MIBG Cardiac Imaging is Less Promising in Differentiating PD from Other Parkinsonism for Early-Onset Patients
Received: 22-May-2019 / Accepted Date: 24-May-2019 / Published Date: 31-May-2019 DOI: 10.4172/2161-0460.1000466
Abstract
Objective: Age at onset has an impact on cardiac meta-iodobenzylguanidine (MIBG) uptake in Parkinson’s disease (PD), but not in other parkinsonism. In the present study, we hypothesized that there is an interaction between age at onset and disease group for heart-to-mediastinum (H/M) ratios.
Methods: Ninety-two patients with parkinsonism, including 60 patients with PD and 32 patients with other neurodegenerative parkinsonism, were retrospectively reviewed. Patients were grouped into the early-onset group (age at onset ≤50 y/o) and late-onset group (age at onset >50 y/o) according to the time they first developed motor symptoms. 131I-MIBG cardiac scintigraphy was done and heart-to-mediastinum ratio was used to quantify cardiac uptake.
Results: There was a statistically significant interaction between disease group and age at onset for both early (P=0.008) and delayed H/M ratio (P=0.043). Subgroup analysis demonstrated that no significant difference was found between PD and other neurodegenerative parkinsonism in the early-onset group for either early (2.03 ± 0.42 vs. 2.01 ± 0.29, P=0.882) or delayed H/M ratio (2.05 ± 0.56 vs. 2.22 ± 0.52, P=0.468). In patients with PD, there was significant difference in H/M ratio for both early (P=0.004) and delayed scan (P=0.002) between early-onset and lateonset patients. In patients with other neurodegenerative parkinsonism, no significant difference was found in H/M ratio for either early (P=0.314) or delayed scan (P=0.902) between early-onset and late-onset patients. Using ROC analysis, H/M ratio can be used to differentiate PD from other neurodegenerative parkinsonism (AUC=0.749 and 0.784 for early and delayed scan, respectively). By deselecting early-onset patients, the discrimination power can be further improved (AUC=0.812 and 0.824 for early and delayed scan, respectively) in late-onset patients.
Conclusion: There is an interaction between age at onset and disease group for H/M ratios. 131I-MIBG cardiac sympathetic imaging is less promising in differentiating PD from other parkinsonism for early-onset patients.
Keywords: 131I-MIBG, cardiac sympathetic scintigraphy; Parkinson’s disease; parkinsonism; age at onset; early onset parkinsonism; progressive supranuclear palsy; multiple system atrophy; interaction effect.
Introduction
Progressive Supranuclear Palsy (PSP) and Multiple System Atrophy (MSA) are often misdiagnosed as Parkinson’s disease (PD). The differentiation between them remains challenging despise with consensus diagnostic criteria. Radioactive iodine labeled MIBG cardiac sympathetic imaging has been demonstrated to be promising in differentiating PD from other neurodegenerative parkinsonism [1]. Most patients with PD have reduced cardiac MIBG uptake [2-4], while patients with MSA [5,6] or PSP [7] would have preserved results. Orimo et al. reported a pooled sensitivity of 89.7% and a specificity of 82.6% using delayed H/M ratio for differentiating PD from other neurodegenerative parkinsonism [8]. The H/M ratio, however, is not solely determined by disease group. Previous studies have suggested that many other clinical parameters might have an impact on cardiac MIBG uptake, including age at onset [9], disease duration [10], disease severity [11], and clinical phenotype [12]. Hamada et al. demonstrated a significant reverse correlation between H/M ratio and age at onset [9]. Earlier onset PD patients had a higher H/M ratio than those with later onset age. By contrast, there’s no publication indicating such difference in patients with MSA or PSP. These results indicate that the effect of age at onset on H/M ratio may be different between PD and other neurodegenerative parkinsonism. The diagnostic efficiency of H/M ratio in distinguishing PD from other neurodegenerative parkinsonism might be compromised in patients with early-onset group.
In the present study, we hypothesis that there is an interaction between age at onset and disease group for H/M ratios. Radioactive iodine labelled MIBG cardiac sympathetic imaging is less promising in differentiating PD from other parkinsonism for early-onset patients.
Methods
Study population
We retrospectively reviewed 92 patients with parkinsonism, including 60 patients with PD and 32 patients with other neurodegenerative parkinsonism (9 patients with PSP and 23 patients with MSA). The diagnosis of PD, MSA, PSP was based on widely accepted criteria [13- 15]. Patients with a history of ischemic heart disease, chronic heart failure, diabetes mellitus, or tricyclic antidepressants medication within 1 month were excluded from this study. No participant demonstrated abnormal cognitive status based on neurological system physical evaluation. Clinical and demographic data of patients, including age, age at onset, gender, Hoehn-Yahr stage, and disease duration, were collected. Age at onset was defined as the time when patients first developed motor symptoms. Patients were further grouped into earlyonset group (age at onset ≤50 y/o) and late-onset group (age at onset >50 y/o).
131I-MIBG cardiac scintigraphy
After a 60-min resting period, patients received an intravenous injection of 111MBq 131I-MIBG. A 5-min planar image of the chest was obtained in an anterior view after 15 min for early imaging and 4 h for delayed scan. A double-head Infinia Hawkeye γ-camera (GE Healthcare) fitted with a high-energy general-purpose collimator was used. Photopeak energy was centered at 364 KeV with a 20% window and the data was stored on a 256 × 256 matrix.
The images were reviewed by an experienced nuclear medicine specialist. Quantitative analysis was used to assess cardiac MIBG uptake. Regions of Interest (ROIs) were manually drawn over heart contour (H) and upper mediastinum (M) for each planar image. Average counts per pixel in the ROIs were used to calculate heart-to-mediastinum (H/M) ratio.
Statistical analysis
The results were expressed as means ± SD values. The analysis of covariance (ANCOVA), Student’s t-test and chi-square test were used to examine the differences between groups (SPSS, version 22). Two-way analysis of variance (two-way ANOVA) was used to determine whether there is an interaction effect between disease group and age at onset on H/M ratio. Diagnostic performance was evaluated using Receiver- Operating-Characteristic (ROC) analysis. P value <0.05 was considered to indicate statistically significant.
Results
Patient characteristics
The clinical data was summarized in Table 1. There was no significance difference in age, age at onset, or gender between PD and other neurodegenerative parkinsonism. The disease duration of patients in PD group was significantly longer. After controlling for disease duration using one-way ANCOVA, there was statistically significant difference in the adjusted H/M ratio (P<0.001 for both early and delayed scan) between the two groups.
| PD | MSA+PSP | P value | |
|---|---|---|---|
| Number | 60 | 32 | |
| Age | 60.0±10.3 | 57.6±7.6 | 0.244 |
| Age at onset | 0.312 | ||
| ≤50 | 13 | 10 | |
| >50 | 47 | 22 | |
| Gender | 0.745 | ||
| Female | 34 | 17 | |
| Male | 26 | 15 | |
| Disease duration (months) | 51.9±49.5 | 31.5 ± 19.3 | 0.006 |
Table 1: Patients’ clinical data
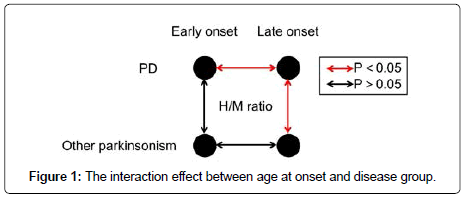
Interaction effect between disease group and age at onset
Using two-way ANOVA, there was statistically significant interaction between disease group and age at onset for both early (P=0.008) and delayed H/M ratio (P=0.043). The effect of disease group on H/M ratio was different between early and late-onset parkinsonism (Figure 1). Subgroup analysis (Table 2) demonstrated that no significant difference was found between PD and other neurodegenerative parkinsonism in early-onset group for either early (2.03 ± 0.42 vs. 2.01 ± 0.29, P=0.882) or delayed H/M ratio (2.05 ± 0.56 vs. 2.22 ± 0.52, P=0.468). In lateonset group, patients with PD demonstrated significant lower H/M ratio compared with other neurodegenerative parkinsonism for both early (1.71 ± 0.32 vs. 2.14 ± 0.34, P<0.001) and delayed images (1.58 ± 0.42 vs. 2.25 ± 0.57, P<0.001). In patients with PD, there was significant difference in H/M ratio for both early (P=0.004) and delayed scan (P=0.002) between early-onset and late-onset patients (Figure 2). In patients with other neurodegenerative parkinsonism, no significant difference was found in H/M ratio for either early (P=0.314) or delayed scan (P=0.902) between early-onset and late-onset patients.
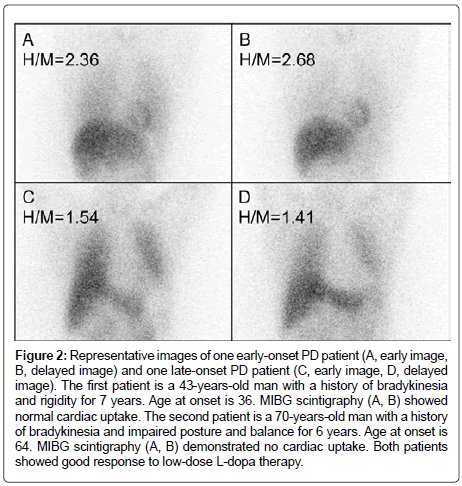
Figure 2: Representative images of one early-onset PD patient (A, early image, B, delayed image) and one late-onset PD patient (C, early image, D, delayed image). The first patient is a 43-years-old man with a history of bradykinesia and rigidity for 7 years. Age at onset is 36. MIBG scintigraphy (A, B) showed normal cardiac uptake. The second patient is a 70-years-old man with a history of bradykinesia and impaired posture and balance for 6 years. Age at onset is 64. MIBG scintigraphy (A, B) demonstrated no cardiac uptake. Both patients showed good response to low-dose L-dopa therapy.
| Early-onset group | Late-onset group | All patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PD | MSA+PSP | P value | PD | MSA+PSP | P value | PD | MSA+PSP | P value | |
| Early H/M ratio | 2.03±0.42 | 2.01±0.29 | 0.882 | 1.71±0.32 | 2.14±0.34 | <0.001 | 1.78±0.36 | 2.10±0.33 | <0.001 |
| Delayed H/M ratio | 2.05±0.56 | 2.22±0.52 | 0.468 | 1.58±0.42 | 2.25±0.57 | <0.001 | 1.68±0.49 | 2.24±0.55 | <0.001 |
Table 2: Subgroup analysis: comparison of H/M ratios between PD and other parkinsonism in early-onset and delayed-onset groups.
Diagnostic efficiency
Using ROC analysis, H/M ratio can be used to differentiate PD from other neurodegenerative parkinsonism (area under curve, AUC=0.749 and 0.784 for early and delayed scan, respectively). By deselecting early-onset patients, the discrimination power can be further improved (AUC=0.812 and 0.824 for early and delayed scan, respectively) in lateonset patients.
Discussion
MIBG cardiac scintigraphy has been widely used in differential diagnosis of parkinsonism. Orimo et al. reported a pooled sensitivity of 89.7% and a specificity of 82.6% using delayed H/M ratio for differentiating PD from other neurodegenerative parkinsonism [8]. The sensitivity and specificity, however, varied significantly among different studies. Reinhardt et al. reported a perfect discrimination power (100% for both sensitivity and specificity) for delayed H/M ratio [16], while Fröhlich et al. demonstrated a limited diagnostic efficiency [17] (80.0% for sensitivity and 23.1% for specificity). This phenomenon has raised a question: what’s the underlying cause for the discrepant performance of MIBG cardiac scintigraphy?
Cardiac MIBG uptake can be impaired by non-parkinsonism related factors, such as medications [18], heart failure [19], and diabetes mellitus [20]. Apart from those, it can also be affected by parkinsonismrelated factors. Satoh et al. reported a correlation between disease duration and H/M ratio in PD patients [10]. Hattori et al. showed a reverse correlation between cardiac MIBG uptake and H-Y stage and UPDRS score [21]. Hamada et al. demonstrated a significant reverse correlation between H/M ratio and age at onset [9]. However, to the best of our knowledge, there’s no literature concerning the interaction effect between disease group (PD versus other neurodegenerative parkinsonism) and clinical parameters for cardiac MIBG uptake in patients with parkinsonism. The interaction effect can be quite essential in image interpretation. Knowing when the images can be trusted is very important in clinical decision-making.
Early-onset Parkinson’s disease refers to patients presenting with a parkinsonian syndrome with onset of motor symptoms before age 50 years. It’s been demonstrated that early-onset PD showed a different genetic and clinical profile from idiopathic PD [22]. In the present study, we investigated the interaction effect between disease group and age at onset. The results showed that the effect of disease group on H/M ratio was different between early and late-onset parkinsonism. In earlyonset patients, there was no significant difference between PD and other neurodegenerative parkinsonism. In late-onset patients, however, significantly reduced cardiac uptake was found in PD compared with other neurodegenerative parkinsonism. It means that a normal cardiac uptake in patients with early-onset parkinsonism can’t be used to rule out the possibility of PD. The mechanism that underlying the preserved MIBG uptake in early-onset PD patient is not clear. It might be due to the different genetic profile between early-onset PD and idiopathic PD.
Lewy bodies are intracytoplasmic eosinophilic inclusions with a hyaline core and a pale halo that is mainly composed of aggregated α-synuclein. It has been suggested that impaired cardiac uptake of MIBG in parkinsonism, especially idiopathic PD, is closely related to the presence of Lewy bodies [23]. Mutations in recessive genes such as PARKIN, PINK1 and DJ1 [24], however, may cause mitochondrial dysfunction and oxidative stress of the neuron [25], which usually present as early onset parkinsonism. Early onset parkinsonism with normal cardiac MIBG uptake was reported in a Korean family with autosomal recessive PARKIN gene mutation [26,27]. Our study further supports these findings from a statistical point of view. Since this is a retrospective study, we weren’t able to test whether there were genetic differences between early-onset and late-onset PD patients in our cohort. Further studies are warranted to explore the inner association.
Conclusions
There is an interaction effect between age at onset and disease group for H/M ratios. 131I-MIBG cardiac sympathetic imaging is less promising in differentiating PD from other parkinsonism for earlyonset patients.
Conflict of interest/disclosure
All authors declare that there is no conflict of interest regarding this work. All authors have approved the final article.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Xu D, Zhu W, Huo L, Zhu S, Li F, et al. (2018) Validation of Iodine-131-meta-iodobenzylguanidine cardiac scintigraphy in Parkinsonism: A preliminary study. Parkinsonism Relat Disord 50: 69-73.
- Yoshita M, Hayashi M, Hirai S (1998) Decreased myocardial accumulation of 123I-meta-iodobenzyl guanidine in Parkinson's disease. Nucl Med Commun 19: 137-142.
- Kashihara K, Ohno M, Kawada S, Okumura Y (2006) Reduced cardiac uptake and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson's disease and dementia with Lewy bodies. J Nucl Med 47: 1099-1101.
- Yoshii F, Ryo M, Baba Y, Koide T, Hashimoto J (2017) Combined use of dopamine transporter imaging (DAT-SPECT) and 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy for diagnosing Parkinson's disease. J Neurol Sci 375: 80-85.
- Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, et al. (2002) Sympathetic cardiac denervation in Parkinson's disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry 73: 776.
- Braune S, Reinhardt M, Schnitzer R, Riedel A, Lücking CH (1999) Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy. Neurology 53: 1020.
- Yoshita M (1998) Differentiation of idiopathic Parkinson's disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci 155: 60-67.
- Orimo S, Suzuki M, Inaba A, Mizusawa H (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson's disease from other neurodegenerative parkinsonism: A systematic review and meta-analysis. Parkinsonism Relat Disord 18: 494-500.
- Hamada K, Hirayama M, Watanabe H, Kobayashi R, Ito H, et al. (2003) Onset age and severity of motor impairment are associated with reduction of myocardial 123I-MIBG uptake in Parkinson's disease. J Neurol Neurosurg Psychiatry 74: 423-426.
- Satoh A, Serita T, Seto M, Tomita I, Satoh H, et al. (1999) Loss of 123I-MIBG uptake by the heart in Parkinson's disease: assessment of cardiac sympathetic denervation and diagnostic value. J Nucl Med 40: 371-375.
- Takatsu H, Nishida H, Matsuo H, Watanabe S, Nagashima K, et al. (2000) Cardiac sympathetic denervation from the early stage of Parkinson's disease: Clinical and experimental studies with radiolabeled MIBG. J Nucl Med 41: 71-77.
- Chung EJ, Kim EG, Kim MS, Bae SK, Seog DH, et al. (2011) Differences in myocardial sympathetic degeneration and the clinical features of the subtypes of Parkinson's disease. J Clin Neurosci 18: 922-925.
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, et al. (2015) MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 30: 1591-1601.
- Wenning GK, Gilman S, Low PA, Brooks DJ, Mathias CJ, et al. (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: 670-676.
- Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, et al. (2017) Advances in progressive supranuclear palsy: New diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol 16: 552-563.
- Reinhardt MJ, Jungling FD, Krause TM, Braune S (2000) Scintigraphic differentiation between two forms of primary dysautonomia early after onset of autonomic dysfunction: value of cardiac and pulmonary iodine-123 MIBG uptake. Eur J Nucl Med 27: 595-600.
- Frohlich I, Pilloy W, Vaillant M, Diederich NJ (2010) Myocardial MIBG scintigraphy: A useful clinical tool? : A retrospective study in 50 parkinsonian patients. Neurol Sci 31: 403-406.
- Jacobson AF, Travin MI (2015) Impact of medications on mIBG uptake, with specific attention to the heart: Comprehensive review of the literature. J Nucl Cardiol 22: 980-993.
- Chirumamilla A, Travin MI (2011) Cardiac applications of 123I-mIBG imaging. Semin Nucl Med 41:374-387.
- Marini C, Bandettini di Poggio M, Pomposelli E, Marchese R, Nobili F, et al. (2010) Whole body and cardiac metaiodobenzylguanidine kinetics in Parkinson disease and multiple system atrophy: Implications for the diagnostic role of imaging. Clin Nucl Med 35: 311-316.
- Hattori T, Orimo S, Hallett M, Wu T, Inaba A, et al. (2014) Relationship and factor structure in multisystem neurodegeneration in Parkinson's disease. Acta Neurol Scand 130: 347-353.
- Domingo A, Klein C (2018) Genetics of Parkinson disease. Handb Clin Neurol 147: 211-227.
- Chung EJ, Kim SJ (2015) (123)I-Metaiodobenzylguanidine Myocardial Scintigraphy in Lewy Body-Related Disorders: A Literature Review. J Mov Disord 8: 55-66.
- Quattrone A, Bagnato A, Annesi G, Novellino F, Morgante L, et al. (2008) Myocardial 123metaiodobenzylguanidine uptake in genetic Parkinson's disease. Mov Disord 23: 21-27.
- Gabilondo I, Llorens V, Rodriguez T, Fernández M, Concha TP, et al. (2019) Myocardial MIBG scintigraphy in genetic Parkinson's disease as a model for Lewy body disorders. Eur J Nucl Med Mol Imaging 46: 376-384.
- Kim YD, Song IU, Kim JS, Chung SW, Lee KS (2010) Cardiac (123)I-metaiodobenzylguanidine Scintigraphy in a Patient with Familial Parkinsonism with Parkin Gene Mutation. J Mov Disord 3: 42-44.
- Kim JS, Lee KS, Kim YI, Lee KH, Kim HT (2003) Homozygous exon 4 deletion in Parkin gene in a Korean family with autosomal recessive early onset parkinsonism. Yonsei Med J 44: 336-339.
Citation: Zhu W, Xu D, Huo L, Wang H (2019) MIBG Cardiac Imaging is Less Promising in Differentiating PD from Other Parkinsonism for Early-Onset Patients. J Alzheimers Dis Parkinsonism 9:466 DOI: 10.4172/2161-0460.1000466
Copyright: © 2019 Zhu W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4105
- [From(publication date): 0-2019 - Nov 08, 2025]
- Breakdown by view type
- HTML page views: 3238
- PDF downloads: 867