Multiple Risk Factors Trigger Smoking Relapse: Insights from a Rat Model of Nicotine Addiction
Received: 02-Oct-2024 / Manuscript No. jart-24-152064 / Editor assigned: 04-Oct-2024 / PreQC No. jart-24-152064 / Reviewed: 18-Oct-2024 / QC No. jart-24-152064 / Revised: 25-Oct-2024 / Manuscript No. jart-24-152064 / Published Date: 30-Oct-2024 QI No. / jart-24-152064
Abstract
Relapse in nicotine addiction is often influenced by environmental cues, drug re-exposure, and stress. These factors frequently co-occur, increasing the likelihood of relapse in abstinent individuals. Using a rat model of nicotine addiction, we examined how combined risk factors impact relapse behavior. Male Sprague-Dawley rats were conditioned to self-administer nicotine through lever presses (0.03 mg/kg/infusion) paired with auditory/visual cues. Following extinction of these responses, we assessed relapse through five conditions: cue re-exposure, nicotine priming, stress, cue/priming, and cue/stress. Results indicated that each factor—nicotine priming, stress, and cue individually reinstated lever responses, with combined exposures producing a stronger effect. These findings underscore the powerful role of nicotine-related cues, drug priming, and stress in relapse, supporting behavioral strategies alongside pharmacological interventions to minimize exposure to these triggers.
keywords
Nicotine addiction; Smoking relapse; Environmental cues; Nicotine priming; Stress and addiction
Introduction
Nicotine addiction, like other forms of substance abuse, is a chronic disorder marked by frequent relapse. Even with treatment options such as nicotine replacement therapy, bupropion, and varenicline, long-term abstinence remains challenging, with success rates between 12.9-26.1%. Despite available treatments, abstinent individuals often relapse due to environmental cues, stress, and drug priming. Environmental cues associated with smoking may have a particularly potent effect due to the high frequency of cue-nicotine pairings in smokers [1]. Stressful life events and nicotine priming similarly decrease abstinence success, making it essential to explore their effects on relapse in a controlled setting. While animal models have demonstrated the role of these factors individually in reinstating drug-seeking behavior, studies examining their combined effects on nicotine-seeking are limited. In this study, we used a pharmacological stressor, yohimbine, to evaluate the individual and combined effects of cues, priming, and stress on nicotine relapse in rats, aiming to shed light on the interplay of these factors [2].s
Materials and Methods
Subject’s
Twenty-two male Sprague-Dawley rats (225-250 g) were used. They were housed in a controlled environment with a reversed light/dark cycle and had unlimited access to water. Following a one-week habituation period, rats were placed on a food-restricted diet throughout the experiment, with training sessions conducted during the dark phase. Experimental protocols adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Self-administration apparatus
Self-administration and reinstatement testing occurred in sound-attenuated chambers equipped with levers and visual stimuli, paired with a drug delivery system for intravenous nicotine injections. Experimental events were computer-controlled.
Food training
After diet restriction, rats were trained to press a lever for food pellets. Successful training (FR1 and FR5 schedules) was achieved in 3-5 sessions [3].
Surgery
following food training, rats were anesthetized and implanted with jugular catheters, allowing a week for recovery. Catheters were flushed daily with heparinized saline to maintain patency and prevent infection.
Results
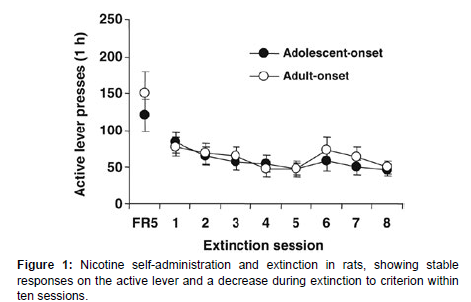
After 30 daily sessions, rats developed stable operant responses for nicotine infusion. By the final sessions, rats averaged 83.0±2.9 responses on the active lever, leading to approximately 14.9±0.5 infusions per hour. During extinction, lever responses decreased, with all rats reaching the extinction criterion within ten sessions (Figure 1). Averaged across the last 3 sessions, response rates were 83.0±2.9 on the active lever and 9.7±s;0.9 on the inactive lever, resulting in 14.9±0.5 nicotine infusions per 1-h session. In the first extinction session, rats emitted 68.1±6.4 responses on the active lever and 6.5 ±1.1 on the inactive lever. During the following extinction sessions, lever responses gradually decreased. All rats reached the extinction criterion within 10 daily sessions. Since rats were divided into two sets for the reinstatement tests in a counterbalanced manner, there was no difference in the number of lever responses and nicotine infusions (data not shown) [4].
Effects of nicotine priming, cue re-presentation, and their combination in the reinstatement tests
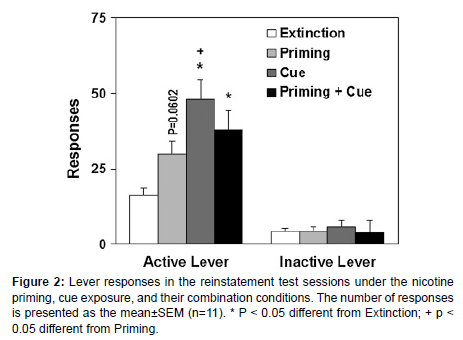
Nicotine priming, cue re-presentation, and their combination play pivotal roles in the reinstatement of nicotine-seeking behavior, demonstrating distinct yet interrelated effects thast have implications for relapse in nicotine addiction. Nicotine priming, achieved by reintroducing a small amount of nicotine, activates reward pathways in the brain, particularly in the dopamine-rich mesolimbic system, reinforcing drug-seeking behaviors. Cue re-presentation, where environmental triggers associated with nicotine are reintroduced, can prompt strong cravings through conditioned responses, even without nicotine itself (figure 2). When combined, these triggers have a synergistic effect, significantly heightening the likelihoods of relapse as they simultaneously stimulate both direct reward pathways and conditioned craving responses. These findings underscore the importance of managing both nicotine exposure and environmental cues in relapse prevention strategies for individuals attempting to quit smoking, suggesting that a comprehensive approach is needed to address both pharmacological and behavioral aspects of nicotine dependence [5-7].
Discussion
The study provides valuable insights into the mechanisms of relapse in nicotine addiction by examining how individual and combined risk factors contrisbute to nicotine-seeking behavior in a rat model. Our findings demonstrate that exposure to nicotine-associated cues, nicotine priming, and stress can independently trigger relapse, with combined exposures exerting an even stronger effect. These results align with human studies suggesting that relapse is often driven by a combination of triggers rather than a single factor. The potency of environmental cues, which were consistently paired with nicotine intake in this model, highlights the profound impact of context-specific cues in promoting relapse. For smokers, environmental stimuli, such as the smell or setting of past smoking experiences, have strong associations with nicotine intake, making them potent triggers. The reinstatement of lever-pressing behavior in response to these cues underscores the need for relapse prevention strategies that address the habitual link between specific cues and nicotine [8-10].
Nicotine priming, a simulated "lapse," and exposure to stress both contributed to relapse in this model. The pharmacological stressor yohimbine, which stimulates stress-like conditions, effectively reinstated nicotine-seeking behavior, supporting the hypothesis that stress is a powerful trigger for relapse. Stressful life events may enhance vulnerability to relapse by activating neurobiological pathways associated with drug-seeking and reward.
The combination of these factors resulted in a significantly greater relapse response than individual factors alone, suggesting an additive or synergistic effect. These findings have direct implications for smoking cessation treatment: interventions might benefit from addressing multiple triggers simultaneously, rather than focusing on a single aspect. Behavioral approaches, such as cue exposure therapy and stress management, may be particularly effective when combined with pharmacological treatments that reduce nicotine cravings. In summary, our study supports the idea that relapses is a multifactorial process influenced by contextual cues, stress, and drug priming [11].
Conclusion
This study provides compelling evidence that multiple risk factors — nicotine-associated cues, nicotine priming, and stress — play a significant role in the relapse process for nicotine addiction. Using a rat model, we observed that these factors not only individually contribute to relapse but also have a cumulative impact when combined, resulting in a stronger relapse response. These findings reflect real-world situations where individuals attempting to quit smoking are frequently exposed to multiple triggers, highlighting the need for multifaceted treatment approaches. In conclusion, this study highlights the complex interplay of risk factors in nicotine addiction relapse and supports a comprehensive approach to smoking cessation that targets both environmental and neurobiological elements. By incorporating strategies that mitigate the effects of cues, manage stress, and address nicotine cravings, future interventions can potentially improve long-term abstinence rates.
Acknowledgement
Nones
Conflict of Interest
None
References
- Chen Y, Zhao G, Zahumensky J, Honey S, Futcher B, et al. (2020)Differential scaling of gene expression with cell size may explain size control in budding yeast.Mol Cell 782: 359-706.
- Cross FR, Umen JG (2015)The Chlamydomonas cell cycle.Plant J 823: 370-92.
- Childs BG, Durik M, Baker DJ, Deursen JM (2015)Cellular senescence in aging and age-related disease: from mechanisms to therapy.Nat Med 2112: 1424-1535.
- Claude K-L, Bureik D, Chatzitheodoridou D, Adarska P, Singh A, et al. (2021)Transcription coordinates histone amounts and genome content.Nature 12:420-502.
- Cockcroft C, Boer GW, Healy MS, Murray AH (2000)Cyclin D control of growth rate in plants.Nature 405: 575-679.
- Conlon I, Raff M (2000)Differences in the way a mammalian cell and yeast cells coordinate cell growth and cell-cycle progression.J Biol 2: 7-9.
- Costanzo M, Nishikawa JL, Tang X, Millman JS, Schub O, et al.(2004)CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast.Cell 1177: 899-913.
- Crane MM, Tsuchiya M, Blue BW, Almazan JD, Chen KL, et al. (2019)Rb analog Whi5 regulates G1 to S transition and cell size but not replicative lifespan in budding yeast.Translat Med Aging 3: 104-108
- Crozier L, Foy R, Mouery BL, Whitaker RH, Corno A, et al. (2021)CDK4/6 inhibitors induce replication stress to cause long-term cell cycle withdrawal.BioRxiv 42: 82-95.
- Cross FR (1988)DAF1, a mutant gene affecting size control, pheromone arrest, and cell cycle kinetics of Saccharomyces cerevisiae.Mol Cell Biol 811: 4675-84.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Bhatria JK (2024) Multiple Risk Factors Trigger Smoking Relapse: Insights from a Rat Model of Nicotine Addiction. J Addict Res Ther 15: 706.
Copyright: © 2024 Bhatria JK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 974
- [From(publication date): 0-0 - Nov 12, 2025]
- Breakdown by view type
- HTML page views: 694
- PDF downloads: 280