Mesenchymal Stem Cell Applications for Ligament Repair after Joint Trauma
Received: 23-Jul-2014 / Accepted Date: 19-Aug-2014 / Published Date: 21-Aug-2014 DOI: 10.4172/2161-0681.1000186
Abstract
Tendon and ligament injuries are the most common problems in adult health accounting for about half of all musculoskeletal injuries. Rupture of the anterior cruciate ligament (ACL) results in the loss of whole joint stability leading to meniscal rupture, cartilage damage and early osteoarthritis. Arthroscopic reconstruction using autografts or allografts has known drawbacks such as ligament laxity, donor site morbidity and long recovery periods. In addition to the appropriate mechanical environment, several biological factors have been implicated in the ACL healing process including specialised growth factors and mesenchymal stem cells (MSCs). However, in order to produce a superior molecular and biomechanical ACL there will be always the need to provide a suitable scaffold to ‘house’ MSCs and to provide adequate biomechanical properties in order for the regeneration process to proceed. Understanding the mechanisms of ACL healing following cellular therapy may lead to novel, more effective and biological-based tissue engineering strategies for ACL reconstruction. The focus of this review is the current knowledge of ACL reconstruction after joint trauma when combining MSC and tissue engineering technologies.
Keywords: Mesenchymal stem cells; Joint trauma; Anterior cruciate ligament repair; Provisional scaffolds; Tissue engineering
315190Introduction
Musculoskeletal trauma continues to cause major disability worldwide. Tendon and ligament injuries represent a major challenge as physiological repair (if there is any) is exceedingly slow and incomplete. Ligaments can sustain high mechanical forces and fundamentally provide joint stability. The anterior cruciate ligament (ACL) is a key knee ligament stabilizer and an ACL tear will result in joint instability, which may in turn cause meniscal tears and degenerative joint disease. Its intra-articular localisation, hypocellularity and hypovascularity afford only a limited healing capacity. In the United States approximately 175,000 ACL cases require surgery each year with an estimated cost of 1 billion dollars [1].
Reconstruction of the ACL has been the topic of vivid discussion amongst surgeons for years. Arthroscopic reconstruction techniques with autografts or allografts have been associated with such drawbacks as ligament laxity, donor site morbidity and a long recovery period. To overcome these shortcomings stem cell- and biomaterial-based technologies have been developed for ligament regeneration. In general, stem cells are immature cells that have the capacity for self-renewal, a high potential for proliferation, and differentiation towards several different cell lineages under appropriate induction conditions. There are two types of stem cell, namely embryonic (pluripotent) and non-embryonic or adult (multipotent) stem cells [2]. Mesenchymal stem cells (MSCs) are multipotential adult stem cells that reside within the connective tissue of most organs [3]. Autologous MSCs can be obtained with minimal invasiveness from a patient’s own bone marrow (BM) and can be added to biological or synthetic scaffolds, to ensure maximal repair potential. Highlighted in this mini-review are recent advances in ACL reconstruction based on MSCs exogenously added, as well as the concept of endogenous MSC recruitment to the injured area.
Physiological repair of injured tendons: is this the case for ACL repair?
Ligaments and tendons are elastic collagenous tissues that contribute to knee motion and possess similar molecular, cellular and hierarchical structure. Normal ligament and tendon healing can be divided into four characteristic phases [4]. Phase I is the post-injury phase where blood enters the affected area until a haematoma develops over the initial 72 hours. During this phase, inflammatory cells (such as monocytes, leukocytes and macrophages) infiltrate the affected area and initiate the healing process by secreting cytokines and growth factors. Next, in phase II reparative fibroblast-like cells slowly proliferate and synthesize tissue-specific collagens (mainly collagen III) forming provisional scaffolds. Finally, phases III and IV are characterised by vascularisation of the newly formed tissue and functional matrix organization, respectively [4].
There are several lines of evidence suggesting that MSCs exist in ligaments and tendons, and contribute to the endogenous phase II of the regeneration process. Firstly, post-injury tendons can occasionally develop fibrocartilage and ossified tissues; tissues descended from MSCs [5,6]. Secondly, human tendon-derived fibroblasts express genes that are related to chondrogenic, osteogenic and adipogenic lineages suggesting their multipotentiality in vitro [7,8]. Furthermore, these MSC-like cells have been referred to as tendon stem/progenitor cells (TSPCs) [9,10].
Whilst the presence of endogenous MSC/TSPCs can explain the spontaneous healing of some tendon pathologies, their involvement in ACL regeneration is likely to be limited. This may be due to the fact that ACL-derived fibroblasts show different biological properties such as low mobility, proliferation, and matrix synthesis capacities compared to TSPCs [11]. Furthermore, the torn ends of ACL retract significantly due to high residual strain making the bridging of the gap more difficult. However, perhaps the most crucial reason as to why ACL does not heal naturally is because its thin synovial sheath disrupts and blood dissipates into the synovial fluid (SF) preventing good haematoma formation [1]. Consequently, phases I and II of the regeneration process cannot proceed and omission of these phases results in a lack of a provisional scaffold formation [1].
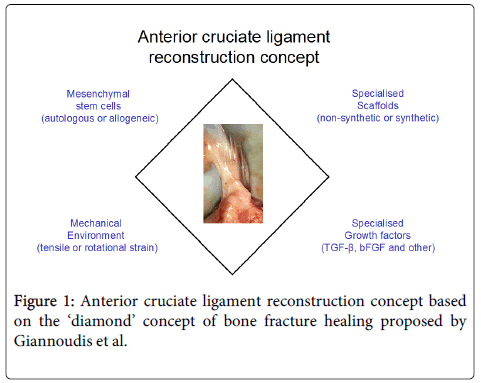
Pioneered by Steadman et al. the use of minimally invasive femoral condyle microfracture to treat ACL injuries in athletes demonstrated encouraging results [12]. The technique involves perforation of the subchondral bone causing bone marrow dissipation within the intra-articular area potentially leading to infiltration of reparative cells into the ACL. The observed repair could also be mediated by SF MSCs that may be released into the damaged area post-injury. Original findings by the Sekiya group showed that post-injury, SF MSCs not only increase in number but are also actively involved in tendon and bone remodelling [13,14]. However, in order to produce a superior molecular and biomechanical ACL there will always be the need to provide an adequate scaffold to ‘house’ these reparative cells and to provide adequate biomechanical properties for the regeneration process to proceed (Figure 1). Therefore, significant research efforts have recently been focused on developing a provisional bioabsorbable scaffold that can be easily implanted resulting in a more effective ACL reconstruction.
Drawbacks of current interventions using scaffolds alone
Even though the current gold standard of ACL treatment, namely the removal and replacement of the ruptured ACL with hamstring tendon or bone-patellar-bone tendon autografts has a satisfactory short-term outcome, a recent prospective cohort study has shown that a large proportion (62%) of patients with a reconstructed ACL present radiographic evidence of post-traumatic osteoarthritis 10-15 years post-implantation [15-18]. Taking into account that most injuries occur during adolescence, the current treatment interventions expose patients to a greater risk of developing premature post-traumatic osteoarthritis before the age of 30. In this context, lack of harvest morbidity, less traumatic surgical technique, decreased postoperative pain and easier early rehabilitation make allografts an attractive alternative. However, another recent cohort study from the United States military academy showed that individuals having undergone an allograft ACL reconstruction were significantly more likely to experience clinical failure requiring surgical revision [19]. This underlines the necessity to develop biocompatible scaffolds to replace auto- and allo-grafts that would not only boost the rapid reconstruction of the damage site but also result in a more histologically and biomechanically normal ACL able to sustain the required mechanical loads long-term.
Two types of scaffold have been used to restore the ruptured ACL, non-synthetic and synthetic. Non-synthetic scaffolds are derived from human or animal connective tissues such as dermis, small intestine submucosa and pericardium. These scaffolds are produced by decellularisation techniques using hypotonic buffers and ultrasonication [20]. Although they possess inherent bioactivities (mostly containing different types of collagen), they commonly display poor mechanical properties, as well as variations in biocompatibility and degradation rates [21]. On the other hand, synthetic scaffolds are normally non-absorbable polymers that possess good mechanical properties but so far have proven to have poor biocompatibility. Several factors contribute to implantation failure of the synthetic scaffolds such as poor incorporation of the scaffold within the bone tunnel, flexural and rotational fatigue of the scaffold’s structure, and loss of scaffold integrity. However, delayed cellular colonisation of synthetic scaffolds is the main cause of the implantation failure [21]. Therefore, the way forward is most likely the development of ACL implants based on scaffolds pre-seeded with MSCs or ‘smart’ scaffolds capable of rapidly attracting and supporting differentiation of resident MSCs, as described below.
Repair of injured ACL by using MSCs and scaffolds
Clinical applications using MSCs as cell therapy support the notion that MSCs are safe and can be applied in both autologous and allogeneic settings [22,23]. Numerous studies of ligament tissue engineering have particularly shown that MSCs can differentiate towards ligament fibroblasts [24-32]. The reparative effects of MSCs directly injected into the intra-articular area (i.e. exogenously added) have been studied in partial ACL rupture rat models. Kanaya et al. showed that direct injection of green fluorescent protein-labelled rat BM MSCs into the intra-articular area accelerated ACL healing by forming superior histological and biomechanical tissue compared to non-treated knees. Interestingly, the exact topography of the infused MSCs was determined as being within the newly formed tissues, proving that injected MSCs had entered the wound area. In a similar study, Oe et al. showed that both uncultured and culture expanded MSCs could be effective for partial ACL tears not only due to their direct differentiation towards fibroblasts but also due to their trophic actions in the wound site mediated by the secretion of high levels of transforming growth factor-ß (TGF-ß) [33].
However, there are drawbacks associated with the repair of injured ACL by MSCs alone. As mentioned above, the post-injury torn ends of an ACL retract due to high strain resulting in the lack of adequate provisional scaffold, leading to poor homing and survival of MSCs in the injured area due to inflammation [34]. Therefore, current efforts are focused on the development of specialised scaffolds that could provide homing signals as well as facilitate the adherence, proliferation and differentiation of MSCs.
In order to improve the osteointegration and biomechanical properties of the provisional biological scaffold, autografts and allografts have been combined successfully with MSCs [35,36]. Synthetic scaffolds, silk-based [25] and collagen-based [37] have been used to treat ACL rupture in porcine models. In both cases the combination of biomaterials with MSCs resulted in biomechanically superior ACL tissues up to one year post-surgery. Various growth factors have been applied to enhance MSC proliferation, differentiation and extracellular matrix deposition on to these scaffolds (Figure 1); these include TGF-ß/epidermal growth factor (EGF) [26], TGF-ß/insulin [26], insulin-like growth factor (IGF-1) [26], platelet-derived growth factor (PDGF) [38], vascular endothelial growth factor (VEGF) [39], basic fibroblast growth factor (bFGF) [31,38] and growth differentiation factor-5 (GDF-5) [40]. Alternatively, BM MSCs encoding TGF-ß1, VEGF or both TGF-ß1/ VEGF growth factors were used in a rabbit model and showed improved performance in promoting angiogenesis and resulted in tissue formation with good mechanical properties 24 weeks post-surgery [30]. Gene therapy is arguably a safer technique compared to the application of various growth factors, as the latter could have undesirable effects on other cell types such as inducing their proliferation or tumorigenesis. For example, recent studies highlighted some adverse effects and complications related to BMP-2 administration in humans [41,42]. However, in order to develop gene therapy approaches for ACL reconstruction, a better knowledge of the mode of action of candidate growth factors on MSCs is needed.
Several studies have demonstrated that mechanical stimulation of MSCs cultured on specialised biomaterials could induce their differentiation towards ligamentous fibroblasts. Early pioneering studies showed that MSCs seeded on to type I collagen or silk matrix and subjected to tensile or rotational strain could differentiate towards fibroblasts by upregulation of ligamentous fibroblast markers such as collagen types I/III and tenascin-C [43-45]. Similarly, Butler et al. showed that MSCs seeded on to type I collagen sponges upregulated their matrix deposition capacity under the action of tensile loading [46]. Recently, Subramony et al. indicated that bFGF stimulation followed by physiologically relevant tensile strain enhanced the differentiation of MSCs into ligamentous fibroblasts and their extracellular matrix production when cultured on nanofibres [47].
In humans, several clinical trials have been performed using MSC transplantation for treating ligament injuries resulting in positive outcomes on the healing process [48]. Therefore, the effects of specialised biomaterials, chemical and mechanical stimuli on the reparative capacity of MSCs need to be further investigated.
Conclusion
Evidence suggests that the addition of MSCs can be an effective tool to achieve ligament regeneration due to their multipotential and trophic properties. However, similar to bone fracture healing [49], ligament healing is a complex physiological process that involves four different factors for optimal tissue restoration: MSCs, scaffolds, growth factors and mechanical stimulus. Therefore, in order to develop a biological scaffold for ACL reconstruction further research has to focus on how the abovementioned factors can be combined effectively to achieve optimal ACL restoration.
References
- Leong NL, Petrigliano FA, McAllister DR (2014) Current tissue engineering strategies in anterior cruciate ligament reconstruction.J Biomed Mater Res A 102: 1614-1624.
- Bajada S, Mazakova I, Richardson JB, Ashammakhi N (2008) Updates on stem cells and their applications in regenerative medicine.J Tissue EngRegen Med 2: 169-183.
- da Silva Meirelles , Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells.Stem Cells 26: 2287-2299.
- Hsu SL, Liang R, Woo SL (2010) Functional tissue engineering of ligament healing.Sports Med ArthroscRehabilTherTechnol 2: 12.
- Oliva F, Via AG, Maffulli N (2012) Physiopathology of intratendinous calcific deposition.BMC Med 10: 95.
- Fenwick S, Harrall R, Hackney R, Bord S, Horner A, et al. (2002) Endochondral ossification in Achilles and patella tendinopathy.Rheumatology (Oxford) 41: 474-476.
- Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, et al. (2003) Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property.Exp Cell Res 287: 289-300.
- de Mos M, Koevoet WJ, Jahr H, Verstegen MM, Heijboer MP, et al. (2007) Intrinsic differentiation potential of adolescent human tendon tissue: an in-vitro cell differentiation study.BMC MusculoskeletDisord 8: 16.
- Lui PP (2013) Identity of tendon stem cells--how much do we know?J Cell Mol Med 17: 55-64.
- Zhang J, Wang JH (2013) Human tendon stem cells better maintain their stemness in hypoxic culture conditions.PLoS One 8: e61424.
- McKean JM, Hsieh AH, Sung KL (2004) Epidermal growth factor differentially affects integrin-mediated adhesion and proliferation of ACL and MCL fibroblasts.Biorheology 41: 139-152.
- Steadman JR, Cameron-Donaldson ML, Briggs KK, Rodkey WG (2006) A minimally invasive technique ("healing response") to treat proximal ACL injuries in skeletally immature athletes. Journal of Knee Surgery 19: 8-13.
- Ju YJ, Muneta T, Yoshimura H, Koga H, Sekiya I (2008) Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing.Cell Tissue Res 332: 469-478.
- Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, et al. (2008) Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans.Rheumatology (Oxford) 47: 1137-1143.
- Oiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, et al. (2010) Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up.Am J Sports Med 38: 2201-2210.
- Georgoulis AD, Ristanis S, Moraiti CO, Paschos N, Zampeli F, et al. (2010) ACL injury and reconstruction: Clinical related in vivo biomechanics.OrthopTraumatolSurg Res 96: S119-128.
- Tzurbakis M, Diamantopoulos A, Xenakis T, Georgoulis A (2006) Surgical treatment of multiple knee ligament injuries in 44 patients: 2-8 years follow-up results.Knee Surg Sports TraumatolArthrosc 14: 739-749.
- Xergia S, McClelland J, Kvist J, Vasiliadis H, Georgoulis A (2011) The influence of graft choice on isokinetic muscle strength 4-24 months after anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy 19: 768-780.
- Pallis M, Svoboda SJ, Cameron KL, Owens BD (2012) Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States military academy. The American Journal of Sports Medicine 40: 1242-1246.
- Ingram JH, Korossis S, Howling G, Fisher J and Ingham E (2007) The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Engineering 13: 1561-1572.
- Chen J, Xu J, Wang A, Zheng M (2009) Scaffolds for tendon and ligament repair: review of the efficacy of commercial products.Expert Rev Med Devices 6: 61-73.
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, et al. (2012) Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials.PLoS One 7: e47559.
- Wang Y, Han ZB, Song YP, Han ZC (2012) Safety of mesenchymal stem cells for clinical application.Stem Cells Int 2012: 652034.
- Hoffmann A, Gross G (2007) Tendon and ligament engineering in the adult organism: mesenchymal stem cells and gene-therapeutic approaches.IntOrthop 31: 791-797.
- Fan H, Liu H, Toh SL, Goh JC (2009) Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model.Biomaterials 30: 4967-4977.
- Fan H, Liu H, Toh SL, Goh JC (2008) Enhanced differentiation of mesenchymal stem cells co-cultured with ligament fibroblasts on gelatin/silk fibroin hybrid scaffold.Biomaterials 29: 1017-1027.
- Gross G, Hoffmann A (2013) Therapeutic strategies for tendon healing based on novel biomaterials, factors and cells.Pathobiology 80: 203-210.
- Liu H, Fan H, Toh SL, Goh JC (2008) A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds.Biomaterials 29: 1443-1453.
- Fan H, Liu H, Wang Y, Toh SL, Goh JC (2008) Development of a silk cable-reinforced gelatin/silk fibroin hybrid scaffold for ligament tissue engineering.Cell Transplant 17: 1389-1401.
- Wei X, Mao Z, Hou Y, Lin L, Xue T, et al. (2011) Local administration of TGFß-1/VEGF165 gene-transduced bone mesenchymal stem cells for Achilles allograft replacement of the anterior cruciate ligament in rabbits. Biochemical and Biophysical Research Communications 406: 204-210.
- Sahoo S, Toh SL, Goh JC (2010) A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells.Biomaterials 31: 2990-2998.
- Sahoo S, Ang L-T, Cho-Hong Goh J, Toh S-L (2010) Bioactive nanofibers for fibroblastic differentiation of mesenchymal precursor cells for ligament/tendon tissue engineering applications. Differentiation 79: 102-110.
- Oe K, Kushida T, Okamoto N, Umeda M, Nakamura T, et al. (2011) New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats.Stem Cells Dev 20: 671-679.
- Sohni A, Verfaillie CM (2013) Mesenchymal stem cells migration homing and tracking.Stem Cells Int 2013: 130763.
- Soon MYH, Hassan A, Hui JHP, Goh JCH, Lee EH (2007) An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: a short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. The American Journal of Sports Medicine 35: 962-971.
- Lim JK, Hui J, Li L, Thambyah A, Goh J, et al. (2004) Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction.Arthroscopy 20: 899-910.
- Murray MM, Fleming BC (2013) Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. The American Journal of Sports Medicine 41: 1762-1770.
- Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, et al. (2010) bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro.Ann Biomed Eng 38: 225-234.
- Kaux JF, Janssen L, Drion P, Nusgens B, Libertiaux V, et al. (2014) Vascular Endothelial Growth Factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles, Ligaments and Tendons Journal 4: 24-28.
- Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, et al. (2003) GDF-5 deficiency in mice delays Achilles tendon healing.J Orthop Res 21: 826-835.
- Carragee EJ, Baker RM, Benzel EC, Bigos SJ, Cheng I, et al. (2012) A biologic without guidelines: the YODA project and the future of bone morphogenetic protein-2 research. The Spine Journal 12: 877-880.
- Carragee EJ, Hurwitz EL, Weiner BK (2011). A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. The Spine Journal 11: 471-491.
- Altman GH, Horan RL, Lu HH, Moreau J, Martin I, et al. (2002) Silk matrix for tissue engineered anterior cruciate ligaments.Biomaterials 23: 4131-4141.
- Chen J, Altman GH, Karageorgiou V, Horan R, Collette A et al. (2003) Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. Journal of Biomedical Materials Research Part A 67A: 559-570.
- Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, et al. (2002) Cell differentiation by mechanical stress.FASEB J 16: 270-272.
- Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, et al. (2008). Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. Journal of Orthopaedic Research 26: 1-9.
- Subramony SD, Su A, Yeager K, Lu HH3 (2014) Combined effects of chemical priming and mechanical stimulation on mesenchymal stem cell differentiation on nanofiber scaffolds.J Biomech 47: 2189-2196.
- Ahmad Z, Wardale J, Brooks R, Henson F, Noorani A, et al. (2012) Exploring the application of stem cells in tendon repair and regeneration.Arthroscopy 28: 1018-1029.
- Giannoudis PV, Einhorn TA, Marsh D (2007) Fracture healing: the diamond concept.Injury 38 Suppl 4: S3-6.
Citation: Kouroupis D, Churchman SM, Giannoudis PV, Jones E (2014) Mesenchymal Stem Cell Applications for Ligament Repair after Joint Trauma. J Clin Exp Pathol 4:186. DOI: 10.4172/2161-0681.1000186
Copyright: © 2014 Kouroupis sD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16102
- [From(publication date): 9-2014 - Sep 03, 2025]
- Breakdown by view type
- HTML page views: 11399
- PDF downloads: 4703