Malignant Transformation of Osteoblastoma of the Capitate: A Case Report
Received: 02-Aug-2022 / Manuscript No. joo-22-70980 / Editor assigned: 05-Aug-2022 / PreQC No. joo-22-70980 (PQ) / Reviewed: 22-Aug-2022 / QC No. joo-22-70980 / Revised: 29-Aug-2022 / Manuscript No. joo-22-70980 (R) / Published Date: 06-Aug-2022 DOI: 10.4172/2472-016X.1000178
Abstract
Osteoblastoma is classically described as a more aggressive form benign bone forming tumors that mainly occurs in the axial skeleton and spine. There have been few case reports and case series of this lesion occurring in the carpal ones, and only three reported cases occurring in the capitate, which were all treated successfully with curettage and bone grafting. Here we present the first reported case of a patient who was diagnosed with osteoblastoma of the capitate who had disease recurrence despite two attempts at curettage and bone grafting followed by capitate resection, ultimately leading to malignant transformation.
Introduction
Osteoblastoma, osteoma, and osteoid osteoma, are a group of benign lesions that are characterized by tumor cells that produce osteoid or mature bone. Although osteoid osteoma and osteoblastoma appear histologically very similar, there are key differences in clinical presentation and distribution. While osteoid osteoma are classically characterized by nocturnal pain improved with non-steroidal antiinflammatory medications, osteoblastomas occur mainly in the axial skeleton, namely in posterior elements of the spine, pelvis, and long bones [1]. However, there have been rare instances of involvement in the hand and wrist, with a few case reports describing occurrence in various carpal bones [2, 3]. Due to its rarity, there are very few studies and primary literature available to guide treatment of osteoblastoma of the carpal bones. This case report intends to shed light on this issue by describing an aggressive osteoblastoma that originated the capitate and recurred despite multiple attempts at surgical intervention, eventually undergoing malignant transformation into an osteosarcoma.
Case
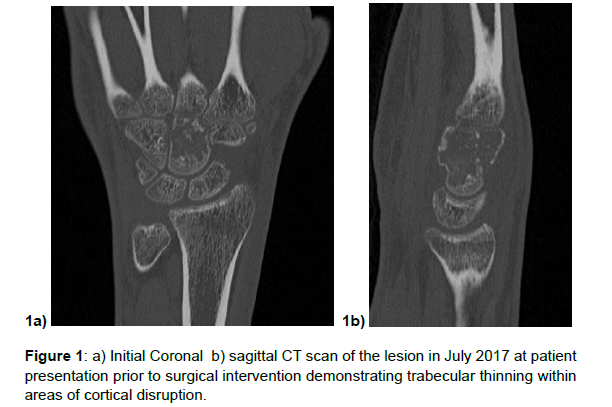
The patient is a 19 year old female who presented in May of 2017 with a gradual several month onset of wrist pain. Despite conservative efforts her pain continued thus xrays were performed which demonstrated a lytic lesion of the capitate, followed by an MRI which demonstrated decreased signal on T1 and increased signal on T2 with extensive marrow replacing lesion of the capitate. At first this finding appeared similar to avascular necrosis of the capitate, however CT scan was obtained demonstrating trabecular thinning with areas of cortical disruption within the capitate, this initially appeared to be then more consistent with a low grade cartilaginous tumor such as a chondroblastoma or enchondroma (Figure 1a and 1b).
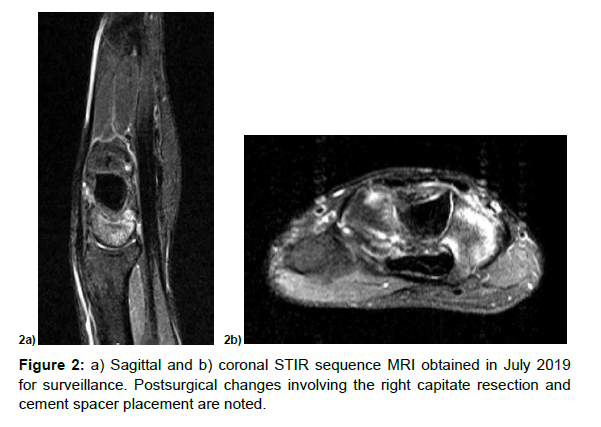
Postsurgical changes involving the right capitate resection and cement spacer placement are noted. There is bone marrow edema within the hamate, lunate and scaphoid and surrounding edema in the surrounding soft tissues.
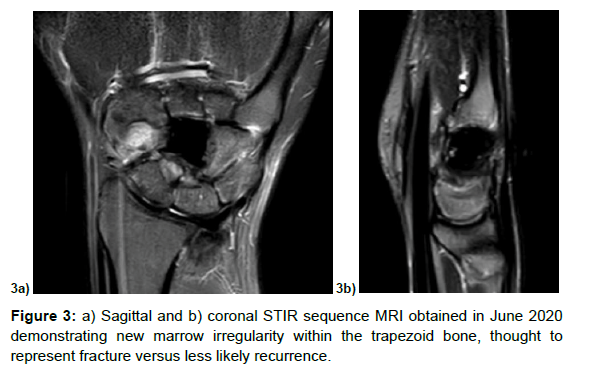
One year later she noticed pain in her volar thumb, thus repeat MRI and CT demonstrated marrow irregularity within the trapezoid consistent with non-displaced fracture (Figure 3a and 3b). Although there was noted to be marrow edema within the base of the third metacarpal, hamate, and lunate, this appeared consistent with reactive post-surgical changes of the adjacent bones. The patient wished to pursue conservative management at this point with removable splinting.
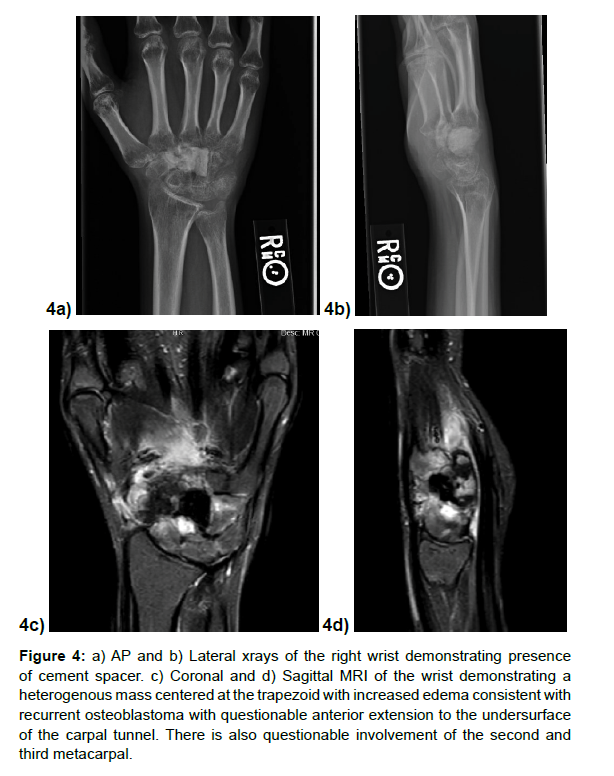
She continued to notice pain, but her symptoms improved with application of a short arm cast, thus she was initially offered a total wrist fusion. Prior to surgery, a repeat surveillance MRI in April 2021 demonstrated interval mass formation in the 2nd and 3rd metacarpal base, trapezoid, trapezium, and scaphoid bones (Figure 4a-4d). She was then referred to our institution for further management. Given involvement of multiple carpal bones, it was recommended she undergo total carpectomy with removal of proximal second and third metacarpals in a staged fashion, followed by placement of a cement spacer. If final pathology report demonstrated clean margins, she would undergo ipsilateral iliac crest bone graft followed by total wrist fusion.
Figure 4: a) AP and b) Lateral xrays of the right wrist demonstrating presence of cement spacer. c) Coronal and d) Sagittal MRI of the wrist demonstrating a heterogenous mass centered at the trapezoid with increased edema consistent with recurrent osteoblastoma with questionable anterior extension to the undersurface of the carpal tunnel. There is also questionable involvement of the second and third metacarpal.
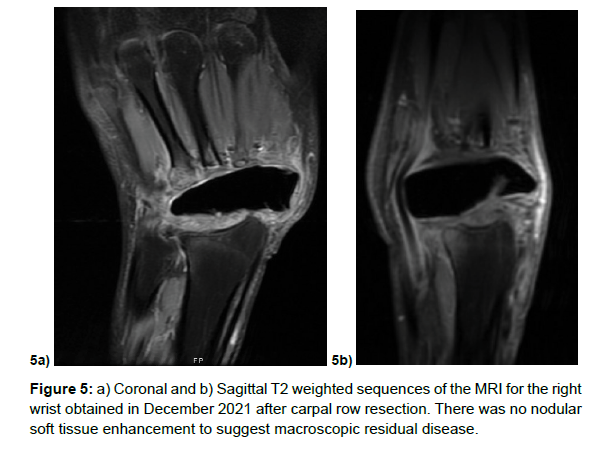
She underwent the first stage of the procedure on August 2021 successfully, the remnants of the proximal carpal row were exposed demonstrating a conglomerate mass with remnants of the lunate and triquetrum in addition to the remnants of the distal carpal row. There appeared involvement of the second metacarpal on gross inspection, thus a sample was obtained. The patient tolerated the procedure well and was discharged home the same day. Unfortunately the final pathology demonstrated High-grade osteosarcoma (3.1 cm) arising from osteoblastoma and involving multiple bones with extraosseous invasion, with second metacarpal positive for neoplasm. After a multidisciplinary tumor board discussion, the consensus diagnosis was consistent with malignant transformation of osteoblastoma to high grade osteosarcoma. In light of this diagnosis, further oncologic workup did not reveal evidence of lung metastasis. She was offered a limb salvage option consisting of ray amputation of the index finger following by ring external fixator of the wrist prior to starting chemotherapy. The alternative option with the goal of reducing possibility of recurrence would have been to pursue trans-radial amputation. The patient wished to proceed with trans-radial amputation. She was seen by medical oncology where standard MAP chemotherapy regimen was started consisting of doxorubicin and cisplatin for week 1-3, followed by high-dose methotrexate on week 4 and 5 of a 5 week cycle. She then proceeded with with two cycles of chemotherapy prior to trans-radial amputation, followed by approximately 4 cycles of chemotherapy following surgery. Other than two admissions to correct elevated methotrexate and leuvocorin levels, her chemotherapy course was largely uncomplicated and surveillance MRI demonstrated no acute findings (Figure 5a and 5b).
After completion of initial chemotherapy regimen, she was underwent a trans-radial amputation on April 2022 with regenerative peripheral nerve interface onto the median, ulnar, and dorsal radial sensory nerve harvested from a vastus lateralis muscle graft with Orthopedic oncology and Plastic surgery. She tolerated the procedure well and discharged home on post operative day one. Final pathology showed no residual viable osteosarcoma and her most recent surveillance imaging demonstrated no disease recurrence.
Discussion
Osteoblastoma accounts for 1% of all primary bone tumors and 3% of all benign bone tumors [1]. Although similar to osteoid osteoma, an osteoid osteoma tends to regress while osteoblastoma tends towards progression and malignant transformation [4]. In a series of 98 cases of osteoblastoma, patients who were symptomatic tended to experience a dull, localized pain, with a median duration of symptoms of 6 months before they presented for treatment [5].
Although characterized as a benign lesion, there is a category of more aggressive osteoblastomas which can result in disease recurrence despite surgical treatment as in the case of this patient. Even though histologically similar to conventional osteoblastoma, aggressive lesions have been reported to generally lack perifocal bone sclerosis in addition to having a thin shell of newly formed periosteal bone covering the lesion [6]. Additionally aggressive osteoblastomas are characterized by “epithelioid” osteoblasts twice the size of osteoblasts with cells that are rounded, have larger nuclei, and abundant cytoplasm. Furthermore the bone trabeculae of these aggressive cells are wider and more irregular than conventional osteoblastoma while tending to have no cement lines [3]. Few studies have been published in order to diagnose more aggressive osteoblastomas. Traditionally the presence of epithelioid osteoblasts, lace- or sheetlike osteoid production, and a permeative pattern of tumor growth in osteoblastomas is thought to be associated with an aggressive clinical behavior [7]. Unfortunately, despite these histological characteristics a study of 55 patients with osteoblastoma found no association between the histologic features and disease outcome. The authors concluded that aggressive behavior of osteoblastoma was not related to particular histologic features, but rather to the skeletal location [7].
Treatment of these lesions should take into account their aggressive nature and recurrence rate despite removal. In a review of 20 cases of osteoblastoma, the most frequent location was in the posterior elements of the spine, followed by the long bones of the extremities and the talus [8]. The authors found intralesional curettage the most common modality of treatment with a 13% recurrence rate. Other studies estimate this recurrence rate at 10-20% [9]. Although often treated successfully with curettage, wide excision should be considered along with careful long term follow up due to the possibility recurrence or malignant transformation.
Unfortunately due to their low prevalence, there are very few studies of malignant transformation of an osteoblastoma, however a few case reports shed light on this issue. The authors of one study describe the case of a 49 year old patient who was initially diagnosed with a high grade osteoblastoma originating from a right petrous apex lesion [10]. Despite two attempts at resection, radiation, and chemotherapy the patient developed a lower abdominal mass diagnosed as an osteosarcoma and the patient died from burden of disease. Another case report was described of a 13 year old patient diagnosed with an aggressive osteoblastoma in the left proximal tibia [11]. Despite curettage and bone grafting, the lesion recurred two years later with repeat biopsy showing osteosarcoma. He then underwent resection and reconstruction with megaprosthesis, however after 14 months of chemotherapy the patient died due to complications of lung metastases. Lastly another case was reported of a 57 year old patient with an aggressive left proximal humerus lesion that was initially diagnosed with osteoblastoma with focal malignant transformation to well differentiated osteosarcoma [12]. Despite undergoing proximal third humerus resection, extensive local soft tissue recurrence occurred and at 8 months the patient developed pulmonary metastasis. However chemotherapy was able to reduce soft tissue size and recurrence while halting the lung metastasis already present. Interestingly to aid in diagnosis, the use of DNA microdensity of biopsies has shown promise in distinguishing the stage of osteoblastoma malignant transformation [13]. Most malignant transformations of aggressive osteoblastomas have occurred in recurrent tumors, suggesting that osteoblastomas may primarily undergo early malignant transformation. Those that do undergo transformation require aggressive surgical approach followed by chemotherapy in the hope of minimizing disease burden.
In regards to osteoblastoma of the hand, similarly few studies exist other than a series of case reports. Out of 26,800 total primary bone tumors, one study found only 0.16% primary neoplasms of carpal bones, with the most common locations including the scaphoid and capitate, which together accounted for 52% of all neoplasms [2, 14]. A case series of five cases of benign osteoblastoma in the hand demonstrated 3 in metacarpals and two in phalanxes, with clinical courses varying considerably, however swelling with an absence of response to salicylates was found to be a persistent finding in all patients [15]. In all cases, osteoblastoma was not considered as a primary diagnosis, however curettage and bone grafting was successful in all cases with no case of malignant transformation. Apart from curettage with bone grafting, treatments in more advanced or recurrent cases include bone resection, followed by additional staged procedures such as structural bone graft replacement, four-corner fusion and proximal row carpectomy for scaphoid resection [16].
Several case reports have been reported regarding low grade and aggressive osteoblastoma in the scaphoid treated with scaphoidectomy [17, 18]. A few case reports in regards to the hamate demonstrate the varying clinical presentations. One case of a 26 year old patient with a grade 2 osteoblastoma occupying three quarters of the hamate underwent resection of hamate and at 12 months had no dislocation of carpal bones or disease recurrence [19]. On the contrary, another case report describes the treatment of osteoblastoma of the hamate in a 13 year old patient that recurred 6 months after initial curettage [16]. The authors described a two stage procedure with the first stage consisting of segmental resection including ulnar carpal bones, distal ulnar head, ulnar third of the distal radius, and bases of the ring and small metacarpals, where the bony defect was filled with cement and K wires. After absence of recurrence several months later, the second stage consisted of cement removal with harvest of a bicortical vascularized iliac crest flap used to fill the defect and anastamosed to the radial artery. This was followed by a Sauve-Kapandji operation to restore pronation and supination with an absence of recurrence at 3 years with good function. The authors discuss that local head generated during polymerization of the PMMA may destroy tumor cells. Had the recurrence not occurred in the patient of the above case, this would have likely been the preferred treatment plan to restore function.
Sadly there are only three case reports of osteoblastomas in the capitate, one of which was a smaller lesion that was successfully treated with curettage and no bone graft [20]. The other two cases describe successful treatment with intralesional resection bone grafting, one of which employed an ipsilateral olecranon bone graft [21, 22]. Our case is the first reported case of an osteoblastoma of the capitate that not only recurred but also underwent malignant transformation into an osteosarcoma. This case is intended to serve as a guide for recognition and management of recurrent aggressive osteoblastoma of not just the capitate but of the hand in general.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Greenspan A (1993) Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Skeletal Radiol 22: 485-500.
- Murray PM, Berger RA, Inwards CY (1999) Primary neoplasms of the carpal bones.J Hand Surg Am 24: 1008-1013.
- Dorfman HD, Weiss SW (1984) Borderline osteoblastic tumors: problems in the differential diagnosis of aggressive osteoblastoma and low-grade osteosarcoma. Semin Diagn Pathol 1215-34.
- Jackson RP, Reckling FW, Mants FA (1977). Osteoid osteoma and osteoblastoma. Similar histologic lesions with different natural histories. Clin Orthop Relat Res 128: 303-313.
- Kroon HM, Schurmans J (1990) Osteoblastoma: clinical and radiologic findings in 98 new cases.Radiology175: 783-790.
- https://www.worldcat.org/title/17546115
- Della RC, Huvos AG (1996) Osteoblastoma: varied histological presentations with a benign clinical course: an analysis of 55 cases.Am J Surg Pathol20: 841-850.
- Saglik Y, Atalar H, Yildiz Y, Basarir K, Gunay C (2007) Surgical treatment of osteoblastoma: a report of 20 cases.Acta orthopaedica Belgica73: 747.
- Lucas DR, Unni KK, McLeod RA, O'Connor MI, Sim FH (1994) Osteoblastoma: clinicopathologic study of 306 cases.Hum Pathol25: 117-134.
- Kraft CT, Morrison RJ, Arts HA (2016) Malignant transformation of a high-grade osteoblastoma of the petrous apex with subcutaneous metastasis. Ear Nose Throat J 95: 230-233.
- Gorgunn O, Salduz A, Kebudi R, Ozger H, Bilgic B (2016) Malignant transformation of aggressive osteoblastoma to ostesarcoma.Joint Diseases and Related Surgery27:108-112.
- Kunze E, Enderle A, Radig K, Schneider-Stock R (1996) Aggressive osteoblastoma with focal malignant transformation and development of pulmonary metastases. A case report with a review of literature.Gen Diagn Pathol 141:377-392.
- Grace J, McCarthy S, Stankovic R, Marsden W (1993) Malignant transformation of osteoblastoma: study using image analysis microdensitometry.J Clin Pathol46: 1024-1029.
- Murray PM, Berger RA, Inwards CY (1999) Primary neoplasms of the carpal bones.J Hand Surg Am24: 1008-1013.
- Farzan M, Mortazavi SMJ, Spar R (2006) Hand Osteoblastoma. Tehran Univ Med J64: 85-90.
- Winters H, Wuisman P (1999) Recurrent osteoblastoma of the hamate bone. A two-stage reconstruction with a free vascularized iliac crest flap. J Hand Surg Br 24: 501-505.
- Ragois P, Leclerc P, Hallonet D (2000) Aggressive osteoblastoma of the carpal scaphoid bone.Rev Chir Orthop Reparatrice Appar Mot86: 94-97.
- Fanning JW, Lucas GL (1993) Osteoblastoma of the scaphoid: a case report. J Surg Orthop Adv 18: 663-665.
- https://www.worldcat.org/title/1189030880
- Afshar A (2012) Osteoblastoma of the capitate bone.J Hand Microsurg4: 34-38.
- de Lima LGD, de Castro UB, Martins GPF (2021) Osteoblastoma do capitato: Relato de caso. Revista Brasileira de Ortopedia 4: 529-531.
- Kaptan C, Atmaca H (2014) Osteoblastoma of the os capitatum.Case Rep Orthop 2014: 241716.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ahmady AA, Ruse SM, Siegel GW, Ozer K (2022) Malignant Transformation of Osteoblastoma of the Capitate: A Case Report . J Orthop Oncol 8: 178. DOI: 10.4172/2472-016X.1000178
Copyright: © 2022 Ahmady AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2242
- [From(publication date): 0-2022 - Dec 16, 2025]
- Breakdown by view type
- HTML page views: 1776
- PDF downloads: 466