Research Article Open Access
Optimization and Validation of a Reverse-Phase High Performance Liquid Chromatography Assay with Ultra-Violet Detection for Measuring Total L-Ascorbic Acid in Food and Beverage Products
Olivia L Parbhunath*, Fanie Rautenbach, Glenda Davison and Jeanine L MarnewickFaculty of Health and Wellness Sciences, Oxidative Stress Research Centre, Cape Peninsula University of Technology, South Africa
- *Corresponding Author:
- Olivia L Parbhunath
Oxidative Stress Research Centre
Faculty of Health and Wellness Sciences
Cape Peninsula University of Technology
P.O. Box 1906, 7538, South Africa
Tel: +2721 9538417
Fax:+27 21 959 8490
E-mail: ParbhunathO@cput.ac.za
Received date: July 29, 2014; Accepted date: August 21, 2014; Published date: August 26, 2014
Citation: Parbhunath OL, Rautenbach F, Davison G, Marnewick JL (2014) Optimization and Validation of a Reverse-Phase High Performance Liquid Chromatography Assay with Ultra-Violet Detection for Measuring Total L-Ascorbic Acid in Food and Beverage Products. J Anal Bioanal Tech 5:201 doi: 10.4172/2155-9872.1000201
Copyright: © 2014 Parbhunath OL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In accordance with national and international regulatory standards, namely ISO/IEC 17025, the validation of chromatography methods is becoming necessary. This study provides an optimized and fully validated reversephase high performance liquid chromatography (RP-HPLC) assay with ultra-violet (UV) detection for the measurement of L-ascorbic acid (L-AA) in fruit, vegetable and food products.
Several commercial fruit juices and teas, fresh fruit and vegetables and food extract products were analyzed using a high performance liquid chromatographic system with UV detection. Chromatographic separation of L-AA was achieved on a reverse phase C18 150 mm×4.6 mm, 0.5 μm column with UV detection of 245 nm at room temperature. Distilled water/acetonitrile/formic acid (99: 0.9: 0.1, v/v/v) at a flow rate of 1 mLmin-1 was used as the mobile phase, in isocratic mode. Samples were extracted in 4.5% metaphosphoric acid solution and filtered through a 0.45 μm membrane. The method was validated for accuracy, precision, linearity, range, limit of detection, limit of quantification, specificity, stability, robustness and system suitability in accordance with ISO 17025 validation requirements. Validation results demonstrated a linear response within a range of 5 to 125 μg/mL with a correlation coefficient of 0.999 was obtained. Mean recoveries ranged from 99 to 103% and 92 to 96% for L-AA standards and samples, respectively. The method was found to be precise (COV’s <5%) and specific with no interferences from coexisting peaks. The LOD and LOQ were 0.61 μg/mL and 1.84 μg/mL respectively.
The successful optimization and validation of the proposed method should make it easily applicable for routine laboratory analysis of L-AA measurement in various fruit and vegetable products.
Keywords
Validation; L-ascorbic acid; High performance liquid chromatography; International Organization for Standards (ISO 17025)
Introduction
Vitamin C or L-ascorbic acid (L-AA) plays a pivotal role in many biological and metabolic processes. Collagen, carnitine and hormone production; bone formation; protection of the immune system; reduction in cholesterol due to its involvement in cholesterol metabolism include some of L-AA’s important physiological roles. Of paramount importance is vitamin C’s antioxidant role in counteracting reactive oxygen species (ROS) and reducing oxidative stress and possibly oxidative damage. The water-solubility of vitamin C allows it to exert its antioxidant activities both within and outside of the cell, subsequently protecting the cell from potential DNA, proteinand lipid damage normally caused by ROS. Several studies have demonstrated a reduced incidence of cataracts, cardiovascular disease and cancer with the intake of L-ascorbic acid [1,2]. Therefore, it is evident that vitamin C is vital to the normal functioning of the human biological systems.
Aside from its biological benefits, L-ascorbic acid also has application within the food and beverage industry. It has been widely used within the food industry as a preservative due to its powerful reducing action, thereby increasing the shelf-life of food and beverage products [3]. The incorporation of fatty esters of vitamin C in cosmetics and is derived from the positive association between its intake/ application and the reduced incidence of certain pathological diseases [4]. Dietary sources rich in vitamin C include green leafy vegetables, peppers, broccoli, brussel sprouts, citrus and tropical fruits [5].
The vast applications and health benefits associated with L-ascorbic acid, has spiked a significant amount of interest within the food industry,resulting in an escalation in L-ascorbic acid assay requests. Several analytical methods have been reported for the analysis of L-ascorbic acid in food and beverage products, some of which include titration, electrochemical methods [6], spectrophotometry [7], potentiometric methods, enzymatic methods and chromatographic methods [8]. However, advantages such as optimal separation potential, ease of operation, rapid analysis time and high accuracy and sensitivity have contributed to the use of high pressure liquid chromatography (HPLC) as a preferred method for vitamin C analysis [9,10]. Further, factors such as robustness, cost effectiveness, simplicity and a common frequency range for which many analytes absorb light, favours the use of ultra-violet (UV) detection as a preferred detection method [11].
Equally important to the selection of an analytical method is the quality, reliability, and regularity of results produced by such a method [12]. Hence, the process of method validation is clearly warranted as a means to verify that the HPLC method employed is acceptable for the procedure/purpose it is intended for [13]. Additionally, national government legislation (Regulation 146/2010 as part of the Foodstuffs, Cosmetics and Disinfectants Act of South Africa, Act 54 of 1972) and several international regulatory organizations namely, the Food and Drug Administration (FDA), World Health Organisation (WHO), U.S. Department of Agriculture (USDA), Centers for Disease Control and Prevention (CDC) and the Codex Alimentarius Commission (CAC) are compelling food and beverage manufacturers to use validated analytical methods to analyze products [14].
The literature reveals validation of UV-HPLC methods for the measurement of vitamin C have been performed largely on pharmaceutical products [15-17]. Few UV-HPLC methods have been validated for the measurement of vitamin C in food products [18,19].
The aim of this study is to validate a reversed-phase HPLC method in accordance with ISO/IEC 17025 validation requirements using UV detection for the quantification of L-AA in several food samples as well as some commercial beverages.
Materials and Methods
Chemicals and reagents
The chemicals formic acid, sulphuric acid (H2SO4), hydrochloric acid (HCL) and sodium hydroxide (NaOH) and methanol were purchased from Merck (Johannesburg, South Africa). Meta-phosphoric acid (MPA) and L-ascorbic acid (L-AA) was purchased from Sigma- Aldrich (Johannesburg, South Africa). Acetonitrile (gradient grade for liquid chromatography) was purchased from Saarchem (Johannesburg, South Africa). HPLC grade water was obtained from a Millipore Synergy water purification system (Cape Town, South Africa). A standard stock solution of L-AA (1 mg/ml) was prepared in 4.5% MPA prior to analysis each day and stored away from light at 4°C when not in use.
Equipment
All analyses were carried out on an Agilent 1200 Series HPLC system purchased from Agilent Technologies (Johannesburg, South Africa). The chromatographic system was equipped with a reverse phase C18 column (150 mm×4.6 mm, 0.5 μm in particle size) purchased from YMC Co., Ltd. (Cape, South Africa), a quaternary pump and a UV detector set at 245 nm. Two different isocratic mobile phases were tested: (a) 0.01% solution of sulphuric acid adjusted to pH 2.6 [10] and (b) distilled water/acetonitrile/formic acid (99: 0.9: 0.1, v/v/v) adjusted to pH 2.6. The flow rate was set at 1 ml/min and the injection volume was 20 μL. The analytical column temperature was maintained at room temperature. Nylon 0.45 μm syringe filters were purchased from GVS Filter Technologies (Johannesburg, South Africa).
Preparation of standards
Several standard solutions of varying concentrations (1 to 300 μg/ ml) were prepared from diluting the L-AA stock solution (1 mg/mL) with the 4.5% MPA to determine a suitable calibration standard range for routine analysis.
Sample extraction and preparation
Onions, berries, apples, tomatos, camu powder, breakfast cereal and some commercial beverages (dragonfruit flavoured vitamin water, orange flavoured vitamin water, pressed berry juice, pressed orange juice, tropical juice) were purchased from several retail outlets within the Cape Town metropolis, South Africa. These samples were chosen to evaluate the effect of different matrices on the method performance parameters of the assay. The extraction of L-AA from food, fruit and vegetable samples was performed as described by Odriozola-Serrano and co-workers [18] with slight modifications. A representative portion of each food product (weight varied from 40 to 200 mg, depending on colour and texture) was added to 25 mL of 4.5% MPA solution and thoroughly homogenized in a DI 25 Basic dispersion unit (Merck chemicals (Pty) Ltd. South Africa) for approximately 1 min. The homogenate was then centrifuged at 4000 rpm for 2 min. The supernatant was filtered through a Nylon 0.45 μm syringe filter and the resulting extracts were aliquoted into 1.5 mL eppendorf tubes and stored at -80°C until the time of analysis. Prior to analysis, the extracted samples were defrosted in a cold water bath, before being appropriately diluted with 4.5% MPA. Diluted sample extracts were then stored away from light at 4°C until the time of injection. Beverage samples were aliquoted into 1.5 mL eppendorf tubes on the day they were purchased and stored at -80°C until the time of analysis. Prior to analysis, they were appropriately diluted in 4.5% MPA and stored away from light at 4°C until the time of injection.
Quality Control (QC) preparation
A 250 mL synthetic juice formulation comprising sucrose (19 g), citric acid (1 g) and sodium citrate (0.023 g) was prepared, while 250 mg of ascorbic acid was added to give a final concentration of 1 mg/ mL. Three QC samples (6.5, 55 and 115 mg/mL) were subsequently prepared in 4.5% metaphosphoric acid and assayed in duplicate. Subsequently, several aliquots of each QC sample was prepared and stored at -40°C until time of analysis. Prior to analysis, the frozen QC aliquots were defrosted in a cold (21°C) water bath.
Pre-Validation Components
Equipment and analyzer qualification
The installation, operational and performance qualification of the Agilent 1200 Series HPLC system was performed at the laboratory site by Agilent Technologies (Cape Town, South Africa). All other equipment (pipettes, thermometers, analytical balances, pH meter, Millipore water purification system, water baths and centrifuges) and glassware were serviced and calibrated by an accredited metrology service (Cape Metrology Field, Cape Town, South Africa). Subsequently the performance of all equipment and glassware were verified on a continuous basis to ensure functioning was optimal and in agreement with manufacturer specifications at all times of analysis. Verification procedures included cleaning, calibration and testing the performance of equipment with certified reference materials (CRM’s). Verification forms were created and all actions documented on a routine basis in terms of repeatability, intermediate precision, accuracy, possible deviations from acceptable criteria and any troubleshooting performed.
Reagent, standards and mobile phase stability
The stability of the extraction solvent (4.5% MPA stock solution) and L-AA standards (20 and 75 μg/mL) was tested. The L-AA standards were prepared in duplicate and one was stored at room temperature (RT) and the other at 4°C prior to and between analyses. The standard samples were then assayed at 0, 4 and 8 hr. Results were evaluated for significant (p<0.05) differences for retention times and peak absorbance area (PA).
The stability of the mobile phase [distilled water/acetonitrile/ formic acid (99: 0.9: 0.1, v/v/v)] was established by analysis of a standard sample (10 μg/mL) at 24 hr intervals for 72 hr using the same mobile phase which was kept at room temperature, and comparing the results with that obtained from a freshly prepared standard (10 μg/mL) solution using a freshly prepared mobile phase. Results were evaluated for significant (p<0.05) differences for retention times and PAA.
Quality Control (QC): Monitoring of method performance
An aliquot of each QC sample was assayed in duplicate for a period of thirty days. Twenty data points were then selected from which the mean, three standard deviations, coefficient of variation (COV) and acceptable tolerance limits were determined [20]. Subsequently, the performance of the HPLC method was evaluated over twenty two days using the QC samples.
Mobile phase optimization
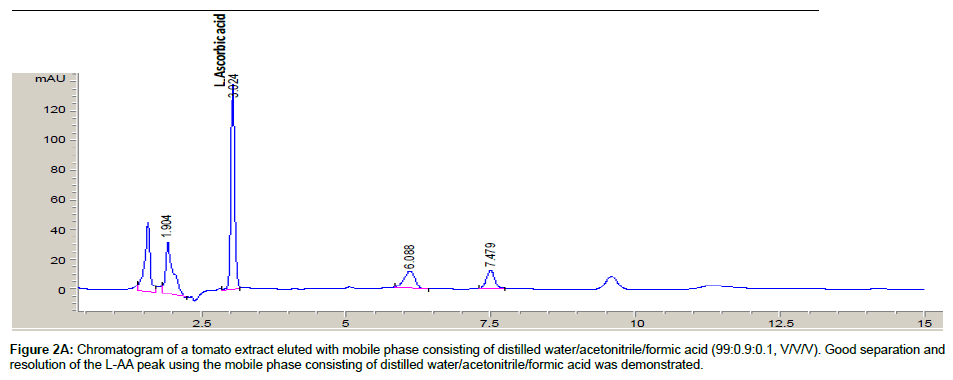
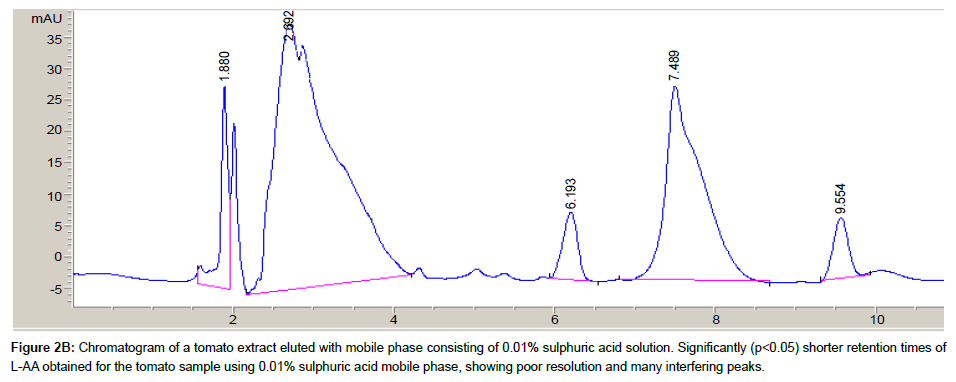
Both mobile phases [distilled water/acetonitrile/formic acid (99: 0.9: 0.1, v/v/v) and 0.01% solution of sulphuric acid] were evaluated for optimal separation of L-AA from other sample component peaks. A tomato sample extract was assayed with both mobile phases and the resulting chromatograms were evaluated.
Method Performance Parameters
Range and linearity
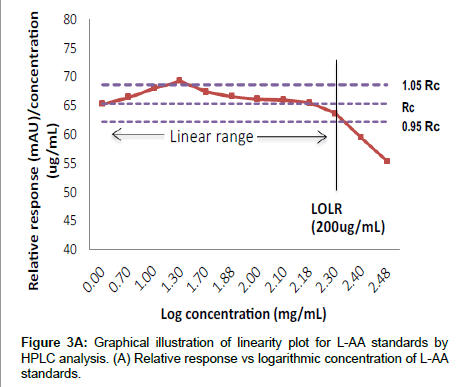
To determine the range for which L-AA can be quantified with acceptable accuracy, precision and linearity, a series of standards (1 to 300 μg/mL) prepared from a standard stock solution of L-AA (1 mg/ mL) was assayed in duplicate over five days. The results were evaluated by two statistical approaches. The first approach involved plotting the relative peak absorbance area (mAU) against the logarithmic concentration (μg/mL) of L-AA standards. A horizontal line should encompass the entire linear range, with positive and negative digressions at low and high concentrations, respectively. Parallel lines are constructed at 95% and 105% of the horizontal relative response line and the intersection points illustrate where the method is nonlinear [13]. In the latter approach, the peak absorbance areas were plotted against the L-AA concentrations (μg/mL) and the results were assessed using least squares linear regression [21]. Subsequently five samples (camu powder extract, dragonfruit flavoured vitamin water, orange flavoured vitamin water, pressed berry and tropical juices) were appropriately diluted at four different concentrations within the linear standard calibration range. Their responses were evaluated for acceptable linearity, accuracy and precision.
Precision and accuracy
The precision of the assay was evaluated by intermediate precision, intra-assay precision and repeatability of injection [21]. Intermediate precision was performed by assaying three L-AA standards (10, 50 and 125 μg/mL) in triplicate over three separate days. Intra-assay precision was performed by assaying QC samples (6.5, 55 and 115 μg/mL) in duplicate three times between other sample runs on the same day. Injection repeatability was performed by injecting a QC sample (55 μg/mL) six times. The mean retention times and L-AA concentrations were calculated. The COV’s were calculated and assessed for acceptable precision. The accuracy of the method was verified by carrying out recovery studies [22]. The spiked-placebo recovery method was performed by assaying three replicates of QC (6.5, 55 and 115 μg/mL) samples. The standard addition recovery procedure was performed at two concentration levels for each sample tested. The concentrations of ascorbic acid added to the samples were: 55 and 115 μg/mL to camu powder extract, 7 and 60 μg/mL to tomato extract and 5 and 10 μg/mL to onion extracts. For each addition level, three determinations were performed and the recovery of L-AA was calculated.
Limit of detection (LOD) and quantification (LOQ)
The LOD and LOQ of the method were determined from the L-AA standard calibration lines that were used to establish linearity (5 to 125 μg/mL) and calibration lines containing concentration levels close to the approximate LOD [23]. Two samples (apple extract and a QC sample) were diluted to concentration levels at or around the LOQ concentration and assayed in triplicate. The responses were evaluated for accuracy, precision and linearity.
Specificity
The specificity of the method was assessed in two ways. The chromatogram of the L-AA standard (50 μg/mL) was compared to those obtained for sample extracts (cereal and tomato extracts). They were evaluated for differences in retention times and the resolution of the L-AA peak from other peaks. In a second experiment, a tomato sample extract was exposed to stress conditions by incubating the sample at 80°C for two hours to partially destroy L-AA and generate degradation products. The post stressed sample was injected and the resulting chromatogram was checked for the presence of interfering peak(s) from degradation products close to the retention time of the L-AA peak. The peak purity was determined by the photo-diode array detector.
Sample stability
The stability of samples was determined for short-term, longterm and freeze-thaw stability. Samples containing high and low concentrations of L-AA were evaluated. Short-term stability was established by storing samples at 4°C for a period of 24 hr. Long-term stability was determined by storing samples at -80°C and testing after one week, one month, and two month intervals. The sample extracts were allowed to thaw at room temperature prior to analysis. Freezethaw stability was assessed by thawing and freezing samples over three days. Three freeze-thaw cycles were performed. All samples were initially assayed fresh and the results were compared to results obtained from samples subjected to the stability conditions. Results were evaluated for significant differences.
Robustness
The ability of the HPLC assay to remain unaffected by small, but deliberate changes in chromatographic conditions was evaluated to assess the reliability of the method during routine sample analysis [24]. The method was subjected to a variety of conditions namely, changes in composition and pH of mobile phase and changes in column temperature. Results were compared to those obtained with the optimized HPLC method. Recoveries and precision between results of the optimized method and method with varied conditions were determined.
System suitability
System suitability parameters such as capacity factor, number of theoretical plates, resolution, peak asymmetry factor and selectivity were determined in accordance with the FDA: Reviewer Guidance [21]. A tomato extract sample (3 μg/mL L-AA) was injected five times and the results were evaluated for system suitability according the acceptance criteria set out in the FDA/CDER: Reviewer Guidance.
Statistical Analysis of Results
The mean, standard deviation, and coefficient of variation i.e. the relative standard deviation % SD were determined for all data. The statistical Microsoft Excel® software package was used to analyze data. Analysis of Variance (ANOVA) was used to ascertain whether the means between sample/standard experimental groups differ significantly (p<0.05, significant; p>0.05, not significant) at a 95% confidence level. The Levene’s Test was used to determine normality between sample/standard experimental groups. If data did not show a normal distribution, a logarithmic transformation was applied. Subsequently, if data did not demonstrate a normal distribution, the Kruskal-Wallis test was used. The paired T-test was used to show differences between two sample/standard experimental groups.
Results and Discussion
Reagent, standards and mobile phase stability
Several authors have suggested the use of MPA for optimal extraction and preservation of L-AA [25-27]. Similarly, in the current study MPA was found to extract and stabilize L-AA with acceptable accuracy and precision. The MPA solvent was found to be stable at RT and 4°C for up to 8 hr. This was confirmed by no significant (p>0.05) differences observed between retention times and peak absorbance areas for the two standards assayed. After 4 hr a slight decrease in PAA was observed for the L-AA standard (75 μg/mL) under both RT and 4°C conditions however, this decrease was not significant (p>0.05). Good repeatability (COV<5%) was achieved for PAA and retention time measurements. These results indicate that the extraction solvent (MPA) and the L-AA standards dissolved in the extraction solvent were stable for up to 8hr at both RT and 4°C.
The results obtained from the stability study for the mobile phase indicate that the mobile phase was stable for up to 48 hr at RT. This was confirmed by significantly (p<0.05) shorter retention times achieved with the 72 hr stored mobile phase standard compared to that obtained with the fresh, 24 and 48 hr stored mobile phase. Moreover, the good precision which was expressed as the COV was observed (<7%) for all runs performed. The PAA showed no significant (p>0.05) differences for all runs.
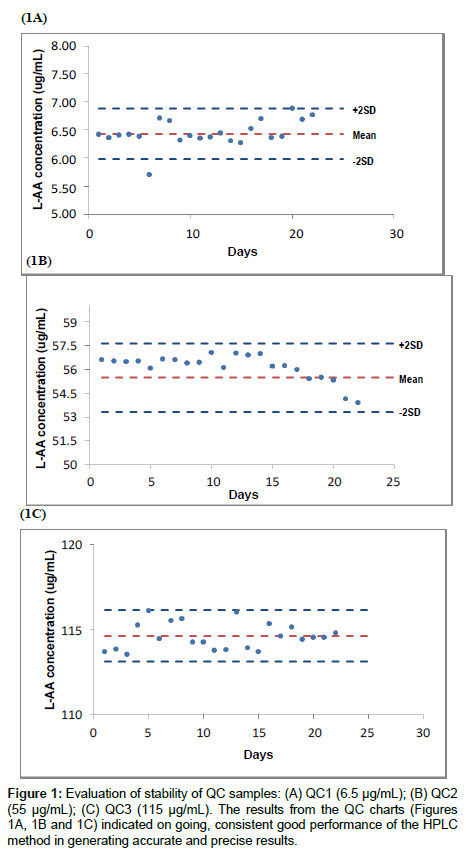
Quality control: Method performance
The inclusion of QC samples is imperative to detect deviation from prescribed tolerance limits. Any deviations outside acceptable tolerance limits implies that the HPLC method does not conform to pre-determined requirements [12]. Figure 1 illustrates the performance of the QC samples over twenty two days. The variations observed with the QC samples were minimal (outlier on day 6 for QC 1(Figure 1A)), and were possibly derived from analytical factors namely, variation in analyst technique, variation in environmental conditions on different days and variability in performance of equipment used. These variations are inherent, however it is important to differentiate between variations of this kind and those that occur due to error such as contamination in the HPLC system, changes in reagents and consumables, poorly functioning equipment and poor analyst technique [28]. The precision of the QC samples reflects the degree of variation of all data points. The closeness of the data points to the true value determines the accuracy of the QC samples [29]. The intermediate precision expressed as the COV fell well within 5% and the accuracy expressed as percent recovery was satisfactory ranging from 99 to 102%. The results indicate that L-AA QC samples were stable for approximately one month at -20°C, demonstrating repeatable and accurate results at a 95% confidence level.
Mobile phase optimization
Conditions such as pH and organic solvent component contribute to the degree of separation of anayte/s within a sample solution [30]. Two mobile phases were evaluated in an attempt to achieve the best separation and resolution between L-AA and other sample components
The chromatograms of a tomato sample extract eluted with both mobile phases are shown in Figures 2A and 2B. Using the mobile phase containing sulphuric acid demonstrated poor resolution of the L-AA peak, hence, the water/acetonitrile/formic acid mobile phase was subsequently used as the mobile phase for all further sample analysis. Similarly, Gorse et al. [31] and Biesaga et al. [32] have observed good separation and resolution of other analytes with various chromatographic methods using mobile phases consisting of organic modifiers.
Method performance parameters
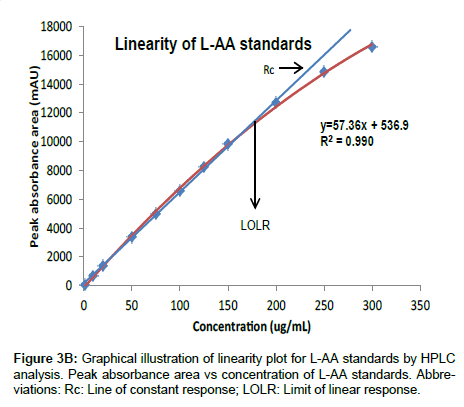
Linearity and range: Figure 3A illustrates the linear relationship between relative responses (mAU) and the logarithmic concentrations for the L-AA standards. The intersection point is at the 200 μg/mL, standard, after which the method becomes non-linear. In the second approach to determine linearity, Figure 3B illustrates the relationship between the peak absorbance area and concentration for the L-AA standards. The results indicate that good linearity (r2=0.999) was observed from 5 to 200 μg/mL for the L-AA standards and is comparable to those obtained in other studies employing HPLC to measure L-AA in food products [18,23,25], however, at high concentrations (>200μg/mL) poor linearity was observed. The poor linearity observed at high concentrations (>200 μg/mL) may be as a result of saturation of the detector [33]. In order to maintain good turn-around times for samples, a narrower calibration standard range of 5 to 125 μg/mL was utilized as the range for all further validation and sample analysis and is in agreement with the ICH’s recommendations for a quantitative analytical method [34]. Furthermore, the intermediate and intra assay precision expressed as the COV was acceptable with all runs falling within 10%. Similarly, good accuracy was observed with percent recovery ranging from 89 to102% for all standards.
Table 1 summarizes the accuracy, precision and linearity of several samples assayed at four concentration levels of the L-AA standard range. A good correlation coefficient (≥ 0.995) was observed for all samples. The precision expressed as the COV between all concentration levels tested was within 10% and the accuracy expressed as percent recovery ranged from 92 to 120%. Hence, the proposed method showed acceptable precision and accuracy, and an excellent correlation between peak absorbance area and concentration for all samples assayed.
| Samples (µg/mL) | Dilution factor | PAA (AU) | Measured L-AA concentrations (µg/mL)1 | Recovery (%) | COV2 | r2 |
|---|---|---|---|---|---|---|
| Dragonfruit vitamin water | 1/50 | 684.25 ± 1.41 | 489.50 | 102.13 | 2.46 | 0.997 |
| (479.28) | 1/25 | 1290.47 ± 0.44 | 474.50 | 99.00 | ||
| 1/9 | 3514.37 ± 3.27 | 461.34 | 96.26 | |||
| 1/4 | 7932.46 ± 0.02 | 479.32 | 100 | |||
| Camu powder extract | 1/45 | 285.54 ± 3.15 | 168.30 | 95.22 | 2.12 | 0.999 |
| (176.74) | 1/8 | 1381.97 ± 71.00 | 163.04 | 92.25 | ||
| 1/4 | 2861.67 ± 9.69 | 171.40 | 96.97 | |||
| 1/2 | 5620.27 ± 21.69 | 169.44 | 95.86 | |||
| Orange vitamin water | 1/4 | 7996.65 ± 7.03 | 480.00 | 100 | 1.87 | 0.999 |
| (480.00) | 1/10 | 3294.70 ± 0.11 | 494.20 | 102.96 | ||
| 1/25 | 1361.94 ± 0.13 | 502.00 | 104.58 | |||
| 1/50 | 684.25 ± 1.41 | 489.50 | 101.98 | |||
| Pressed berry juice | 1/2 | 3784.89 ± 55.15 | 113.74 | 103.23 | 6.28 | 0.995 |
| (110.14) | 1/5 | 1774.24 ± 12.42 | 131.70 | 119.58 | ||
| 1/10 | 870.84 ± 3.67 | 126.30 | 114.67 | |||
| 1/20 | 435.99 ± 2.98 | 120.40 | 109.31 | |||
| Tropical juice | 1/5 | 7548.66 ± 5.79 | 570.03 | 100.00 | 3.81 | 0.999 |
| (570.03) | 1/12 | 3230.86 ± 3.54 | 581.46 | 102.00 | ||
| 1/20 | 1993.46 ± 2.55 | 593.39 | 104.10 | |||
| 1/100 | 396.43 ± 3.71 | 542.43 | 95.16 |
PAA values are means ± SD of two determinations (n=2); 1L-AA concentrations calculated using y=mx+c; 2Precision between four concentration levels. Abbreviations: AU: Absorbance Unit; COV: Coefficient of Variation; PAA: Peak Absorbance Area; r2, Correlation Coefficient
Table 1: Linearity of samples (Peak absorbance area vs. Dilution factor).
Precision and accuracy: Table 2 summarizes the precision and accuracy of the current method using standards and QCs. The method showed satisfactory intermediate precision of the L-AA standards. All the COV values achieved for PAA and retention times were less than 1% and 3%, respectively. Additionally, good intra-assay precision (COV <1%) were observed for both retention times and L-AA concentrations of the QC samples. Similarly, the injection precision was acceptable demonstrating COV’s of less than 1% for both retention times and peak absorbance areas. These results can be compared to those obtained by Kumar et al. [35] who validated various levels of precision (COV’s less than 2%) of an HPLC method for ascorbic acid determination in health drinks. Similarly, Spinola et al. [23] obtained COV’s within 4% for an improved HPLC method for the measurement of L-AA in various fruit and vegetables. In another study, using food commodities to measure ascorbic acid content by HPLC, the average COV obtained was 8.7% and is comparable to those obtained in the present study [36].
The results from accuracy experiments reflect both the efficiency of the L-AA extraction from samples of the method in use and the effects of the sample matrices. Satisfactory recoveries ranging from 99 to 103% (Table 2) and 92 to 96% (Table 3) were achieved for L-AA from both QCs and food and vegetable samples, respectively. These results are similar to those obtained by Odriozola et al. [18] who demonstrated average recoveries of approximately 94 to 105% in fruit and vegetables, and is in agreement with the FDA/CDER’s requirements of being within ± 15% of the target value [29]. Additionally, the results obtained are comparable to average recoveries of 82.2 to 95.9% and 93.3% obtained by Valente et al. [37] and Sanchez et al. [10] for ascorbic acid determination in fruit and vegetables, respectively. The results indicate that the extraction procedure employed was optimal, demonstrating almost complete recovery of L-AA by both recovery methods. Furthermore, the ability of the current method to produce accurate results with good precision was confirmed by the low COV’s (<1%) achieved for samples in Table 3.
| L-AA QC/STD expected concentration (µg/mL) | Ret time (min) | COV | Measured concentration (µg/mL) | COV | REC4 (%) | |
|---|---|---|---|---|---|---|
| Intermediate precision1 | 10 (L-AA STD) | 3.038 ± 0.02 | 0.54 | 9.14 ± 2.46 | 2.46 | |
| 50 (L-AA STD) | 3.041 ± 0.01 | 0.30 | 48.61 ± 2.81 | 2.81 | ||
| 125 (L-AA STD) | 3.042 ± 0.01 | 0.46 | 123.18 ± 1.96 | 1.96 | ||
| Intra-assay precision2 | 6.5 (QC) | 3.023 ± 0.004 | 0.12 | 6.59 ± 1.644 | 0.38 | 101.41 ± 0.40 |
| 55 (QC) | 3.023 ± 0.003 | 0.11 | 56.76 ± 6.780 | 0.19 | 103.19 ± 0.19 | |
| 115 (QC) | 3.022 ± 0.003 | 0.09 | 114.05 ± 8.568 | 0.12 | 99.18 ± 0.14 | |
| Injection precision3 | 55 (QC) | 3.031 ± 0.02 | 0.52 | 54.11 ± 0.49 | 0.49 |
1Intermediate precision values are means ± SD of three determinations (n=3) assayed over three separate days. 2Intra-assay precision values are means ± SD of three determinations (n=3) assayed on the same day. 3Injection precision values are means ± SD of six determinations (n=6). 4Spiked recovery method used for accuracy. Abbreviations: COV: Coefficient of Variation; L-AA STD, L-ascorbic acid Standard; QC: Quality Control; REC: Recovery
Table 2: Precision of the UV-HPLC method for the determination of L-ascorbic acid.
| Sample | Initial concentration (µg/mL) | Concentration after addition (µg/mL) | Recovery (%) | Mean Recovery (%)3 | Mean COV | ||
|---|---|---|---|---|---|---|---|
| Level I1 | Level II2 | Level I | Level II | ||||
| Camu powder extract | 7.51 | 63.52 ± 0.28 | 118.09 ± 2.17 | 93.35 | 92.15 | 92.75 ± 0.85 | 0.92 |
| Tomato extract | 26.22 | 32.83 ± 3.68 | 82.21 ± 2.05 | 94.49 | 93.31 | 93.91 ± 0.84 | 0.89 |
| Onion extract | 1.24 | 6.02 ± 15.03 | 10.74 ± 25.25 | 95.59 | 94.98 | 95.29 ± 0.43 | 0.45 |
155 μg/mL to camu powder extract; 7 μg/mL to tomato extract; 5 μg/mL to onion extracts. 2115 μg/mL to camu powder extract; 60 μg/mL tomato extract; 10 μg/mL to onion extracts. 3Recovery mean ± Standard deviation (n=3 in each level)
Table 3: Standard addition recovery method of the UV-HPLC assay to determine L-ascorbic acid in food products.
For the commercial fruit juices, the ascorbic acid result ranged from 11.3 to 13.17 mg/100 ml for pressed berry juice and was comparable to the manufacturer’s declaration of 12 mg/100 ml. The range of results obtained for the dragon-fruit and orange vitamin waters (46.1 to 48.9 mg/100 ml and 48.0 to 50.2 mg/100 ml respectively) differed substantially from the manufacturer’s claim of 17.57 mg/100 ml. A possible reason for this could be that the product is produced in different locations and could be subject to climatic, storage and maturity stage of fruit extracts added to these water products, whilst the nutritional package labelling may not be updated accordingly.
Limit of detection and quantification:The LOD and LOQ corresponded to 0.61 μg/mL and 1.84 μg/mL, respectively which implied that good sensitivity, accuracy and precision was achieved at this lower concentration level and is comparable to comparable to higher LOD and LOQ values (1.7 and 5.7 μg/mL) reported by Odriozola-Serrano et al. [18] who demonstrated the various UVHPLC methodologies to analyze ascorbic acid fruits. The LOD and LOQ values were calculated from the regression equation obtained from the 0.1 to 5 μg/mL linear range, due to the lower standard error achieved for this linear range. The standard error for the intercepted point for the 0.1 to 5 μg/mL linearity range was significantly (p<0.05) lower (9.65) than that obtained for the 5 to 125 μg/mL linearity range (29.64). In this previous study, a standard error of 36.98 was reported for the calibration standard line and is comparable to the current study. Similarly in another study, Sawant et al. [30] reported LOD and LOQ values of 1.42 and 4.32 μg/mL respectively for the analysis of ascorbic acid in Phyllanthus Emblica, which were calculated from the calibration standard as was demonstrated in the current study
Subsequently, samples diluted to the LOQ level demonstrated good linearity (r2=0.991 and 0.972) for the QC and apple extract samples, respectively. Accuracy which was expressed as percent recovery was satisfactory for QC (87 to 103%) and apple extract samples (91 to 99%) for all concentration levels tested. Additionally, acceptable precision (<10%) within and between dilutions were observed. These findings demonstrate acceptable accuracy (within ± 20% of target value), precision (within 20% of the COV) and linearity at the limit of quantification for samples tested [29].
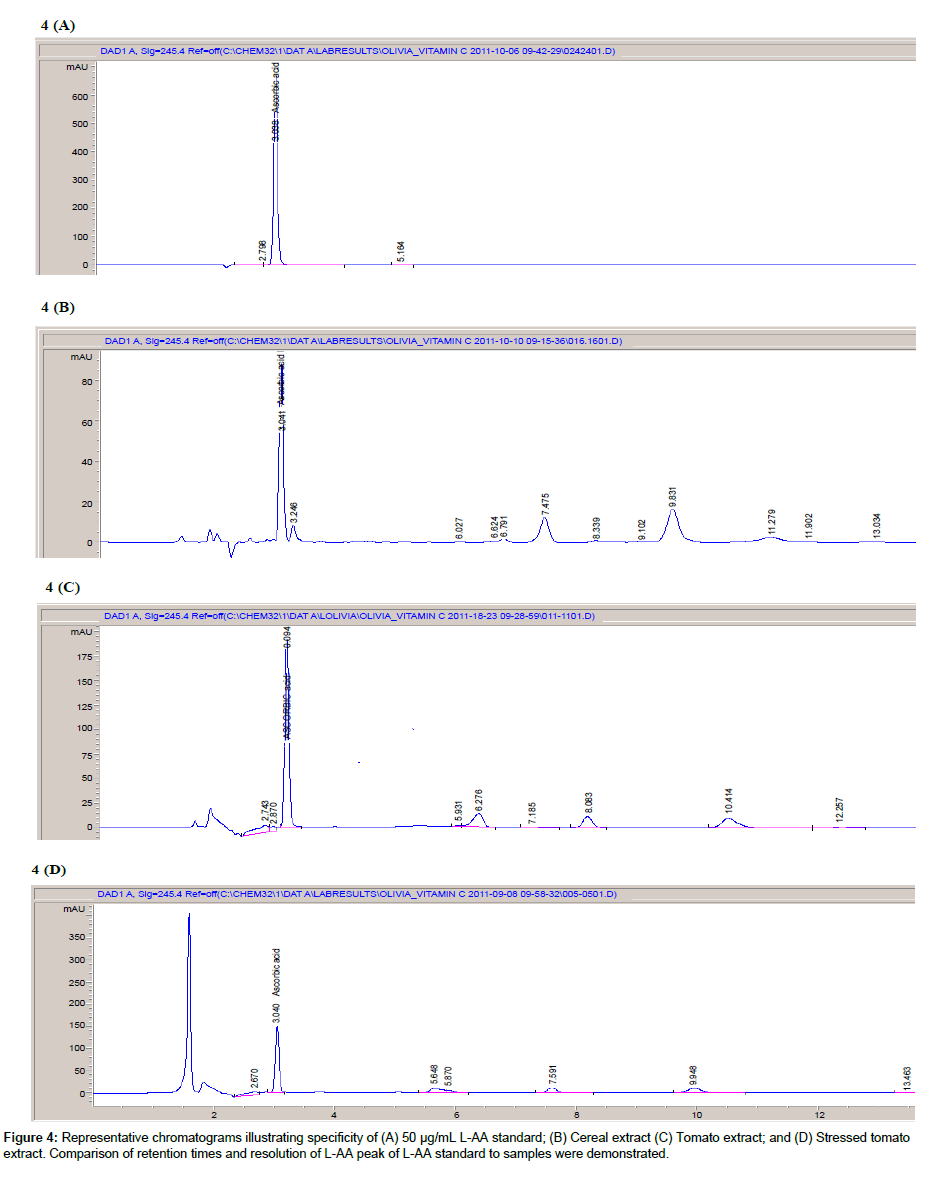
Specificity: Figure 4 shows representative chromatograms of samples (cereal and camu powder) and L-AA standard (50 μg/mL). The retention time of the L-AA standard (Figure 4A) was close to that obtained for sample (Figure 4B and 4C). The sample peaks were sharp and symmetrical and well resolved from other sample components with no co-eluting peaks. Peak purity was 98.25 and 99.64% for onion and cereal samples, respectively. Additionally, the UV-spectrum acquired for both sample extract peaks was the same as those obtained for L-AA standards. The chromatogram of the stress-induced tomato extract (Figure 4D) gave similar retention times to those obtained for the 50μg/mL L-AA standard. Considering the controversy surrounding the sensitivity of using an HPLC method to reliably detect and quantify various sample components, the chromatograms of the current method still demonstrated well resolved L-AA peaks for all samples assayed. Any degradation products and sample matrix components possibly present were not visible on the sample chromatograms. The UV-spectrum for the stress-induced sample was identical to that of the L-AA standards. Good peak purity was obtained for tomato (99.67%) sample. Under the method’s test conditions, L-AA appeared to be well resolved from other sample components and thus proves the specificity of the method for the determination of L-AA.
Sample stability:
All samples were found to be stable at 4°C for 24 hr (99 to 102% recovery). Similarly, no significant (p>0.05) differences were observed between results of fresh samples and samples stored at -80°C for a week, a month and two months. Samples showed good preservation of L-AA at -80°C for up to two months. These findings are in agreement with Scherer et al. [38] who reported stability of L-AA in fruit juices stored at 5°C for at least the first two days. Significant (p<0.05) losses of vitamin C content during the freeze-thaw cycles in the current study were in agreement with some other studies that reported similar losses during the thaw-out process, despite a slight variation in storage and temperature conditions [39-41]. in L-AA was observed when samples were thawed out three times at room temperature. The onion sample demonstrated the most significant (p<0.05) loss in L-AA. This could be due to the possibility that no preservatives were added to the onion sample in comparison to commercially available fruit juices that may contain preservatives that protect L-AA [44]. Hernández et al. [39] recommended the addition of antioxidants to slow down oxidation in certain fruit extracts. From the results of the stability study, it is evident that freezing vegetable and beverage products resulted in no significant (p>0.05) L-AA losses, however thawing out at room temperature resulted in significant (p<0.05) L-AA losses. Hence, it is recommended that frozen samples be thawed out in a microwave to reduce significant L-AA losses. The addition of an antioxidant should be considered during the extraction of L-AA in fruit and vegetables.
Robustness: The results of the robustness study in Table 4 demonstrated that all varied conditions applied to the method, produced good recoveries of L-AA. Results were not significantly (p>0.05) different from those obtained from the optimized method for most samples tested. Camu powder extract was the only sample that produced significantly (p<0.05) higher results than that obtained with the standard optimized method when eluted with the adjusted mobile phase composition [distilled water/acetonitrile/formic acid; (80.9: 19: 0.1, v/v/v)]. The precision which was expressed as the COV was acceptable (<5%) between results obtained with the optimized method and those achieved with the adjusted method. Therefore the ability of the optimized method to remain unaffected by small changes in parameters thereby producing accurate and precise results indicates the robustness of the method.
| Samples/standards/QC | Changes | Retention time | Response | |
|---|---|---|---|---|
| pH 2.81 | COV | REC (%) | COV4 | |
| Camu powder | 0.075 | 102.13 | 2.42 | |
| ;Tropical juice | 0.094 | 98.94 | 0.62 | |
| Mix berry juice | 0.635 | 96.83 | 1.90 | |
| Pressed orange juice | 0.099 | 102.48 | 1.42 | |
| Distilled water/acetonitrile/formic acid (80.9: 19: 0.1, v/v/v)2 | ||||
| QC (60 µg/mL) | 0.33 | 99.92 | 0.73 | |
| Camu powder | 0.12 | 103.56a | 2.03 | |
| Onion | 4.64 | 99.47 | 3.89 | |
| Column temperature: 20°C3 | ||||
| Camu powder | 0.33 | 102.61 | 1.51 | |
| 10 µg/mL L-AA standard | 0.27 | 100.25 | 1.09 | |
| 20 µg/mL L-AA standard | 0.20 | 98.93 | 2.64 | |
| 50 µg/mL L-AA standard | 0.29 | 99.44 | 1.88 | |
| 100 µg/mL L-AA standard | 0.01 | 97.16 | 3.08 | |
| Column temperature: 26°C | ||||
| 5 µg/mL L-AA standard | 0.29 | 101.31 | 2.30 | |
| 20 µg/mL L-AA standard | 0.78 | 97.59 | 2.97 | |
| 50 µg/mL L-AA standard | 2.13 | 97.83 | 2.56 | |
| 100 µg/mL L-AA standard | 0.05 | 96.06 | 3.48 | |
| 125 µg/mL L-AA standard | 0.07 | 97.86 | 1.34 | |
| QC sample (60µg/mL) | 0.18 | 99.83 | 0.72 | |
| camu powder | 0.26 | 101.71 | 1.00 | |
1Optimized HPLC method at pH 2.6. 2Mobile phase composition for optimized method: distilled water/acetonitrile/formic acid (99: 0.9: 0.1, v/v/v). 3Column temperature of optimized method: 23°C.
4Precision of assay performed at optimal conditions and with variations. aSignificantly (p<0.05) higher recovery obtained with modified mobile phase
Table 4: Evaluation of the robustness of the HPLC method for L-AA determination.
One important factor observed was that small changes of the organic component present in the mobile phase could result in significant changes in retention time. The results show that the retention times for samples eluted with the adjusted mobile phase were shorter than those obtained with the standard mobile phase, and were possibly due to the increased polarity of the mobile phase [45]. Hence, this should be taken into consideration when preparing the mobile phase.
System suitability testing:Table 5 summarizes the results of the system suitability tests. The results show that all parameters evaluated fell within their respective limits. The precision, expressed as the coefficient of variation (COV) of the retention time (0.018) was less than 1% and is in keeping with the FDA’s acceptance limit [21]. The precision of the response (COV=1.470) fell within 2% and is in compliance with the United States Pharmacopeia (USP) requirements [46]. Hence, the results of the system suitability tests indicate that the entire HPLC system is performing optimally and within the validated method performance limits.
| Retention time (min)1 | PAA (AU) | Height (AU) | Capacity factor (K´) | Theoretical plates (N) | Resolution (Rs) | Peak asymmetry factor (As) | Selectivity factor (ȴ) | ||
|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 3.035 | 257.86 | 55.09 | 7.62 | 10561 | 7.91 | 0.89 | 1.45 |
| 2 | 3.034 | 263.41 | 55.27 | 7.47 | 10749 | 7.53 | 0.86 | 1.45 | |
| 3 | 3.035 | 255.99 | 55.20 | 7.48 | 10850 | 7.89 | 0.92 | 1.45 | |
| 4 | 3.034 | 253.12 | 55.21 | 9.18 | 10747 | 7.91 | 0.88 | 1.46 | |
| 5 | 3.035 | 258.70 | 55.88 | 7.36 | 10752 | 7.7 | 0.9 | 1.46 | |
| Mean ± SD | 3.035 ± 0.001 | 257.8 ± 3.8 | 55.3 ± 0.3 | 7.482 ± 0.092 | 10731.8 ± 104.9 | 7.788 ± 0.169 | 0.890 ± 0.022 | 1.454 ± 0.005 | |
| COV | 0.018 | 1.470 | 0.568 | 1.234 | 0.978 | 2.172 | 2.512 | 0.377 |
1The retention time for the unresolved peak is 0.357 ± 1.33 SD. Abbreviations: AU, absorbance unit
Table 5: System suitability testing for the HPLC assay for the determination of L-AA in food and beverage products.
Conclusion
The proposed optimized and validated method demonstrated an excellent technique for measurement of L-AA in food and beverage products. The extraction method proved an effective means for the isolation of L-AA from a variety of fruit and vegetable sample matrices. The results from the validation study confirmed a good performance of the method with regard to ISO 17025 validation requirements namely, accuracy, precision, linearity, specificity, robustness and stability. The successful optimization and validation of the proposed method should make it easily applicable for routine analysis of L-AA measurement in various fruit and vegetable products. Furthermore, the validation procedure applied in this study could be applied to samples other than food and beverage, such as pharmaceutical products and biological samples.
Acknowledgements
I would like to thank the Oxidative Stress Research Centre at the Cape Peninsula University of Technology for their support of the scientific research.
References
- Enstrom JE (1997) Vitamin C in Health and Disease. Marcel Dekker Inc., New York.
- Weber P, Bendich A, Schalch W (1996) Vitamin C and human health--a review of recent data relevant to human requirements. Int J Vitam Nutr Res 66: 19-30.
- Rock CL, Jacob RA, Bowen PE (1996) Update on the biological characteristics of the antioxidant micronutrients: vitamin C, vitamin E, and the carotenoids. J Am Diet Assoc 96: 693-702.
- Yan Y, Bornscheuer UT, Schmid RD (1999) Lipase-catalyzed synthesis of vitamin C fatty acid esters. Biotechnol Lett 21: 1051-1054.
- Davey MW, Montagu M, Inzé D, Sanmartin M, Kanellis A, et al. (2000) Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agri 80: 825-860.
- Calokerinos A, Hadjiioannou T (1983) Direct potentiometric titration of thiosulfate, thiourea, and ascorbic acid with lodate using an iodide ion-selective electrode. Microchem J 28: 464-469.
- Liu TZ, Chin N, Kiser MD, Bigler WN (1982) Specific spectrophotometry of ascorbic acid in serum or plasma by use of ascorbate oxidase. Clin Chem 28: 2225-2228.
- Arya S, Mahajan M, Jain P (2000) Non-spectrophotometric methods for the determination of Vitamin C. Anal Chim Acta 417: 1-14.
- De Quirós A, Fernández-Arias M, López-Hernández J (2009) A screening method for the determination of ascorbic acid in fruit juices and soft drinks. Food Chem 116: 509-512.
- Sánchez-Mata MC, Cámara-Hurtado M, Díez-Marqués C, Torija-Isasa ME (2000) Comparison of high-performance liquid chromatography and spectrofluorimetry for vitamin C analysis of green beans (Phaseolus vulgaris). Eur Food ResTechnol 210: 220-225.
- Toomula N (2011) Development and Validation of Analytical Methods for Pharmaceuticals. J Anal Bioanal Tech 2: 127.
- Masson P (2007) Quality control techniques for routine analysis with liquid chromatography in laboratories. J Chromatogr A 1158: 168-173.
- Huber L (1999) Validation of HPLC methods. BioPharm 12: 64-66.
- Taverniers I, De Loose M, Van Bockstaele E (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends in Analytical Chemistry 23: 535-552.
- Dudhwal R, Jagadish P, Bhat K, Mane A (2011) Simultaneous Estimation of Ascorbic Acid and Calcium Pantothenate in Multivitamin and Multimineral Tablets by Reverse-Phase HPLC. Pharma Chem 3: 375-381.
- Maia AM, Baby AR, Yasaka WJ, Suenaga E, Kaneko TM, et al. (2007) Validation of HPLC stability-indicating method for Vitamin C in semisolid pharmaceutical/cosmetic preparations with glutathione and sodium metabisulfite, as antioxidants. Talanta 71: 639-643.
- Mitic S, Kostic D, Naskovic-okic D, Mitic MN (2011) Rapid and Reliable HPLC Method for the Determination of Vitamin C in Pharmaceutical Samples. Trop J Pharm Res 10: 105-111.
- Odriozola-Serrano I, Hernández-Jover T, Martín-Belloso O (2007) Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem 105: 1151-1158.
- Van de Velde F, Pirovani ME, Cámara MS, Güemes DR, Bernardi CMH (2011) Optimization and Validation of a UV–HPLC Method for Vitamin C Determination in Strawberries (Fragaria ananassa Duch.), Using Experimental Designs. Food Anal Methods 5: 1097-1104.
- Association of Official Analytical Chemists (2002) AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals.
- Gao L, Li J, Kasserra C, Song Q, Arjomand A, et al. (2011) Precision and accuracy in the quantitative analysis of biological samples by accelerator mass spectrometry: application in microdose absolute bioavailability studies. Anal Chem 83: 5607-5616.
- Australian Pesticides and Veterinary Medicines Authority (2004) Guidelines for the Validation of Analytical Methods for Active Constituent, Agricultural and Veterinary Chemical Products.
- Spínola V, Mendes B, Câmara JS, Castilho PC (2012) An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal Bioanal Chem 403: 1049-1058.
- Shabir GA (2003) Validation of high-performance liquid chromatography methods for pharmaceutical analysis. Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J Chromatogr A 987: 57-66.
- Burini G (2007) Development of a quantitative method for the analysis of total L-ascorbic acid in foods by high-performance liquid chromatography. J Chromatogr A 1154: 97-102.
- Gennaro M, Bertolo P (1990) L-ascorbic acid determination in fruits and medical formulations by ion interaction reagent reverse phase HPLC technique. J Liq Chromatogr 13: 1419-1434.
- Graham WD, Annette D (1992) Determination of ascorbic and dehydroascorbic acid in potatoes (Solanum tuberosum) and straberries using ion-exclusion chromatography. J Chromatogr A 594: 187-194.
- Westgard JO (2008) Basic Method Validation. 3rd edition, Westgard QC, Inc., Madison.
- Food and Drug Administration (2001) Guidance for Industry: Bioanalytical Method Validation.
- Sawant LP, Prabhakar S, Pandita N (2010) Quantitative HPLC Analysis of Ascorbic Acid and Gallic Acid in Phyllanthus Emblica. J Anal Bioanal Tech 1: 1-4.
- Gorse J, Balchunas A, Swaile D, Sepaniak M (2005) Effects of organic mobile phase modifiers in micellar electrokinetic capillary chromatography. J High Resolut Chromatogr 11: 554-559.
- Biesaga M, Ochnik U, Pyrzynska K (2007) Analysis of phenolic acids in fruits by HPLC with monolithic columns. J Sep Sci 30: 2929-2934.
- Bidlingmeyer BA (1993) Practical HPLC methodology and applications. Wiley & Sons, New York.
- International Conference on Harmonisation (2005) Validation of Analytical Procedures: Text and Methodology Q2(R1) Part I and II.
- Kumar KR, Kumar PP, Rao NM (2011) Development and validation of RP-HPLC method for the estimation of ascorbic acid in health drinks. J Chem Pharm Res 3: 363-374.
- Frenich AG, Torres ME, Vega AB, Vidal JL, Bolaños PP (2005) Determination of ascorbic acid and carotenoids in food commodities by liquid chromatography with mass spectrometry detection. J Agric Food Chem 53: 7371-7376.
- Valente A, Albuquerque TG, Sanches-Silva A, Costa HS (2011) Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res Int 44: 2237-42.
- Scherer R, Rybka ACP, Ballus CA, Meinhart AD, Godoy HT (2012) Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem 150-154
- Hernández Y, Lobo MG, González M (2006) Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chem 96: 654-664.
- Nursal B, Yücecan S (2000) Vitamin C losses in some frozen vegetables due to various cooking methods. Nahrung 44: 451-453.
- Pilar Cano M, Fuster C, Antonia Marín M (1993) Freezing preservation of four Spanish kiwi fruit cultivars (Actinidia chinensis, Planch): chemical aspects. Z Lebensm Unters Forsch 196: 142-146.
- Holzwarth M, Korhummel S, Carle R, Kammerer DR (2012) Evaluation of the effects of different freezing and thawing methods on color, polyphenol and ascorbic acid retention in strawberries ( Fragaria x ananassa Duch.). Food Res Int 241-248.
- Oszmianski J, Wojdylo A, Kolniak J (2009) Effect of l-ascorbic acid, sugar, pectin and freeze–thaw treatment on polyphenol content of frozen strawberries. Food Sci Technol 42: 581-586.
- Haddad P (1977) Vitamin C content of commercial orange juices. An analytical project. J Chem Educ 54: 192-193.
- Hao Z, Xiao B, Weng N (2008) Impact of column temperature and mobile phase components on selectivity of hydrophilic interaction chromatography (HILIC). J Sep Sci 31: 1449-1464.
- Dolan JW (2004) System Suitability.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18330
- [From(publication date):
September-2014 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 13388
- PDF downloads : 4942