucogen synthase kinase 3B (GSK3B). GSK3B, when phosphorylated, stabilizes B-catenin. When this occurs, B-catenin has a greater tendency to migrate to the nucleus and activate transcription. One of B-catenin’s targets is SLUG. SLUG is a modulator that represses transcription of e-cadherin reducing its availability for placement in the cell membrane [13,14]. P53 is also involved in regulation of ecadherin expression by its effects on the levels of SLUG. P53 also regulates SLUG levels by promoting MDM2 mediated ubiquitination and proteasome degradation of SLUG. When p53 is mutated or absent, this system does not function, increasing levels of SLUG and thus reducing the levels of e-cadherin [13]. P53 also influences e-cadherin expression by influencing levels of certain families of micro-RNA. Studies analyzing micro-RNA levels in the epithelial and mesenchymal components of MMMT’s have found that a family of micro-RNA’s, known as miR-200 is significantly reduced in the mesenchymal component versus the epithelial component [15]. The miR-200 family, which is directly induced by p53, serves as posttranslational repressors of Zeb. Zeb represses e-cadherin in a manner similar to SLUG, and when miR-200 levels are reduced, transcription of Zeb increases, leading to repression of e-cadherin [13,16]. The net effect is the loss of e-cadherin resulting in loss of cohesion and cell polarity and subsequently resulting in diversion into two different tissue types forming a MMMT as what was seen in the present case.
Although cancer is thought to arise from a cell with mutations that activate oncogenes and deactivate tumor suppressor genes, it has become widely accepted that through the evolution of tumors, subsequent changes occur that, eventually, would result in a tumor with genetic and phenotypic diversity [17]. Our case appears to be an example of a endometrioid carcinoma within which certain changes occurred in one of the tumor cells that eventually gave rise to a giant cell carcinoma; while another cell within the same tumor, went down a pathway that resulted in a undifferentiated carcinoma within which some of those cells, according to current evidence, underwent transdifferentiation into a co-existing malignant mesenchymal neoplasm resulting in a MMMT. This case makes a nice example of how in the process of evolution; certain tumors can develop strikingly different morphologic and clinical characteristics.
References
- Jones MA Young RH, Scully RE (1991) Endometrial adenocarcinoma with a component of giant cell carcinoma. Int J GynecolPathol 10: 260-270.
- Mulligan AM Plotkin A, Rouzbahman M, Soslow RA, Gilks CB, et al. (2010) Endometrial giant cell carcinoma: a case series and review of the spectrum of endometrial neoplasms containing giant cells. Am J SurgPathol 34: 1132-1138.
- Kurita T Kashimura M, Hamada T, Hisaoka M (2006) Giant cell carcinoma of the uterine cervix: a case report. Int J GynecolPathol 25: 298-300.
- Bhattacharyya A, Gon S, Bandyopadhyay G, Bipasa M, Prosenjit G (2012) Giant Cell Carcinoma of Endometrium: A Rare Clinical Entity. Iranian J Pathol 7: 197-202.
- Vasenwala SM Beg S, Ansari HA, Haider N, Sharma R (2012) Endometrial adenocarcinoma with osteoclastic giant cells in stroma: a case report and review of literature. Arch GynecolObstet 285: 1157-1160.
- Akbulut M Tosun H, Soysal ME, Oztekin O (2008) Endometrioid carcinoma of the endometrium with choriocarcinomatous differentiation: a case report and review of the literature. Arch GynecolObstet 278: 79-84.
- Manglik N Sawicki J, Saad A, Fadare O, Soslow R, et al. (2012) Giant cell tumor of uterus resembling osseous giant cell tumor: case report and review of literature. Int J SurgPathol 20: 618-622.
- Patai K Illyes G, Varbiro S, Gidai J, Kosa L, et al. (2006) Uterine leiomyosarcoma with osteoclast like giant cells and long standing systemic symptoms. GynecolOncol 102: 403-405.
- Wang JC Su CC, Xu JB, Chen LZ, Hu XH, et al. (2007) Novel microdeletion in the transforming growth factor beta type II receptor gene is associated with giant and large cell variants of nonsmall cell lung carcinoma. Genes Chromosomes Cancer 46: 192-201.
- Tong SY Lee JM, Choi YJ, Lee JK, Kim JW, et al. (2012) The comparison of clinicopathological characteristics in primary malignant mixed mullerian tumour  with epithelial endometrial carcinoma. Aust N Z J ObstetGynaecol 52: 44-48.
- Lopez-Garcia MA Palacios J (2010) Pathologic and molecular features of uterine carcinosarcomas. SeminDiagnPathol 27: 274-286.
- de Jong RA Nijman HW, Wijbrandi TF, Reyners AK, Boezen HM, et al. (2011) Molecular markers and clinical behavior of uterine carcinosarcomas: focus on the epithelial tumor component. Mod Pathol 24: 1368-1379.
- Voutsadakis IA (2012) Epithelial to mesenchymal transition in the pathogenesis of uterine malignant mixed Müllerian tumours: the role of ubiquitin proteasome system and therapeutic opportunities. ClinTranslOncol 14: 243-253.
- Saegusa M Hashimura M, Kuwata T, Okayasu I (2009) Requirement of the Akt/beta-catenin pathway for uterine carcinosarcoma genesis, modulating E-cadherin expression through the transactivation of slug. Am J Pathol 174: 2107-2115.
- Castilla Mà Moreno-Bueno G, Romero-Pérez L, Van De Vijver K, Biscuola M, et al. (2011) Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol 223: 72-80.
- Mirantes C Espinosa I, Ferrer I, Dolcet X, Prat J, et al. (2013) Epithelial-to-mesenchymal transition and stem cells in endometrial cancer. Hum Pathol 44: 1973-1981.
- Marusyk A Polyak K (2010) Tumor heterogeneity: causes and consequences. BiochimBiophysActa 1805: 105-117.
Citation: Johannesen E, Nguyen V (2015) Malignant Mixed Mullerian Tumor with an Endometrioid Adenocarcinoma with a Component of Giant Cell Carcinoma: A Case Report and Literature Review. J Clin Exp Pathol 5:212. DOI: 10.4172/2161-0681.1000212
Copyright: © 2015 Johannesen E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17266
- [From(publication date): 4-2015 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 12418
- PDF downloads: 4848