Morphofunctional Correlation of Excitatory and Depressor Synaptic Processes in Hippocampus, Amygdala and Basal Meynert Nucleus Neurons in Dynamics of Development of Alzheimer's Disease Model Induced by Aβ25-35
Received: 28-Sep-2017 / Accepted Date: 18-Oct-2017 / Published Date: 25-Oct-2017 DOI: 10.4172/2161-0460.1000391
Abstract
Objective: Compensatory mechanisms are responsible for the clinical signs of suppression of neurodegeneration. Intervention into their mechanisms on an example of the ratio of excitatory and depressor synaptic responses will contribute to the development of therapeutic strategies.
Methods: After 12-28 weeks (w) of experiment on the model of Alzheimer’s disease (AD), an activity of single neurons of hippocampus (H), Amygdala (Am) and, nucleus basalis of Meynert (NBM) to high frequency stimulation (HFS) of entorhinal cortex (EC) was recorded. The high frequency stimulation of H resulted in an activity of single neurons of the Am and NBM. By means of on-line selection and special mathematical analysis, tetanic potentiation (TP) and depression (TD) with further combination into posttetanic uni and multidirectional sequences, were revealed. In morpho- and histochemical study, the method of revelation of Сa2+- dependent phosphorylation was used.
Results: After 12 weeks of experiment on the model of AD, a heavy TD of NBM and Аm neurons to HFS of H, as well as a weak (TD) in H and NBM neurons to HFS of EC were found. TP occurred by the activation of ЕС in the H (TP PTP) and in Am (TP PTD) neurons, equal to and above the norm in neurons of Am to HFS of H. In the neurons of NBM to HFS of EC, the weakest excitation to HFS of H was detected. In the neurons H to HFS of EC, Am and NBM to HFS of H, after 13-28 weeks, TD and tetanic excitation in all cases were low, which indicating on depletion of compensatory opportunities. Morpho- and histo-chemical changes of H, Am and NBM neurons on the model of AD were characterized by total tendency to structural-metabolic dysfunctions, with distortion of forms, central chromatolysis, presence of light ectopic nucleus with increased nucleolus, change reaction of neurofibrills, lack of reaction processes, accumulation of hyper phosphorylated entities and, presence of spaces with the lack of cellular reaction.
Conclusion: The absence of expressed depression, presupposed by us as a protector in the present study, makes it necessary to involve pharmacological interventions with a view to its strengthening, and therefore is the subject of the next reports. Electrophysiological data have been confirmed morphologically.
Keywords: Alzheimer’s disease; Hippocampus; Rostral amygdalopiriform area; Basal nucleus of meynert; Single spike activity; Acid phosphatase
Introduction
Accumulation of β-amyloid (Aβ) in the brain is a hallmark of Alzheimer’s disease (AD) and studies have demonstrated that it can induce synapse dysregulation, altered neuronal activity [1-5] and in early AD hallmark of the aberrant regulation in synaptic function including AMPA receptor (AMPAR) synaptic accumulation and synaptic plasticity [6,7]. Previous findings have demonstrated that synaptic plasticity, regarded as the cellular basis for learning and memory [8], is vulnerable to exposure to high levels of Aβ; hippocampal long-term potentiation (LTP) is impaired following exogenous exposure to soluble oligomeric Aβ [2,9,10]. To understand how memory function in AD becomes deregulated, a vast number of studies have looked to determine the mechanisms underlying the Aβ-mediated impairment of synaptic plasticity [11]. Synaptic plasticity is one of the most important properties of the mammalian brain as it underlies learning and memory processes. One of the main forms of synaptic plasticity found at the excitatory synapses is the long-term potentiation (LTP), which requires the activation of postsynaptic glutamate receptors (NMDA and AMPA) and is altered in neurodegenerative diseases [12]. Soluble Aβ potently and selectively disrupts synaptic plasticity causing inhibition of LTP and enhancement of long-term depression (LTD) of glutamatergic transmission in normal rodent hippocampus and future clinical trials of drugs acting through the glutamatergic and cholinergic systems [10,13]. In vivo injection of Aβ is reported to facilitate LTD and LTP reversal (depotentiation) in the CA1 region of the hippocampus [1]. Synapse vulnerability strongly correlates with cognitive decline before detectable neuronal death [14,15] and might contribute to the subsequent neuronal degeneration. The correlation between AD dementia and synaptic degeneration, investigated the ability of soluble Aβ-derived oligomers (ADDLs) to affect synapse composition, structure and abundance, attaching to presumed excitatory pyramidal neurons but not GABAergic neurons [16]. Moreover, in vitro studies performed in hippocampal neurons have reported that application of Aβ peptides, at concentrations below neurotoxic levels, can inhibit LTP induction without affecting basal synaptic transmission [17-19]. Although some downstream targets of Aβ have been identified [20-23], only a limited number of studies has shown the ability of these molecules to fully restore function after significant synapse degeneration [21,22]. The identity of the signaling pathways that could restore synapse function remains poorly understood. On the other hand, as apoptotic death in CA3 and neurogenesis in dentate gyrus is modulated by glutamatergic neurotransmission resulting from NMDA receptor activation and Ca2+ levels fluctuations when interaction with corticosteroids [24]. Homeostatic synaptic plasticity (HSP) serves to restrain neuronal activity within a physiological range. The studies have provided great insight into Hebbian plasticity in AD, however the role of Aβ in HSP remains largely unknown [25]. A major function of HSP is to regulate neuronal activity in a negative feedback manner, thus maintaining neuronal activity or synaptic function [26,27] within a physiological range after changes in network activity [28-31]. Finally, increasing evidence suggests a link between a deficiency in Wnt signaling and AD. The findings demonstrate the remarkable capacity of adult neurons to regenerate functional circuits after substantial synapse loss and highlights that Wnt signaling is a targetable pathway in neurodegenerative diseases [32].
The aim of the study was the micro electrophysiological study the mechanisms of development of synaptic processes in neural structures not only the short-term H, but also the long-term (Rostral amygdalopiriform area - Am and NBM) memory of the brain evoked by activation of the Entorhinal cortex (EC) and H in the form of synaptic potentiation and depression on amyloid model of AD induced by Aβ25-35.
Materials and Methods
Experiments were performed on 26 male adult albino rats, weighing 200-250 g in 2 series of experiments: 1) on intact (n=10) and 2) on AD model (n=16), induced by bilateral intra cerebroventricular (i.c.v) injection of Aβ 25-35. The neuronal activity of H, Am and NBM to high frequency stimulation (HFS) (1 s) of EC, Am and NBM to HFS of H are studied. All experiments were performed according to the “rules of laboratory animal care” (NIH publication for no 85-23, revised 1985; experiments, the authors certify that they were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 or the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, or the European Communities Council Directive of 24 November 1986 (86/609/EEC)). To create a model of AD bilaterally and i.с.v. was injected toxic oligomer Аβ 25-35 according to the stereotaxic coordinates of the rat atlas [33] (AP-1, L ± 1.5, DV+3.5 mm) by 3mcl of its 9-10 M fragments (1 mg/ml at 37°C , aggregated for 4 days) [34] and sustained up to 12-28 weeks till acute experiment. Surgeries under pentobarbital anesthesia (40 mg/kg, i.p.) are carried out. The operative field was treated with 0.1% bicillin (Benzathine benzylpenicillin). In acute experiments animals were additionally immobilized by 1% ditilin (Suxamethonium iodide) (25 mg/kg, i/p) and transferred to artificial respiration. After craniotomy stereotactically oriented stimulating electrodes were implanted in the ipsilateral EC (AP- 11, L ± 3.5, DV+4.0 mm) and H (AP-3,5, L ± 2.0, DV+4.0 mm) and glass recording microelectrode with tip diameter of 1 μm, filled with 2 M NaCl solution, were inserted in H (AP-3.5, L ± 2.0, D+4.0 mm), Am (AP-3.24, L ± 5.4-5.8, DV+9.5-10.2 mm) and NBM (AP-1.8, L ± 3.0, DV+7.4 mm). In general was recorded the activity of 876 neurons from H (236), Am (382) and NBM (258). HFS (100 Hz for 1 s) of EC and CA1 of H was performed by rectangular current shocks of duration 0.05 ms, 0.10-0.16 and 0.16- 0.18mA. We performed software analysis of the single spike activity of H (88), Am (89) and NBM (90) neurons in the norm and H (205), Am (293) and NBM (178) neurons on the model of AD. On-line recording is made on the basis of the program, which provides a selection of spikes by amplitude discrimination, followed by the “raster” of pre- and post-stimulus spiking from numerous neurons, as well as diagrams of the averaged spike frequency (developed by Kamenetski). After selection the pulse flow was subjected to programmed mathematical analysis. For selected and comparable groups of neuronal activity spiking were built summed and averaged (PETH Average) and frequency histograms (Frequency Average). To determine the statistical significance of differences in the duration of inter spike intervals before and after the stimulus was used nonparametric test the homogeneity of two independent samples - twosample “Wilcoxon-Mann-Whitney” test. Since the number of recorded spikes were quite large (up to several hundreds of spikes per 20 s interval after the stimulus), was used a modification of this test, taking into account its asymptotic normality - z-test. Critical values comparing to tabulated values of a normal distribution with a significance level of 0.05, 0.01 and 0.001 (for different tests) are shows, that after the HFS for most samples of neuronal activity spiking there is a statistically significant alteration at least 0.05 level of significance.
Morphohistochemical studies were carried out by the method of detecting the activity of Ca2+ dependent acid phosphatase (AP) [35] on the basis of the Hamori method. This methodological approach is based on the identification of intracellular phosphorus-containing compounds having key positions in metabolic energy processes for preservation and self-reproduction of vital systems. The method is reproducible, adequate and selectively identifies AP, which is the most important Ca2+-dependent marker of neuron activity. And that is an important criterion of reliability of its use and in a comparative aspect, it is highly reproducible both on intact and on pathological material. In addition to the histochemical value, the method represents a definite morphological interest. The resulting picture is adequate, has a great informativeness and allows to judge about certain links in the metabolism of the structures under investigation and about morphohistochemical changes in the brain. On the light-colored background of the preparation, neural structures are clearly identified, constantly reproducible, that is an important criterion for the reliability of the method. The pieces of the brain were fixed in 5% neutral formalin prepared on 0.1 M phosphate buffer pH 7.4. For better stabilization of phosphorus and in order to prevent autolysis, fixation was performed at 4°C for 48 h. Frozen slices 60 μ thick were prepared from pieces of the brain, washed in distilled water and transferred to an incubation mixture for selective detection of nerve cells: 20 ml of a 0.38% solution of lead acetate, 5 ml of 1 M acetate buffer pH 5.6 and 5 ml of a 2% solution of sodium β-glycerophosphate. The incubation was carried out in a thermostat at 37°C for 1 to 2 h. Then, the washing of the sections in distilled water, the development in sodium sulfate solution and after repeated washing, the embedding in the balsam with the subsequent description of the preparations.
Results
Comparative analysis of the impulse activity of single H, Am and NBM neurons in norm (n=322), on the model of AD after 12 (n=581) and 13-28 (n=197) weeks revealed the formation of excitatory and depressor responses in neurons of H, Am and NBM to HFS of EC, in neurons of Am and NBM to HFS of H as tetanic potentiation (TP) and depression (TD), followed by post-tetanic mixed uni (TD PTD, TP PTP) and multidirectional (TD PTP, TP PTD) manifestations of activity. On the basis of the average number of spikes (PETH), with conversion into inter spike intervals and frequency in Hz (Frequency Average) and compared with pre-stimulus level the depressor post-stimulus manifestations of neuronal activity to HFS of EC identified in the following ranges. A 12 weeks later after the injection of Aβ 25-35 tetanic depression in depressor sequences in H neurons to HFS of EC within a 3-fold underestimation of pre-stimulus activity, twice less than norm (6-fold) (Figure 1A); in the depressor-excitatory sequence about 4-fold and also below the norm (Figure 1B). After 12 weeks TD in the TD PTD in Am neurons to HFS of EC were within 23-fold underestimated, while in the norm it reached only 4.25-fold, i.e., much higher and in TD PTP - 22 times also above norm (4.66-fold) (Figure 1C). In NBM neurons to HFS of EC after 12 weeks TD in the TD PTD determined by the order of 2.3-fold underestimation, respectively, which was much lower than norm (15-fold); TD in TD PTP - within 3-fold underestimated, respectively, is also much lower than the norm (Figure 1D). In Am neurons to HFS of H TD in TD PTD after 12 weeks reached 3.5-fold underestimation but above the norm (2-fold) (Figure 1E); TD in TD PTP after 12 weeks - 3-fold underestimation, also exceeding the norm (about 2-fold) (Figure 1F). Finally, after 12 weeks on the model of AD in NBM neurons TD in TD PTP and PTP to HFS of H understatement was within 4 and 2.5-fold underestimation, respectively, which turned out to be somewhat higher than norm (4- and 2-fold, respectively) (Figures 1G and 1H). Thus, in the corresponding posttetanic sequences tetanic depression in the model of AD 12 weeks later in H neurons to HFS of EC did not reach the norm, in Am neurons to HFS of EC significantly exceeded the norm, and in NBM neurons to HFS of EC also significantly, but did not reach the norm, and in NBM neurons to HFS of H was equal to it (Figure 1).
Figure 1: A-E- integrated averaged peristimulus (PETH Average) and frequency histograms (Frequency Average) of tetanic and post-tetanic depressor (TD PTD, depressor-excitatory (TD PTP), excitatory (TP PTP) and excitatory-depressor spike activity of neurons in the H (A, F), the Am (B, G) and NBM (C, H) to HFS of EC, in Am (D, I) and NBM (E, J) to HFS of H on AD model 12 weeks after the administration of Aβ 25-35,compared with the norm. The remaining marks are in the figure.
The excitatory post-stimulus tetanic reaction (Figure 2) changed as follows: TP in excitatory s sequences (TP PTP) in H neurons to HFS of EC later 12 weeks after β 25-35 injection reached exceeded of prestimulus level 8-fold, that turned out to be higher than normal (4.5- fold) (Figure 2A); TP in excitatory-depressor sequences (TP PTD) in this period of testing reached 2.5-fold exceeding, much below normal (10-fold) (Figure 2B). In Am neurons to HFS of EC after 12 weeks TP in TP PTP is calculated within the 10.25- fold higher and above the norm (7.83-fold); TP in TP PTD reached 14-fold elevation, much higher than the norm (2.14-fold) (Figure 2C). In NBM neurons, to HFS of EC reached only the 1.38- fold elevation and lower than the norm (2.25- fold); TP in TP PTD - 1.2-fold, far below the norm (13.0-fold) (Figure 2D). In Am neurons to HFS of H 12 weeks later TP in TP PTP detected by about 1.43-fold elevation, lower than normal (2.66-fold); TP in TP PTD in the same period is calculated within a 1.14-fold, which was below the norm (3-fold) (Figures 2E and 2F). In NBM neurons, to HFS of H TP in TP PTP after 12 weeks was within the 1.12-fold elevation, not reaching the norm (2-fold); TP in TP PTD in the same period had 1.5-fold excess and equal the norm (1.5-fold) (Figures 2G and 2H). In other words, in the H neurons to HFS of EC excitatory tetanic effects after 12 weeks in both sequences were higher and significantly below the norm, respectively. In Am neurons to HFS of EC after 12 weeks the situation was the reverse - with some excess and a much higher than norm. In NBM neurons, to HFS of EC after 12 weeks in excitatory sequence excess was below the norm, and in excitatory-depressor - much lower. In Am and NBM neurons to HFS of H generally changes were in insignificant ranges. In Am neurons to HFS of H TP in both sequences did not reach the norm.
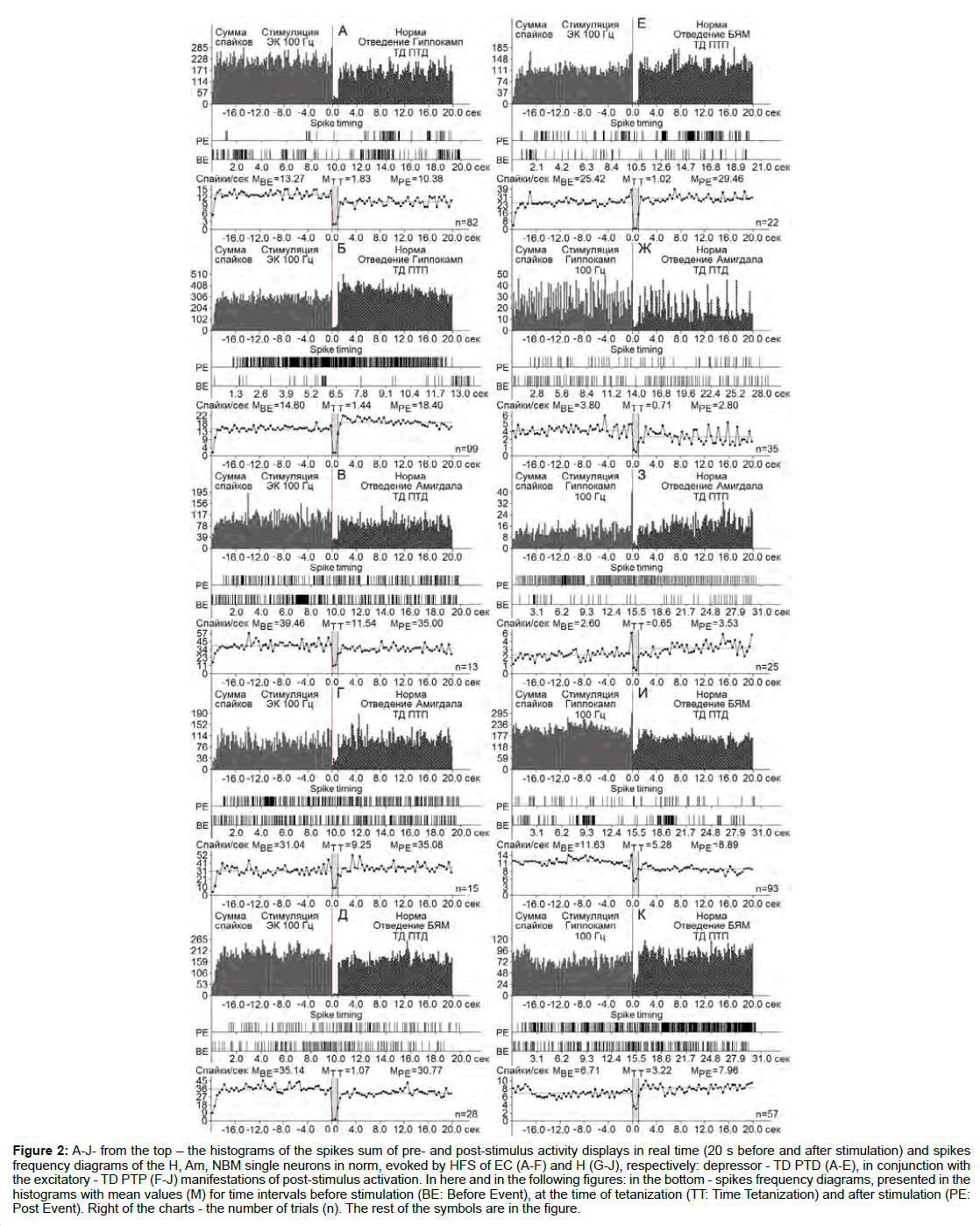
Figure 2: A-J- from the top - the histograms of the spikes sum of pre- and post-stimulus activity displays in real time (20 s before and after stimulation) and spikes frequency diagrams of the H, Am, NBM single neurons in norm, evoked by HFS of EC (A-F) and H (G-J), respectively: depressor - TD PTD (A-E), in conjunction with the excitatory - TD PTP (F-J) manifestations of post-stimulus activation. In here and in the following figures: in the bottom - spikes frequency diagrams, presented in the histograms with mean values (M) for time intervals before stimulation (BE: Before Event), at the time of tetanization (TT: Time Tetanization) and after stimulation (PE: Post Event). Right of the charts - the number of trials (n). The rest of the symbols are in the figure.
Finally, in NBM neurons to HFS of H at 12 week, on the contrary, the excess did not reach the norm in the exciting sequence, and in excitatory-depressor - was in the normal range.
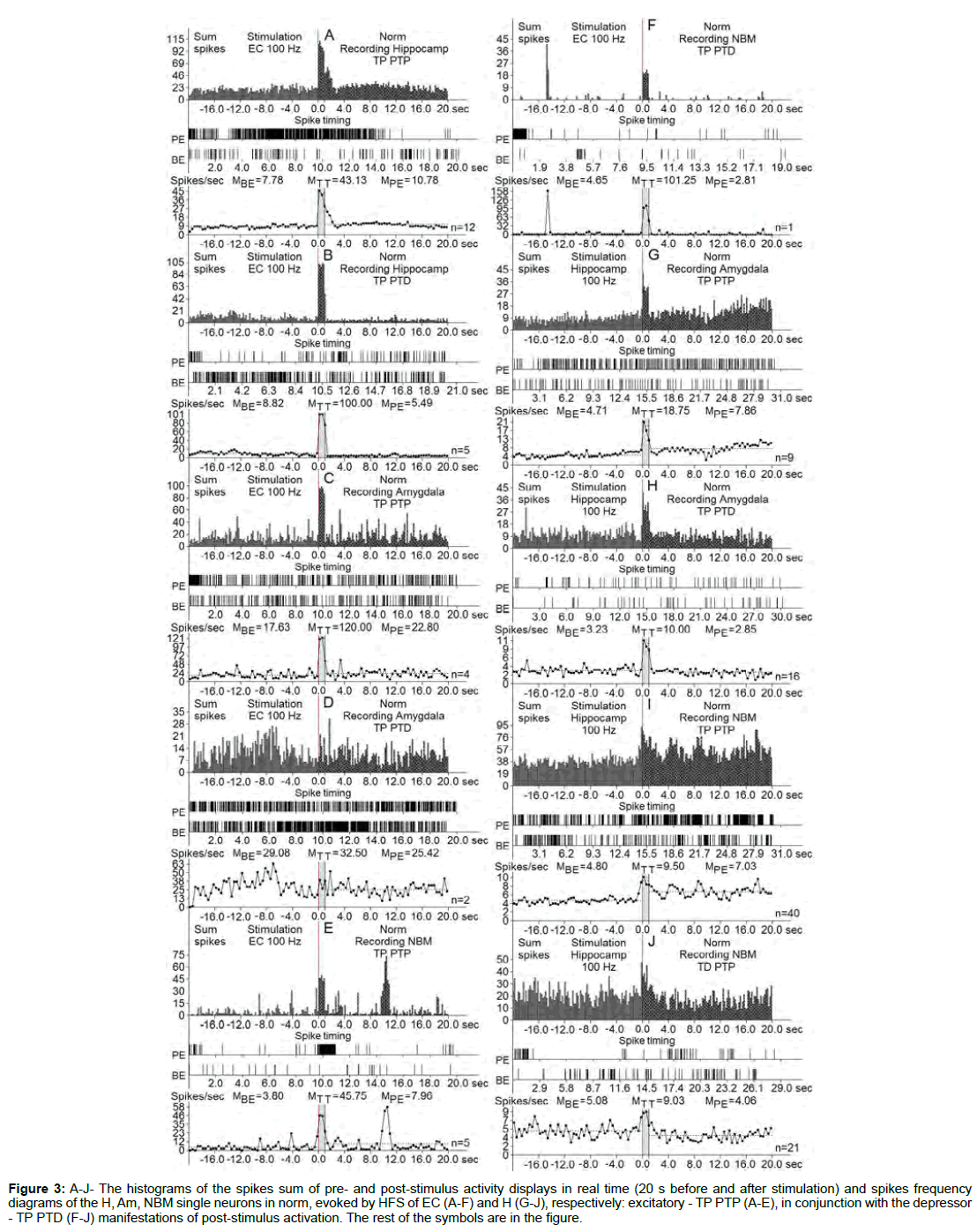
Figure 3: A-J- The histograms of the spikes sum of pre- and post-stimulus activity displays in real time (20 s before and after stimulation) and spikes frequency diagrams of the H, Am, NBM single neurons in norm, evoked by HFS of EC (A-F) and H (G-J), respectively: excitatory - TP PTP (A-E), in conjunction with the depressor - TP PTD (F-J) manifestations of post-stimulus activation. The rest of the symbols are in the figure.
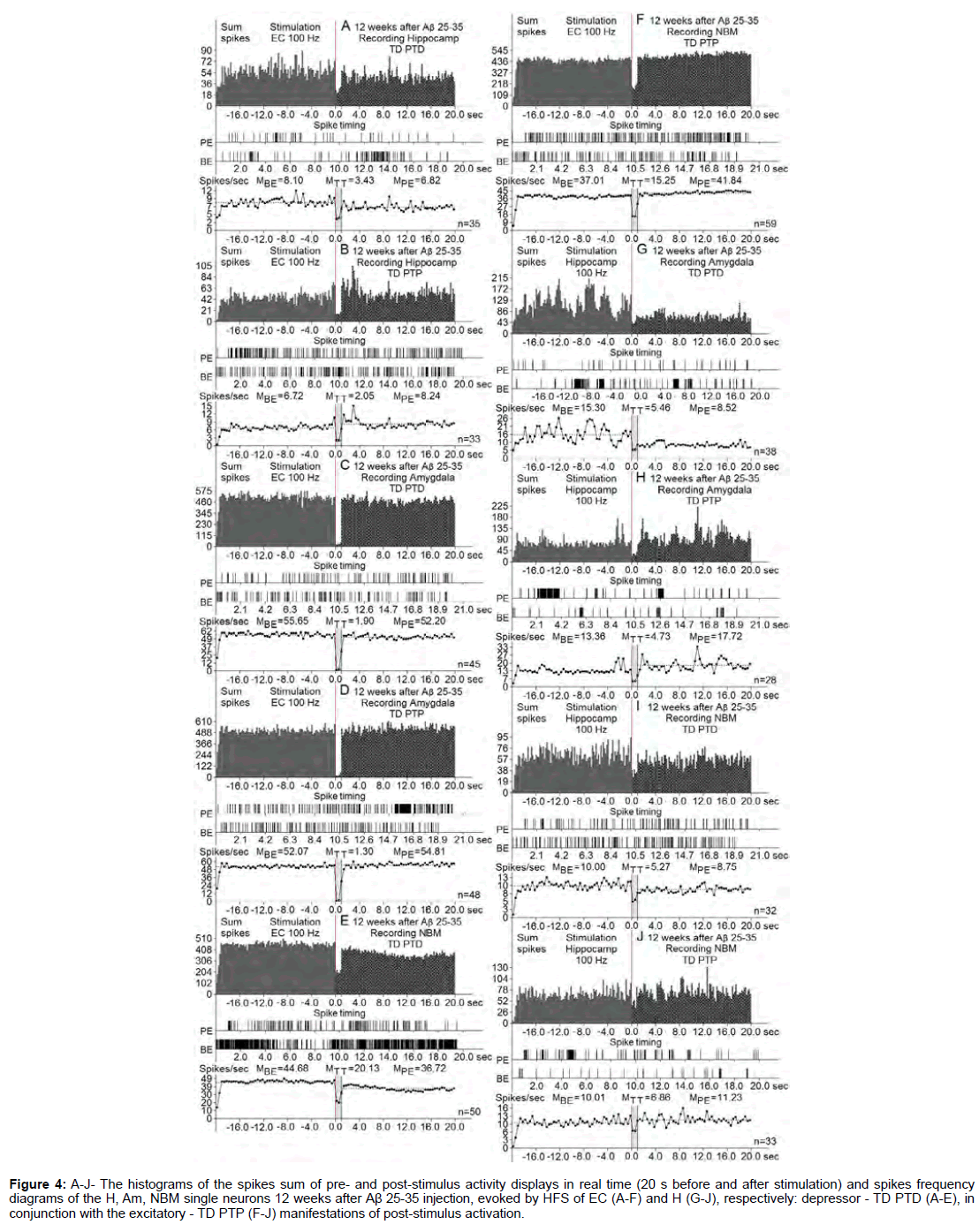
Figure 4: A-J- The histograms of the spikes sum of pre- and post-stimulus activity displays in real time (20 s before and after stimulation) and spikes frequency diagrams of the H, Am, NBM single neurons 12 weeks after Aβ 25-35 injection, evoked by HFS of EC (A-F) and H (G-J), respectively: depressor - TD PTD (A-E), in conjunction with the excitatory - TD PTP (F-J) manifestations of post-stimulus activation.
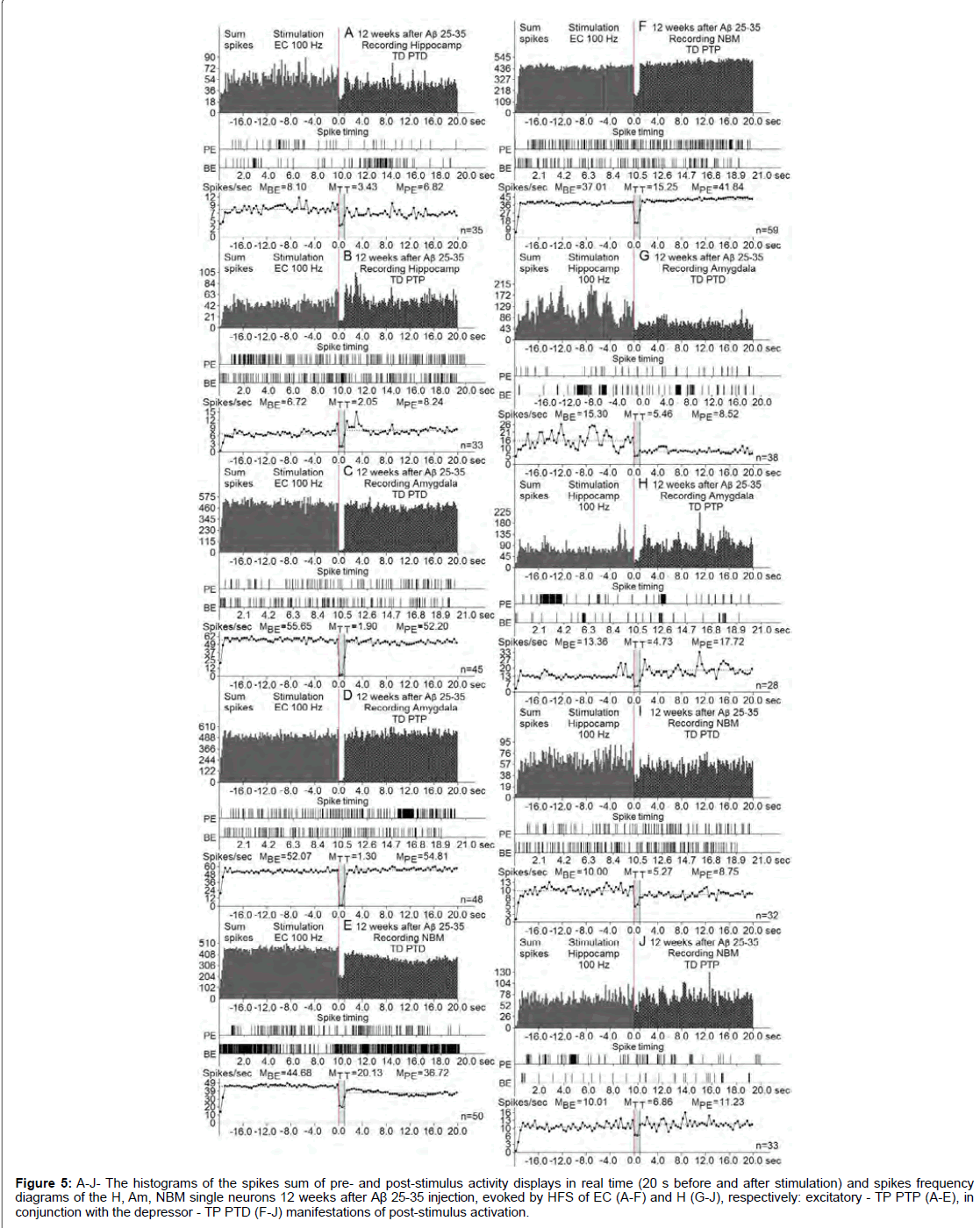
Figure 5: A-J- The histograms of the spikes sum of pre- and post-stimulus activity displays in real time (20 s before and after stimulation) and spikes frequency diagrams of the H, Am, NBM single neurons 12 weeks after Aβ 25-35 injection, evoked by HFS of EC (A-F) and H (G-J), respectively: excitatory - TP PTP (A-E), in conjunction with the depressor - TP PTD (F-J) manifestations of post-stimulus activation.
At the norm and model of AD at 12 weeks after administration of Aβ 25-35 constructed the histograms of spikes sum and diagrams of averaged spike frequency the pre- and post-stimulus displays of activity of structures studied in real time (20 seconds before and after stimulation) on the basis of raster of pre- and post-stimulus depressor and excitatory manifestations of spike activity, with details of the numerical values, including the time of HFS (Figures 2-5) This type of analysis give possibility to judge on relative intensity of pathology, compared with the norm of above-mentioned uni- and multidirectional post-stimulus tetanic and post-tetanic manifestations of activity of these structures in the frequency terms against the pre-stimulus level.
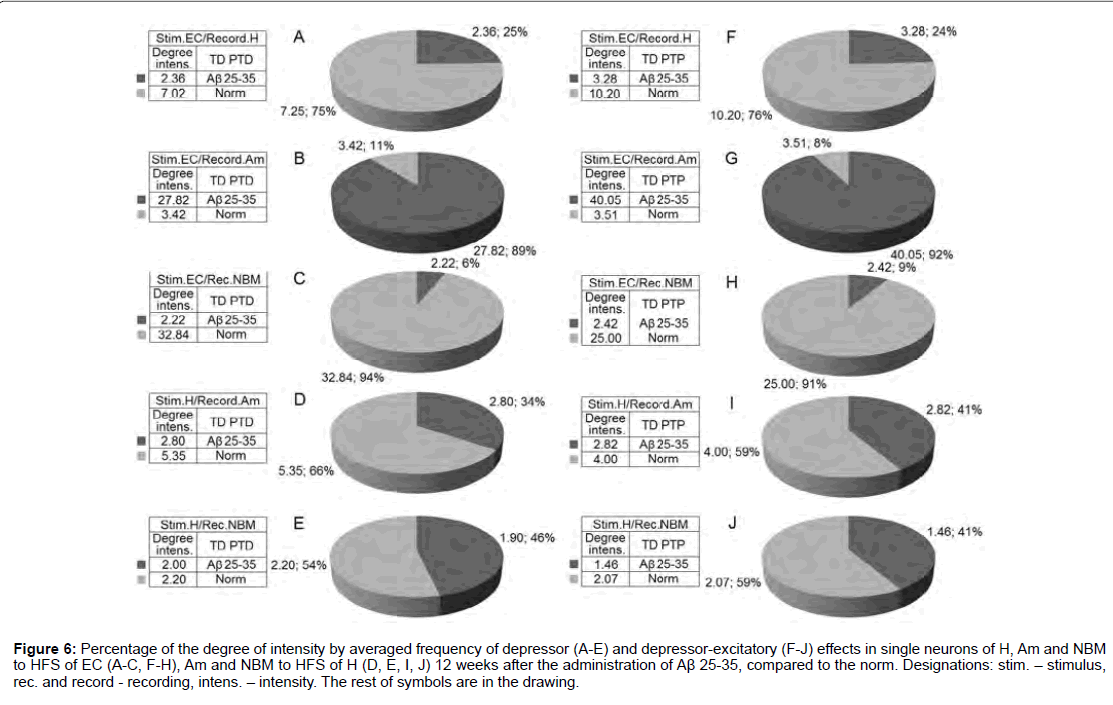
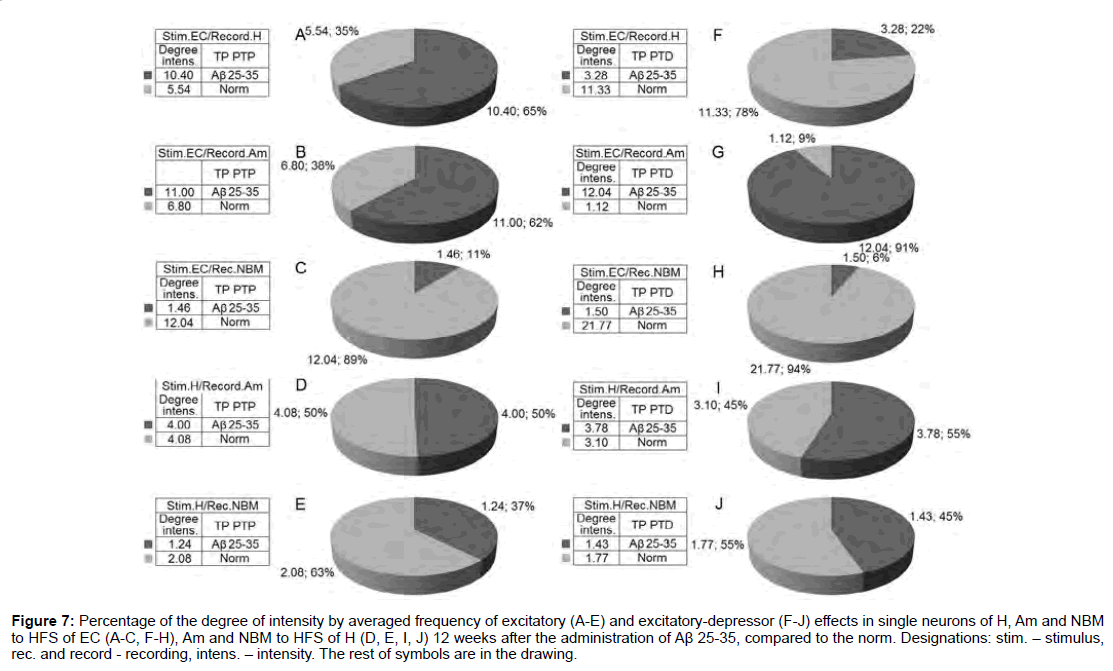
For a final conclusion, based on the corresponding calculations the degree of relative manifestation of the indices of decrease and increase of tetanic depression and excitation frequency in uni- and multidirectional post-stimulus depressor and excitatory manifestations in the mentioned period of testing in the pathology and for comparison with the norm were composed disk diagrams (Figures 6 and 7), in which they are presented graphically for all of the above mentioned cases.
Figure 6: Percentage of the degree of intensity by averaged frequency of depressor (A-E) and depressor-excitatory (F-J) effects in single neurons of H, Am and NBM to HFS of EC (A-C, F-H), Am and NBM to HFS of H (D, E, I, J) 12 weeks after the administration of Aβ 25-35, compared to the norm. Designations: stim. - stimulus, rec. and record - recording, intens. - intensity. The rest of symbols are in the drawing.
According to mention diagrams, we may come to the following conclusion (Figure 6). On the model of AD of 12 weeks remoteness recorded the most powerful deepening of tetanic depression as an example of both sequences in Am neurons to HFS of EC and approaching to the norm in NBM neurons to HFS of H. Expressed but almost half do not reach the norm in Aм neurons to HFS of H. There’s even is less expressed in H neurons to HFS of EC. Considerably less pronounced in NBM neurons to HFS of EC diametrically opposite to neurons of Am evoked by HFS of EC. Tetanic excitation in these experimental conditions, compared with the norm, changes in the following proportions (Figure 7). Powerful tetanic excitation took place by activation of EC in neuron of H (excitatory sequence) and Am (in excitatory-depressor sequences), equal to and above the norm in the Am neurons. HFS of H on the contrary, in NM neurons to HFS of EC were detected weakest excitation and relatively weak - to HFS of H in both sequences.
Of interest the quantitative correlation, compare with norm, poststimulus depressor and excitatory reactions. To 12 weeks trials on the model of AD depressor effects (including aggregate with post-tetanic excitatory) experienced the sharp increase in neurons of Am and NBM at HFS EC and in neurons Am at HFS H, but fell in neurons H at HFS EC (especially in depressor-excitatory sequences), as well as - NBM at HFS Н, but in inverse proportion (more in excitatory sequences).
As regards post-stimulus excitatory reactions, then it in all studied structures predominantly was sharply increased, with the exception of NBM at HFS of H. In other words, depressor effects up to 12 weeks increased with protective function in excitatory - compensatory order. However with increase of the trial’s time on the model of AD from 12 weeks to 28 weeks of both depressor and excitatory reactions sharply fell, that indicates about depletion of compensatory possibilities, what it was expected.
On rats in norm and when i.c.v. introduction of amyloid peptide Aβ 25-35 a comparative study of the morphohistochemical state of the H, amygdala complex (AM) and NBM was performed.
Figure 7: Percentage of the degree of intensity by averaged frequency of excitatory (A-E) and excitatory-depressor (F-J) effects in single neurons of H, Am and NBM to HFS of EC (A-C, F-H), Am and NBM to HFS of H (D, E, I, J) 12 weeks after the administration of Aβ 25-35, compared to the norm. Designations: stim. - stimulus, rec. and record - recording, intens. - intensity. The rest of symbols are in the drawing.
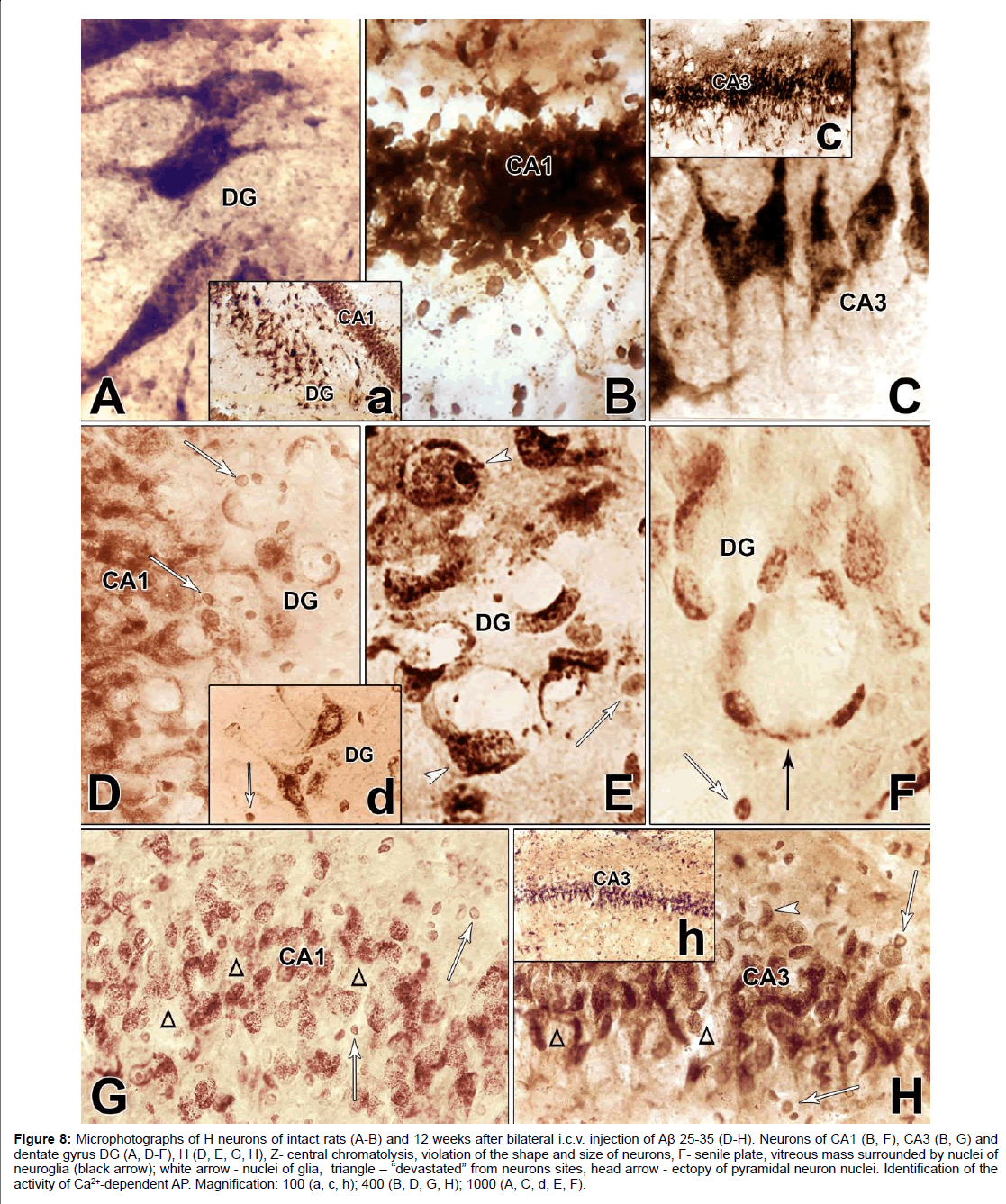
In intact rats on frontal sections of H a layered pattern of alternating white and gray matter is visible. Pyramidal cells of H differ in size, shape, branching nature of the processes. The largest neurons are distinguished in the dentate gyrus, they are scattered and characterized by high activity of AP (Figure 8A). The neurons of the fields CA1 and CA3 are arranged in rows, and very crowded, are distinguished by the intensity of staining and the very dense arrangement of the neurons (Figures 8B and 8C). In form they are polygonal, triangular and oval and they clearly stand out basal dendrites. Their apical dendrites are thickened and traced without branches at some distance from the body (Figures 8A and 8C).
Figure 8: Microphotographs of H neurons of intact rats (A-B) and 12 weeks after bilateral i.c.v. injection of Aβ 25-35 (D-H). Neurons of CA1 (B, F), CA3 (B, G) and dentate gyrus DG (A, D-F), H (D, E, G, H), Z- central chromatolysis, violation of the shape and size of neurons, F- senile plate, vitreous mass surrounded by nuclei of neuroglia (black arrow); white arrow - nuclei of glia, triangle - “devastated” from neurons sites, head arrow - ectopy of pyramidal neuron nuclei. Identification of the activity of Ca2+-dependent AP. Magnification: 100 (a, c, h); 400 (B, D, G, H); 1000 (A, C, d, E, F).
In the AD model, the morphological pattern of neurodegenerative changes in H was characterized by the absence of continuity of the ranges (Figures 8D-8H), some regions of H were more intensely affected, while others remained intact. In the initial stage of cellular lesions, in the cytoplasm a gradual disappearance of the lead phosphate sediment is observed. There is a violation of the structural nature of neurons, complete lack of response of neurofibrils. Neurons lose their characteristic shape and are rounded, swollen, and in most of them stop reacting the processes (Figures 8D-8H), although some large pyramidal cells still have preserved thickened apical dendrites (Figures 8D and 8H). In the cytoplasm of swollen neurons devoid of processes, a gradual disappearance of the granular sediment is occurs. This process begins with the central part of the cell body (Figures 8D-8H) and resembles the picture of central chromatolysis observed in the Nissl method.
In some neurons, it is found in the form of a “rosette,” creating a picture of a split neuron (Figure 8E). At the end, it gives a picture of the cell shadow (Figure 8D) or completes disappearance (Figures 8G and 8H). The process recalls the “disappearance of cells”, described by Nissle in “Zellschwund”, and therefore Fünfgeld gave it the name “cell disease in the form of their disappearance” [36].
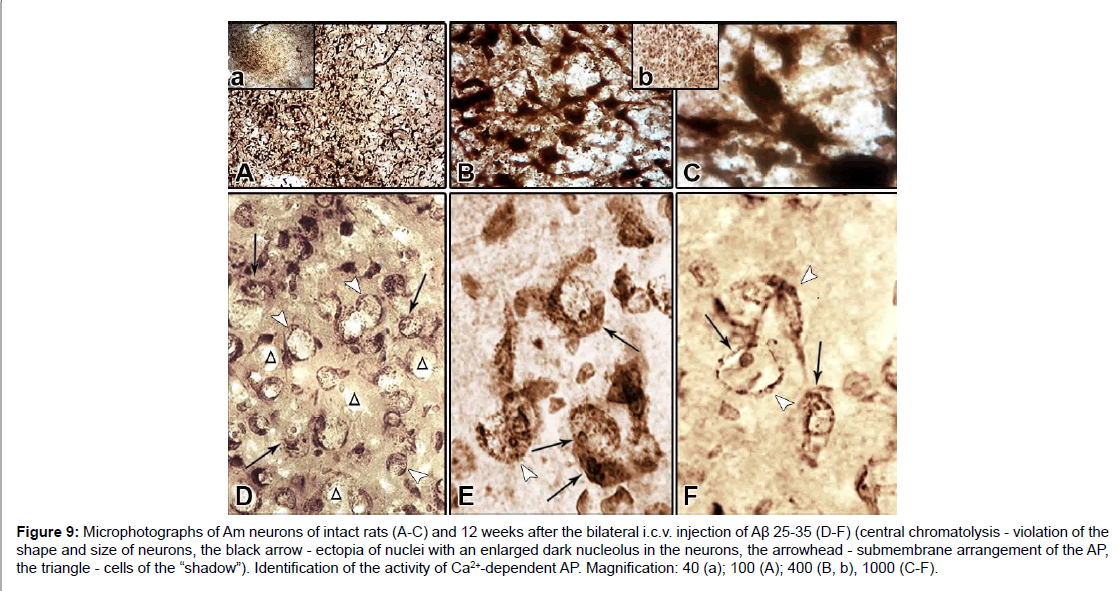
Expressed degenerative changes are noted in the field CA1. There is a complete absence of the reaction of the processes, violation of the shape and size of the neurons, very characteristic are circular “devastated” from the neurons sites (Figure 8G). In the intercellular space of the dentate gyrus and in the fields CA3 and CA1, glial cell nuclei are found (Figures 8D-8H). In the field CA3, the bodies of neurons are rounded and swollen. The morphological picture is characterized by uneven granularity in the cytoplasm of cells, a decrease in phosphatase activity of neurons in the fields CA1, CA3 H (Figures 8G and 8H), most of them are subjected to chromatolysis, although it is fair to note that the nuclei of neurons occupy a central location (Figure 8H). Large pyramidal cells of the dentate gyrus are more resistant to amyloid, but they also tend to clear the cytoplasm. In such swollen pyramidal cells, an ectopic, disproportionately large swollen nucleus is found, surrounded as a rim by a dark layer of sediment (Hamoripositive granulation) (Figure 8D). It seems that the nucleus searches for optimal conditions for survival and often it is so strongly pressed under the cell membrane that the cell reminds a ring (Figure 8E). In such cases, the nuclear membrane disappears. It is necessary to note one more form of lesion, morphologically similar to the senile plaque. These are quite large round formations, inside of which are visible nonstructural clumps surrounded by a light vitreous mass, which, in turn, is surrounded by a crown surrounded by thickened fibers and nuclei of neuroglia. Such single formations were found in the dentate gyrus of H (Figure 8F). Analysis of morphohistochemical data in neurons of Am showed that they are diverse in form in intact rats. The shape of the cells is round, oval, polygonal, the passage of processes is well traced, small nuclei are visible, mainly with one nucleolus. Part of the cytoplasm is filled with granules of the deposit; the other part is colored homogeneously and looks lighter. The cell membrane is richly littered with granules, and in the processes they are arranged in one row. There are neurons with low activity of AP. In general, no uniform staining of neurons is observed in Am (Figures 9A-9C). Dark and light cells, according to most researchers, are different functional states of the same cell. By their structural organization, they have features similar to neurosecretory cells of H [37]. Results of morphogistochemical study of Am rats have shown that with the i.c.v. of amyloid peptide Aβ 25- 35, neurons lose their characteristic shape and are rounded, swollen, hypertrophied and in most of them the processes cease to respond (Figures 9D-9F).
Figure 9: Microphotographs of Am neurons of intact rats (A-C) and 12 weeks after the bilateral i.c.v. injection of Aβ 25-35 (D-F) (central chromatolysis - violation of the shape and size of neurons, the black arrow - ectopia of nuclei with an enlarged dark nucleolus in the neurons, the arrowhead - submembrane arrangement of the AP, the triangle - cells of the “shadow”). Identification of the activity of Ca2+-dependent AP. Magnification: 40 (a); 100 (A); 400 (B, b), 1000 (C-F).
The nuclei of cells that are in the swelling stage are also swollen and take an eccentric position. In such neurons clearly appears enlarged dark-colored nucleolus (Figures 9E and 9F). Characteristic is the uneven distribution of the AP sediment. In the cytoplasm of swollen neurons devoid of processes, a gradual disappearance of the granular sediment starts beginning from the central part of the cell body. Cells with centrally located swollen light nuclei and annular sub-membrane arrangement of AP are detected; the cell membrane of such neurons is clearly expressed, at the expense of bordering with the deposit granules (Figures 9D and 9E). In some places, there is a picture of cell shadow or its complete disappearance (Figures 9D and 9F). Among the cells subjected to chromatolysis, neurons with AP activation in the cytoplasm and nucleus with a preserved apical dendrite are also occasionally found (Figure 8D).
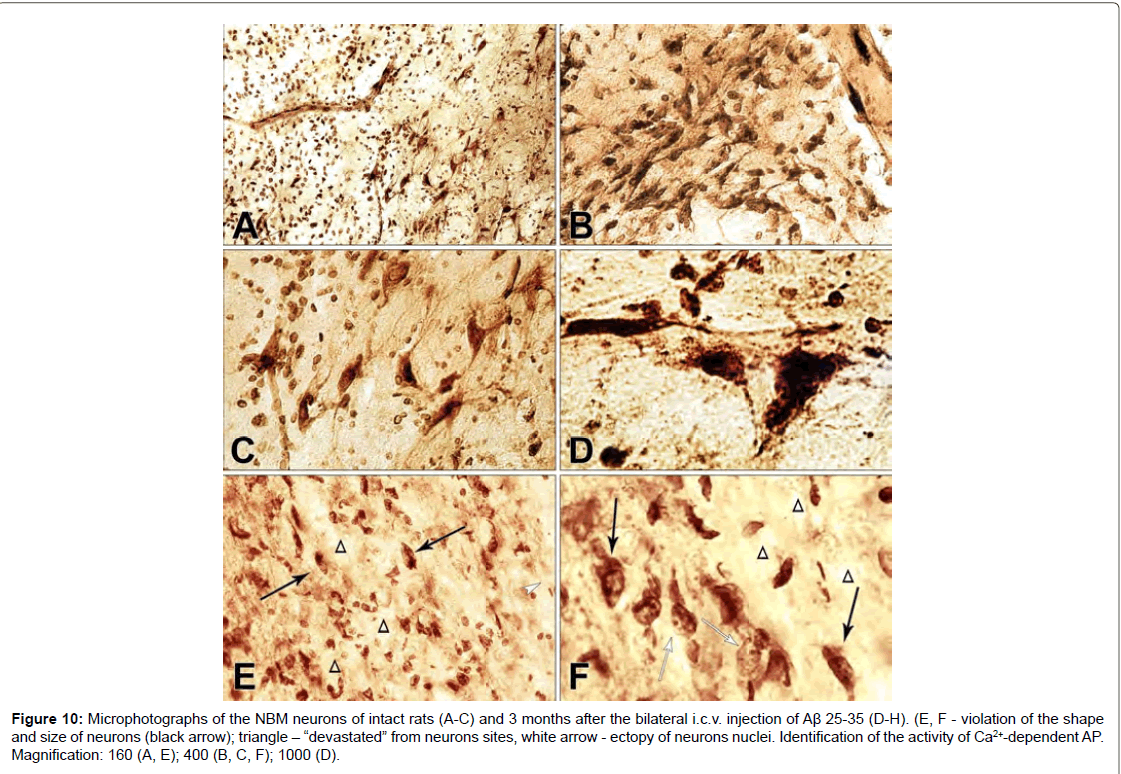
Figure 10: Microphotographs of the NBM neurons of intact rats (A-C) and 3 months after the bilateral i.c.v. injection of Aβ 25-35 (D-H). (E, F - violation of the shape and size of neurons (black arrow); triangle - “devastated” from neurons sites, white arrow - ectopy of neurons nuclei. Identification of the activity of Ca2+-dependent AP. Magnification: 160 (A, E); 400 (B, C, F); 1000 (D).
On the frontal slices of intact rats NBM, very fine colored nerve cells of a triangular and polygonal shape with outgrowths emerging from acute angles and branching are detected, which can be traced on a quite long distance from the body (Figures 10A-10D). In intact rats in all neurons of the brain, the nuclei occupy a central location and characteristic is the presence of reactive phenomena from the glial elements. A similar morphological picture is observed in the neurons of the NBM (Figures 10B and 10C). The results of the morphohistochemical study of NBM in rats with i.c.v. injections of Aβ 25-35 revealed lesions of the nervous tissue, primarily affecting the soma and the processes of nerve cells. There is a rarefaction in the density of the location of nerve cells, the shape of the neurons is disturbed, they wrinkle and the processes are not detected (Figures 10E and 10F). The process of lesion begins with the fact that the cell decreases from the very beginning in size, its lateral and basal surfaces are somewhat fall. Nerve cells have the appearance of black, angular or rod-like formations. The processes become thinner and shorten. The apical and lateral dendrites coil or disappear. Clumps of tigers approach, merge into a compact, darkly colored mass, the nucleus stretches (Figure 10F). However, among such affected cells, deformed neurons with a light ectopic nucleus are found in significant amounts, in which an enlarged dark nucleolus clearly appears (Figures 10E and 10F).
Discussion
It is possible that a deficit in neurogenesis may contribute to AD pathogenesis since at least one of the two regions ostensibly neurogenic in the adult human brain (the subgranular zone of the dentage gyrus and the ventriculo-olfactory neurogenic system) support high-level functions affected in early AD (associative memory and olfaction respectively). The results suggest that modestly reduced GABA(A) receptor function in immature neurons of the developing and adult brain can serve as a common molecular substrate for deficits in adult neurogenesis and behavior indicative of anxious and depressive-like mood states [38]. Neurogenesis is regulated by several extrinsic factors, many of which also induce the expression of neurotrophins (NF). NF (BDNF, CDNF, GDNF, NGF) and their receptors, which appear to be critical for neuronal degeneration. Indeed, NF prevents cell death in degenerative processes and can enhance the growth and function of affected neurons in these disorders. Several biochemical and molecular mechanisms are involved in the AD and Parkinson’s disease (PD) pathogenesis, such as synaptic dysfunction, NF impairment, energetic deficit triggered by mitochondrial disorder, OS and neuroinflammation [39-41]. In both illnesses, NF play an essential role for the survival of neurons affected by degenerative processes [42,43], NFs prevent the cell death and support the neuronal proliferation.
Therefore, the main findings related to the NF support in the survival, proliferation and maturation of affected neurons in AD and PD, such as cholinergic and dopaminergic neurons, as well as their putative employment as new therapeutic strategy for management of these diseases [44]. Although neurogenesis can be modulated exogenously by growth factors, stimulation of neurogenesis as a mean to treat neurodegeneration is still for the most part speculative [45]. The rest of the disease process, including formation of neurofibrillary tangles containing tau protein (Tp), is proposed to result from an imbalance between Aβ production and Aβ clearance [46]. Оutcomes from numerous studies indicate a vital role of physiological Tp in the synaptic biology including induction of LTP, LTD and dendritic activity. Put together, unlike previously assumed, Tp may have a significantly larger role in imparting synaptic instability and cognitive deficits in AD and other tauopathies [47]. In conclusion, importance should be given to compensatory mechanisms. These include increased activity and hyper perfusion of the affected brain structures, the formation of antibodies to the β-amyloid, synthesis of neuroprotective proteins, activation of antioxidant defense systems and pro-ontogenesis that inhibit neuronal apoptosis, induction of neurogenesis, sprouting of the neuronal terminals, increasing “density” of receptors for neurotransmitters. Even the process of plaque formation may be of a compensatory function, because it reduces the level GABAergic control and decrease of GABA (A) receptors function can serve as a universal molecular substrate for the deficit of neurogenesis in adult brain.
In these experiments, conducted on intact and semichronic (amyloid model of AD) rats after 12 weeks, compared with the norm, activity recordings from single neurons of H, AM and NBM to HFS of EC and H by on-line selection and mathematical analysis revealed the formation of excitatory and depressor responses in the form of tetanic potentiation and depression (TD, TP) to be combined into uni- (TD PTD, TP PTP) and multi-directional (TD PTP, TP PTD) post-tetanic sequence.
The analysis of the noted post-tetanic manifestations of impulse activity of these structures that are calculated based on the average number of spikes (PETH) with conversion into interpulse intervals and frequency in Hz (Frequency Average) revealed the following. Of particular interest are early manifestations of compensatory opportunity of the studied brain structures involved in this pathology. Tetanic depression on the model of AD, 12 weeks after i.c.v. administration of Aβ 25-35 and to HFS of EC, did not reach the norm in neurons of H, significantly exceeded it in Am neurons and in neurons of NBM as well, but did not reach the norm (Figures 10A-10C). To HFS of H in neurons of Am tetanic depression exceeded the norm, but in NBM neurons turned out to be equal to it (Figures 10D and 10E). Excitatory tetanic effects to HFS of EC after 12 weeks in H neurons were higher and significantly below the norm in uni- and multi-directional sequences, respectively. In neurons of Am situation was reversed - with some excess, and much higher than the norm, and in NBM neurons excess was lower and much lower than the norm (Figures 10F-10H). To HFS of H in neurons of Am and NBM, in general, tetanic excitation changed insignificantly: in Am and not reached the norm in both sequences, and in neurons of NBM did not reach the norm in excitatory and in excitatory-depressor case was in the normal range (Figures 10I and 10J).
According to the relative intensity of tetanic and post-tetanic post-stimulus activity manifestations of the studied structures in these experimental conditions, compared with the norm, the histogram analysis of the sum spikes and charts of the average frequency of spikes the pre- and post-stimulus activity manifestations in real time (20 s before and after stimulation) built on the basis of pre- and poststimulus raster of the depressor and excitatory manifestations of spikes activity, with details of the numerical values, including the time of HFS, one can come to the following conclusions (Figures 2-5). By this type of analysis it can be judged on relative intensity of disease, compared with the norm of aforementioned uni- and multi-directional poststimulus tetanic and post-tetanic activity manifestations and of these structures in terms of frequency, relative to pre-stimulus level. On the model of AD of the 12 weeks duration the most powerful deepening of tetanic depression observed in Am neurons to HFS of EC approaching the norm - in NBM neurons to HFS of H, and almost half do not reaching it - in Am neurons to HFS of H, still less pronounced - in H neurons to HFS EC and much less pronounced - in NBM neurons to HFS of EC (diametrically opposed to Am neurons to HFS of EC) (Figure 6). Tetanic excitement changed in the following ratios. Powerful tetanic excitation occurred on the activation of EC in H (in excitatory sequence) and Am neurons (in both sequences), equal to and above the norm to HFS of H (Figure 5). On the contrary, in the neurons of NBM to HFS of EC were detected weakest excitement and relatively weak - to HFS of H in both sequences (Figure 7). In some cases, the absence of strong depression, which we accept as a protector, in this study creates the need to involve pharmacological interventions for its gain that is subject to the following message.
In the present morpho-histochemical studies with Aβ-induced neurodegeneration, the characteristic morphological feature was the “gaps” characterized by the disappearance of the neuronal response in H, Am, and NBM. The picture of neurons, from the view of their structure, was a morphological proof of the disorders of their metabolism. The morphological picture resembled the acute swelling of nerve cells, which refers to a fairly common type of cellular pathology. The cause of this process can be various pathological effects of exo-and endogenous origin. Probably, as a result of the unfolding of metabolic phenomena of the pericarion, the process of respiration of cells is disrupted and the activity of a number of enzymes decreases. In the NBM, the morphological picture resembles the wrinkling of nerve cells, which refers to one of the types of cellular pathology observed in a variety of chronic diseases. However, an important criterion for assessing the degree of cell damage is the degree of damage to the nucleus. However, an important criterion for assessing the degree of cell damage is the degree of damage to the nucleus. The present data indicate that, mainly the nucleus of neurons trend to preserve vital activity by moving to the periphery of the cell, where apparently more favorable conditions are observed. Degenerative processes mainly affect the ectodermic elements of the central nervous system. Senile platelets are a special form of degeneration of the ectodermal tissue. At the beginning of the last century, Eikelenboom et al. [48] found that the key step in plate formation is the extracellular deposition of a foreign substance that provokes an inflammatory reaction accompanied by a regenerative response of the surrounding nerve fibers. Modern findings lead to the view that the amyloid plate is the focus of a non-immunely mediated chronic inflammatory response locally caused by the deposition of fibrillar Aβ [49].
Conclusion
Deepening of tetanic depressor responses apparently is a consequence of their advancement as a protective. According to our own data, depressor response more intensively involved of both in the nonspecific (peripheral and central) and specific neurodegeneration [50- 52]. It is supposed the advancement deepening the tetanic depression as assisting the recovery of the initial ratio of excitatory and depressor responses. It is shown that depressor post-stimulus manifestations in the form TD and PTD mediate GABA or glycine monoamines. Us shows the protective purpose of GABA in studies of nonspecific neurodegeneration, especially in the Deiters’ nucleus (following unilateral labyrinthectomy) [51], in the spinal cord after lateral hemisection [53-56] on peripheral nerve injury [57-61], on amyloid model of AD [50,62,63] and also on the rotenone model of PD [64,65]; in neurons mainly the GABAergic nature, in which early involving depressor reactions are accompanied by the recovery process until its completion. Confirmation the assumption of the universal protective assignment of GABA-ergic inhibition is the fact that in some systems during development of the nervous system GABA promotes proliferation, migration, differentiation and maturation of the synapse, expression of the GABA(A) receptor [66]. According to recent data, it suggested that GABA and glycine can play an important in the developing and mature central vestibular system [67]. In turn, in neurons of the vestibular nuclei by the GABA receptor mediated vestibular compensation after unilateral labyrinthectomy [67-70]. In this paper, where besides H for the first time are studied of Am and NM on the model of AD and also it is possible the involvement of true GABAergic inhibition during tetanic depression, with the aim of its deepening in the structures studied, thus supposed need of protectors for therapeutic intervention in the process of de and regeneration. In confirmation, the modern studies on the cellular and network levels show that synaptic inhibition cannot be assessed only as opposed to synaptic excitation and additionally serves highly specific functions in the nervous system of mammals [71]. Moreover, sustainable deepening of depression in our above-mentioned works is the result of involvement as a protective categories Galarmin or PRP-1 (proine-rich peptide), the hypothalamic neuropeptide-immunomodulator, snake venoms, parathyroid hormone (PTH), and others [50,62,63,72,73] and adrenalectomy [74-76] on the activity of H neurons in the amyloid model of AD.
References
- Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ (2001) Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci 21: 1327-1333.
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, et al. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535-539.
- Tanzi RE (2005) The synaptic Abeta hypothesis of Alzheimer disease. Nat Neurosci 8: 977-979.
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, et al. (2006) AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 52: 831-843.
- Liu SJ, Gasperini R, Foa L, Small DH (2010) Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J Alzheimers Dis 21: 655-666.
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, et al. (2006) AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proc Natl Acad Sci U S A 103: 3410-3415.
- Minano-Molina AJ, Espana J, Martin E, Barneda-Zahonero B, Fado R, et al. (2011) Soluble oligomers of amyloid-beta peptide disrupt membrane trafficking of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor contributing to early synapse dysfunction. J Biol Chem 286: 27311-27321.
- Bliss TVP, Collingridge GL (1993) A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31-39.
- Klyubin I (2005) Amyloid beta protein immunotherapy neutralizes abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med 11: 556-561.
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. (2008) Amyloid-beta protein dimmers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 14: 837-842.
- Huh S, Baek SJ, Lee KH, Whitcomb DJ, Jo J, et al. (2016) The reemergence of long-term potentiation in aged Alzheimer’s disease mouse model. Si Rep 6: 29152.
- Goel A, Lee HK (2007) Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci 27: 6692-6700.
- Ondrejcak T, Klyubin I, Hu NW, Barry AE, Cullen WK, Rowan MJ. Alzheimer's disease amyloid beta-protein and synaptic function. Neuromolecular Med 12: 13-26.
- Shankar GM, Walsh DM (2009) Alzheimer’s disease: Synaptic dysfunction and Abeta. Mol Neurodegener 4: 48.
- Arendt T (2009) Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol 118: 167-179.
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, et al. (2007) Abeta oligomer-induced aberrations in synapse composition, shape and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci 27: 796-807.
- Chen Q, Kagan B, Hirakura Y, Xie C (2000) Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J Neurosci Res 60: 65-72.
- Chen QS, Wei WZ, Shimahara T, Xie CW (2002) Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem 77: 354-371
- Zhao D, Watson J, Xie CW (2004) Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol 92: 2853-2858.
- De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, et al. (2005) Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci U S A 102: 3811-3816.
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, et al. (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med 15: 331-337.
- Cissé M, Halabisky B, Harris J, Devidze N, Dubal DB, et al. (2011) Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature 469: 47-52.
- Bie B, Wu J, Yang H, Xu JJ, Brown DL, et al. (2014) Epigenetic suppression of neuroligin 1 underlies amyloid-induced memory deficiency. Nat Neurosci 17: 223-231.
- Karst H, Joëls M (2001) Calcium currents in rat dentate granule cells are altered after adrenalectomy. Eur J Neurosci 14: 503-512.
- Jang SS, Chung HJ (2016) Emerging link between alzheimer’s disease and homeostatic synaptic plasticity. Neural Plast 2016: 7969272.
- Hou Q, Gilbert J, Man HY (2011) Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron 72: 806-818.
- Fong MF, Newman JP, Potter SM, Wenner P (2015) Upward synaptic scaling is dependent on neurotransmission rather than spiking. Nat Commun 6: 6339.
- Davis G, Bezprozvanny I (2001) Maintaining the stability of neural function: A homeostatic hypothesis. Annu Rev Physiol 63: 847-869.
- Burrone J, O’Byrne M, Murthy V (2002) Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420: 414-418.
- Turrigiano G, Nelson S (2004) Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97-107.
- Gilbert J, Shu S, Yang X, Lu Y, Zhu LQ, et al. (2016) β-Amyloid triggers aberrant over-scaling of homeostatic synaptic plasticity. Acta Neuropathol Commun 4: 131.
- Marzo A, Galli S, Lopes D, McLeod F, Podpolny M, et al. (2016) Reversal of synapse degeneration by restoring wnt signaling in the adult hippocampus. Curr Biol 26: 2551-2561.
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Academic Press, Elsevier.
- Maurice T, Privat A (1998) Sigma1 receptor agonists and neurosteroids attenuate Aβ25-35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience 83: 413-428.
- Meliksetyan IB (2007) Revelation of activity of Cа2+- dependent acid phosphatase in cellular structures of the rats brain. Morphologia 131: 77-80.
- Niculescu IT (1963) Pathomorphology of the nervous system. Med Publ, Bucharest.
- Minibayeva ZR, Kalimullina LB, Sharafutdinova LA (2006) Dark and bright neurons of anterior section oamtgdaloid body of rats brain.
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, et al. (2007) GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci 27: 3845-3854.
- O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci 34: 185-204.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1: a006189.
- Mack JM, Schamne MG, Sampaio TB, Pertile RA, Fernandes PA, et al. (2016) Melatoninergic system in Parkinson's disease: from neuroprotection to the management of motor and nonmotor symptoms. Oxid Med Cell Longev 2016: 3472032.
- Bothwell M (2016) Recent advances in understanding neurotrophin signaling. F1000Res 5: 1885.
- Ibanez CF, Andressoo JO (2017) Biology of GDNF and its receptors - Relevance for disorders of the central nervous system. Neurobiol Dis 97: 80-89.
- Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S (2017) Neurotrophic factors in Alzheimer's and Parkinson's diseases: Implications for pathogenesis and therapy. Neural Regen Res 12: 549-557.
- Galvan V, Bredesen DE (2007) Neurogenesis in the adult brain: Implications for Alzheimer’s disease. CNS Neurol Disord Drug Targets. 6: 303-310.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353-356.
- Jadhav S, Cubinkova V, Zimova I, Brezovakova V, Madari A, et al. (2015) Tau-mediated synaptic damage in Alzheimer’s disease. Transl Neurosci 6: 214-226.
- Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJM, van Gool WA, et al. (2006) The significance of neuroinflammation in un-derstanding Alzheimer’s disease. J Neural Transmission 113: 1685-1695.
- Fischer O (1910) Die presbyoph rene demenz, deren anatomische grund-lade und klinische abgrenzung. Z Ges Neurol u Psychiat 3: 371-471.
- Galoyan AA, Sarkissian JS, Chavushyan VA, Meliksetyan IB, Avagyan ZE, et al. (2008) Neuroprotection by hypothalamic peptide proline-rich peptide-1 in Abeta 25-35 model of Alzheimer's disease. Alzheimers Dement 4: 332-344.
- Galoyan AA, Khalaji N, Hambardzumyan LE, Manukyan LP, Meliksetyan IB, et al. (2010) Protective effects of hypothalamic proline-rich peptide and cobra venom Naja oxiana on dynamics of vestibular compensation following unilateral labyrinthectomy. Neurochem Res 35: 1747-1760.
- Sarkissian JS, Chavushyan VA, Meliksetyan IB, Poghosyan MV, Avakyan ZE, et al. (2007) Protective effect of Naja naja oxiana cobra venom in rotenone-induced model of Parkinson’s disease: Electrophysiological and histochemical analysis. New Armenian Medical Journal 1: 43-56.
- Sarkissian JS, Chavushyan VA, Sulkhanyan RM, Poghossyan MV, Grigoryan YKh, et al. (2004) The study of protective effect of neurosecretory cytokines on the spinal cord neurons under conditions of hemisection. Nejrokhimia 21: 15-26.
- Galoyan AA, Sarkissian JS, Chavushyan VA, Sulkhanyan RM, Avakyan ZE, et al. (2005) Neuroprotective action of hypothalamic peptide PRP-1 at various time survivals following spinal cord hemisection. Neurochem Res 30: 507-525.
- Galoyan AA, Sarkissian JS, Chavushyan VA, Meliksetyan IB, Avakyan ZE, et al. (2007a) Neuroprotective action of new hypothalamic peptide PRP 3 at various time survivals following spinal cord hemisection. Neurochem J (RAS and NAS RA) 1: 160-172.
- Galoyan AA, Sarkissian JS, Chavushyan VA, Meliksetyan IB, Avakyan ZE, et al. (2007b) Morpho-functional study of protective action of new hypothalamic peptide PRP-3 on neurons of spinal cord in different times after lateral hemisection. Neurochem J (RAS and NAS RA) 24: 86-99.
- Galoyan AA, Sarkissian JS, Kipriyan TK, Sarkissian EJ, Chavushyan EA, et al. (2001) Protective effect of a new hypothalamic peptide against cobra venom and trauma induced neuronal injury. Neurochem Res 26: 1023-1038.
- Sarkissian JS, Sulkhanyan RM, Chavushyan VA, Gevorgyan AJ, Avakyan ZE, et al. (2003) Study of protective effect of neurosecrtory cytokins on the neurosecretory cytokins on the spinal cord moto- and interneurons after cutting of nervus ischiadicus. Neirokhimia (RAS and NAS RA) 20: 146-160.
- Sarkissian JS, Yaghjyan GV, Abrahamyan DO, Chavushayn VA, Meliksetyan IB, et al. (2005) Stimulation of peripheral nervr regeneration by hypothalamic proline0rich peptides PRP-1 (Galarmin). Ann Plastic Esthetic Surg 4: 19-30.
- Minasyan AL, Aznauryan AV, Meliksetyan IB, Chavushyan VA, Sarkissian JS, et al. (2011) Morphological-histochemical study of neurodegenerative and regenerative processes in flexor and extensor collaterals of the sciatic nerve after crushing in the presence of PRP-1. Neurochem J 5: 278-284.
- Minasyan AL, Aznauryan AV, Chavushyan VA, Meliksetyan IB, Sarkissian JS (2012) Dynamics of neurodegenerative and regenerative processes in flexor and extensor collaterals of nervus ischiadicus after crush in conditions of proline-rich peptide (PRP-1) action. Neirokhimia (RAS and NAS RA) 29: 63-74.
- Galoyan AA, Sarkissian JS, Chavushyan VA, Abramyan SS, Avakyan ZE, et al. (2004) The study of protective effect of the new hypothalamic proline rich peptide PRP-1 on the morpho-functional changes on the model of Alzheimer’s disease induced by intracerebroventricular administration of beta-amyloid peptide Ab 25-35 in rats. Neirokhimia 21: 265-288.
- Yenkoyan K, Safaryan K, Chavushyan V, Meliksetyan I, Navasardyan G, et al. (2011) Neuroprotective action of proline-rich polypeptide-1 in β-amyloid induced neurodegeneration in rats. Brain Res Bull 86: 262-271.
- Nalbandyan AA, Poghosyan MV, Chavushyan VA, Sarkissian JS (2010) Electrophysiological study of spinal cord motoneurons on the modl of Parkinson’s disease, induced by rotenone. Modern directions of studies of functional interhemispheric asymmetry and plasticity of the brain. Materials of All-Russia conference with the international participation. Publishers of Russ Acad Med Sciences, Moscow.
- Poghosyan MV, Nalbandyan AA, Badalyan BY, Behnam N, Chavushyan VA, et al. (2012) Excitatory and inhibitory synaptic processes in neurons of the spinal cord on the model of Parkinson’s disease, induced by rotenone. Modern directions of studies of functional interhemispheric asymmetry and plasticity of the brain. Materials of All-Russia Conf. with intern participation. Publ of Russ Acad Med Science, Moscow.
- Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition. Nat Rev Neurosci 3: 715-727.
- Tighilet B, Lacour M (2001) Gamma amino-butyric acid (GABA) immunoreactivity in the vestibular nuclei of normal and unilateral vestibular neurectomized cats. Eur J Neurosci 13: 2255-2267.
- Giardino L, Zanni M, Fernandez M, Battaglia A, Pignataro O, et al. (2002) Plasticity of GABA(a) system during ageing: Focus on vestibular compensation and possible pharmacological intervention. Brain Res 929: 76-86.
- Johnston AR, Him A, Dutia MB (2001) Differential regulation of GABA (a) and GABA (b) receptors during vestibular compensation. Neuroreport 12: 597-600.
- Yamanaka T, Him A, Cameron SA, Dutia MB (2000) Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurons after unilateral labyrinthectomy. J Physiol 2: 413-424.
- Birke G, Draguhn A (2010) No simple brake-the complex functions of inhibitory. Pharmacopsychiatry 43: S21-31.
- Khudaverdyan DN, Mnatsakanyan VR, Chavushyan VA, Avetisyan ZA, Sarkissian JS (2008) The plasticity of hippocampal neurons under conditions of Aß (25–35) induced model of Alzheimer’s disease and action of parathyroid hormone. Materials of All-Russian conference with the international participation Structure-functional neurochemical and immunochemical patterns of asymmetry and the brain plasticity. Russian Acad Sci Press, Moscow.
- Mnatsakanyan VR, Khudaverdyan DN, Meliksetyan IB, Chavushyan VA, Sarkissian JS (2009) Electrophysiological and histochemical study of effects of parathyroid hormone on Aβ1–42 induced model of of Alzheimer’s disease. Materials of the International scientific conference. The modern problems of integrative activity and plasticity of the nervous system, devoted 80-years from the day of birth V.V. Fanardjian.
- Chavushyan VA, Stepanyan HY, Meliksetyan IB, Avetisyan ZA, Simonyan KV, et al. (2010) Electrophysiological and morpho-histochemical study of action of adrenalectomy on the functions of hippocampus on the amyloidal model of Alzheimer’s disease in rats. Materials of All-Russian conference with the international participation. Modern direction of studies of functional interhemispheric asymmetry and brain plasticity. Russian Acad Sci Press, Moscow.
- Chavushyan VA, Stepanyan HY, Minasyan AL, Sarkissian JS (2012) The ratio of excitatory anf inhibitory processes in amygdale after unilateral adrenalectomy in combined with model of Alzheimer’s disease , induced by Aβ 25-35. Modern directions in study of functional interhemispheric asymmetry and plasticity of the brain. Materials of Russian Academy medical Sciences with the intern. participation. Publishers of Russ Acad med Sciences, Moscow.
- Chavushyan VA, Meliksetyan IB, Sarkissian JS, Stepanyan HY, Avetisyan ZA, et al. (2013) Electrophysiological and morpho-histochemical study of action of adrenalectomy on hippocampal neurons. Journal of Comparative and Ontogenic Physiology 2013: 153-161.
Citation: Sarkissian JS, Minasyan AL, Sahakyan KT, Danielyan MH, Stepanyan HY, et al. (2017) Morphofunctional Correlation of Excitatory and Depressor Synaptic Processes in Hippocampus, Amygdala and Basal Meynert Nucleus Neurons in Dynamics of Development of Alzheimer’s Disease Model Induced by Aβ25-35. J Alzheimers Dis Parkinsonism 7: 391. DOI: 10.4172/2161-0460.1000391
Copyright: ©2017 Sarkissian JS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5616
- [From(publication date): 0-2017 - Jul 05, 2025]
- Breakdown by view type
- HTML page views: 4744
- PDF downloads: 872