New Description of the Rostral Migratory Stream in Adult Mice Brains Delineating the Starter of the Stream as an Infundibulum: A New Described Limb, by H&E and Antidoublecortin Antibody
Received: 14-Nov-2016 / Accepted Date: 10-Jan-2017 / Published Date: 15-Jan-2017
Abstract
Background: Neural stem cells are not confined to the embryonic development of the brain as believed in the past; in contrasts, it continues life- long in the adult mammalian brains even in non-pathological state. Neural stem cells and their progeny, the neuroblasts, are residents in the subventricular zone (SVZ) of the lateral ventricle in the adult mammalian brains. The neuroblasts after they were produced in the SVZ would migrate in a well-defined pathway emerged from the SVZ and directed towards the olfactory bulb (OB). This pathway is called the Rostral Migratory Stream (RMS), and its main component, are the neuroblasts.
Aims of study: To trace the chain arrangement of neuroblasts along the RMS, using Hematoxylin and Eosin and the specific marker of the neuroblasts “antidoublecortin antibody”, describing grossly how the pathway emerges from SVZ proceeding rostrally to the OB in the adult mice brains.
Materials and methods: Adult mice brains from both brain sexes were used in this experiment to view both coronal and sagittal sections upon which Hematoxylin and Eosin and immunohistochemical staining were exploited. Antidoublecortin antibody, the specific marker of the immature neurons, was used in the immunohistochemical staining of this study.
Results: This study revealed clustering of the neuroblasts in the SVZ and while this special arrangement carried out at the SVZ, the neuroblasts changed their arrangement when being traced sagittaly into chain-like strip of cells forming a sigmoidal shape stream. The chain of the neuroblasts in the stream demonstrated changing in shape and direction throughout its length with special arrangement at its starter from the lateral ventricle forming a funnel shape limb before it joined the rest of stream. It delineated here four limbs along its pathway.
Conclusion: The neuroblasts take different arrangement through their period of life from their site of origin to their final destination the olfactory bulb through the RMS. In the stream the neuroblasts follow a sigmoidal shape pathway described here as four limbs instead of 3 ones in previous studies. The new described part is the funnel shape limb which is named the infundibulum at which the neuroblasts in the stream starting up their migration from SVZ before they join the next limb, the vertical limb.
Keywords: Subventricular zone; Rostral migratory stream; Neuroblasts; Neural stem cells
76709Introduction
The discovery of neural stem cells in the adult mammalian brain shattered the long-standing belief that neurogenesis is restricted to embryonic and early postnatal periods [1,2]. The continuous generation of new neurons in the adult mammalian brain throughout life occurs in few discrete regions, called neurogenic niches [3-5]. One of these neurogenic niches in the adult rodent brain is the walls of the lateral ventricle the SVZ [6-8], at which the neurogenic niche separated from the ventricle cavity by a layer of ependymal cells; therefore, the adult neurogenic region was referred to as a subventricular zone SVZ [1]. SVZ is considered to be the largest germinal region in the adult mammalian brain [3,9,10]. The neural stem cells in the SVZ correspond to a subpopulation of slowly dividing astroglial cells [11], adjacent to the layer of ependymal cells [12]. In this region, neural stem cells form transit amplifying cells that divides rapidly to produce the neuroblasts [13]. Throughout life neuroblasts migrate through more complex and frequently extensive territories and must integrate into circuits that are already fully functional [1]. The rostral migratory stream (RMS) is the pathway that the neuroblasts committed to after their production in the SVZ in physiological conditions. This pathway follow specific fate to the OB and neuroblasts after reaching there, attain full maturity and integrate into the OB layers circuitry, as the cells in the OB layers continuously undergo programmed cell death and thereby replaced by those newly arriving neuroblasts [14].
Aim of Study
In this research, the RMS had been explored to describe and reevaluate the shapes of its limbs from its emergence till its termination with ordinary staining, the hematoxylin and eosin and to confirm it by the neuroblasts specific marker the antidoublecortin antibody.
Materials and Methods
The study has been executed at the histology laboratory and animal house of College of Medicine/Baghdad University. Thirty six apparently healthy male and female adult mice (Micromys minutus) aging >60 days, weighing 30-40 gm., collected from the Animal House of the National Centre of Researches and Drug Monitoring/Baghdad; breeded at the Animal House/College of Medicine – Baghdad University. Breeding had been done in well aerated cages; given ad libitum access to food and water. All mice were anesthetized by Ketamine (75 mg/kg body weight) intraperitoneally by insulin syringe and waited until fully anesthetized, then perfused intracardially with 4% paraformaldehyde [Selfine-India] prepared in Phosphate Buffered Saline PBS [Fluka-GERMANY]. The Calvaria dissected and brain harvested en block. The harvested brain were sliced and trimmed with small sharp scalpel, to obtain a slice of no more than 3-5 mm so that penetration of the fixative could proceed rapidly. After dissecting the brains coronally and sagittaly, they were fixed for 20 hours in the same fixative used for the perfusion. Processing has been done according to James et al. [15]. Tissue slices were washed from fixative with running tab water for 5 minutes, and then dehydrated with ascending graded conc. of ethanol alcohol [Scharlau-Spain] begins with 70% (2 changes 15 min each), 80% (2 changes 15 min each), 90% (2 changes 15 min each), absolute ethanol... (2 changes 15 min eah) and clearing in chloroform [Riedel-de Haen-Germany], (2 changes 15 min each). Brain slices were impregnated with molten paraffin at 61-63°C [Scharlau-Spain, melting point (56-58°C)]; 2 changes each with 1 hour in an oven [Memmert- Germany], then embedding in the same paraffin. Sectioning had been done with Microtome [Histoline laboratories MRS-3500-Italy] by disposable microtome blade [Pathocutter-Japan]. Sections thicknesses were 5 micrometers. Sections were mounted on charged slides [AFCO-China]. Deparaffinization had been done by Xylene [Scharlau-Spain] (2-3 changes, each for 15 min), and rehydration with descended grades of ethanol alcohol100%, 90%, 80%, 70%, one change, each for 2 minutes; washed with tab water for 2-3 min then the slides were immersed in Harris hematoxylin staining by Harris hematoxylin [SYRBIO-S.A.R] for 2 min washed with running tab water for 5 min then immersed in eosin [SYRBIOS. A.R] for 40 seconds, then dehydrated in ascending concentration of ethanol alcohol 70%, 80%, 90%, 100%, one change each for 1 min. After dehydration, tissues were cleared in xylene, one change for 1 min and finally they were mounted with DPX [HISTOFLUID-GERMANY] and cover slipped. For the immunohistochemical staining, Marker used is Anti-Doublecortin antibody (ab18723) [ABCAM –UK] Rabbit polyclonal to Doublecortin. Detection kit (including the secondary antibody and chromogen) was [Expose Rabbit Specific Detection Kit (ab80437) [Abcam-UK]]. The secondary antibody was goat anti-rabbit polyclonal antibody “readymade”. The primary antibody was diluted to 1:1000 by Phosphate Buffered Saline PBS [Fluka-GERMANY]. Incubation of the primary antibody with the tissue was for 2 hours and secondary antibody for 1 hour. The Incubator used was from [Memmert-Germany]. All incubated at 30°C. Application of DAB had been done in dark for five minutes and counterstaining was done by Harris hematoxylin for 1 minutes. Visualization of the Slides had been done with light microscope [Micros-Austria] and dissecting microscope x10 [KRUSS-GERMANY] (Table 1).
| Parts of the RMS | Neuroblasts/HPF | |
|---|---|---|
| Mean ± St. Error | St. Deviation | |
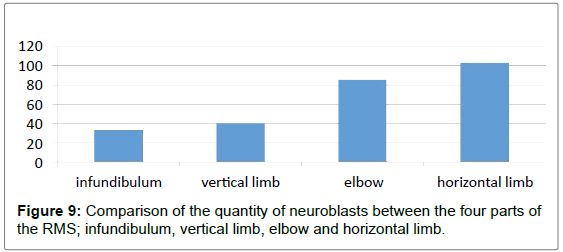
| Infundibuluma | 33.3 ± 4.7586 | 11.6562 |
| Vertical limbsb | 40.39 ± 3.19509 | 7.8263 |
| Elbowc | 86.0 ± 4.6547 | 11.4018 |
| Horizontal limbsd | 103.33 ± 5.5777 | 13.6626 |
• t: a=7.005; b=12.642; c=18.476; d=18.526, df=35.
• (P=>0.001) Statistically strongly significant.
Table 1: Comparison of the quantity of neuroblasts between the four parts of the RMS; infundibulum, vertical limb, elbow and horizontal limb:
Results
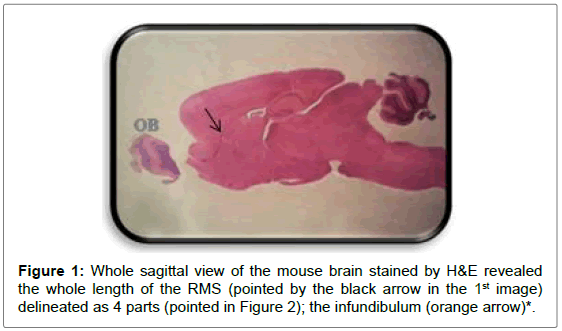
Sagittal sections through the RMS revealed this long pathway emerged from the anterior tip of the lateral ventricle ending in the OB in a sigmoidal shape manner (Figures 1-9).
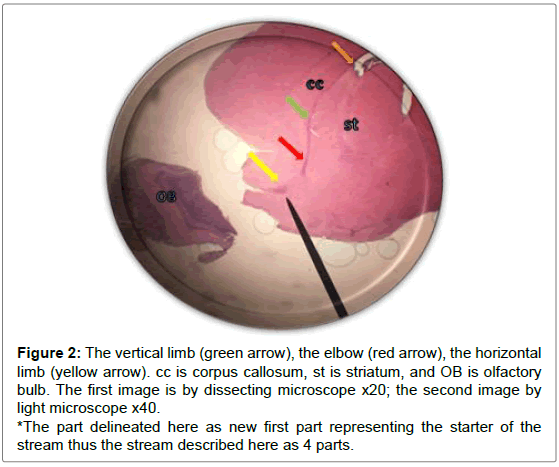
Figure 2: The vertical limb (green arrow), the elbow (red arrow), the horizontal limb (yellow arrow). cc is corpus callosum, st is striatum, and OB is olfactory bulb. The first image is by dissecting microscope x20; the second image by light microscope x40.
*The part delineated here as new first part representing the starter of the stream thus the stream described here as 4 parts.
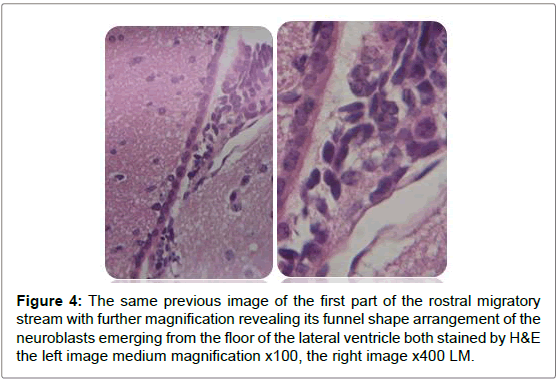
Figure 4: The same previous image of the first part of the rostral migratory stream with further magnification revealing its funnel shape arrangement of the neuroblasts emerging from the floor of the lateral ventricle both stained by H&E the left image medium magnification x100, the right image x400 LM.
Discussion
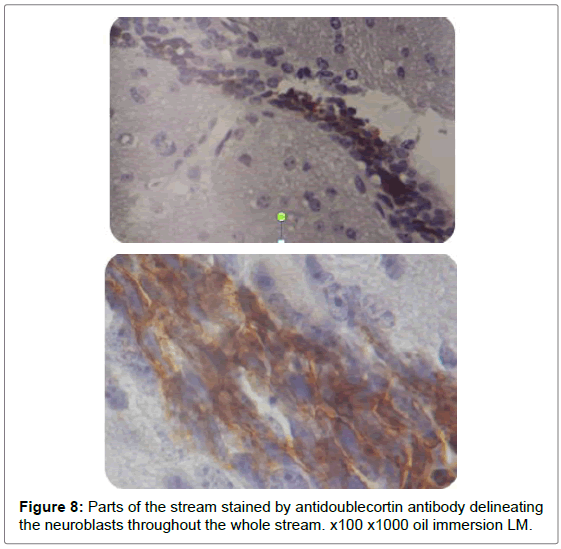
In this research the neuroblasts had been investigated inside the RMS to get information about their shape and behaviors while they migrated through the brain parenchyma. The RMS was demonstrated as a long, relatively restricted pathway through which cells emerged in a caudal-to-rostral direction from the anterior tip of the lateral ventricle towards the olfactory bulb, this outcome was similar to what was described by Carleton et al. [13]. However those studies either used live imaging techniques or electron microscope. This study revealed the cytoarchitecture of the migratory pathway, RMS, by the H&E staining and by antidoublecortin staining. By these two methods the RMS appeared as a stream of elongated cells oriented in the direction of migration). The stream was smooth with no shattering of cells along its whole length and limited by corpus callosum superiorly. The corpus callosum was nicely roofing the RMS.
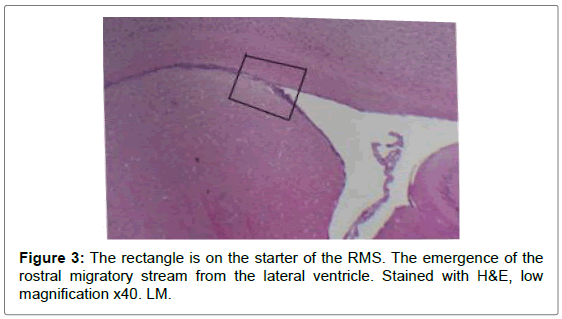
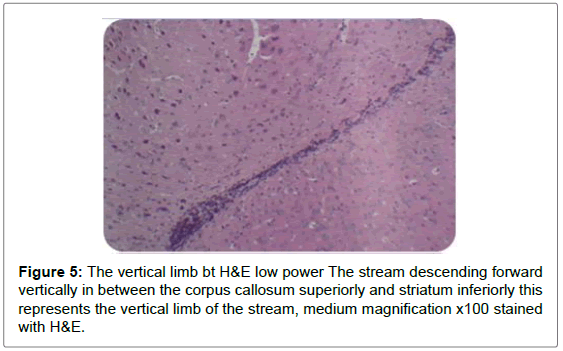
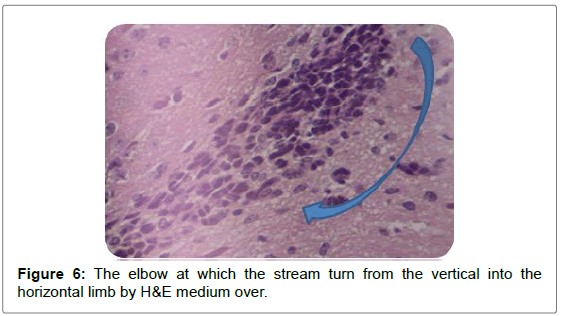
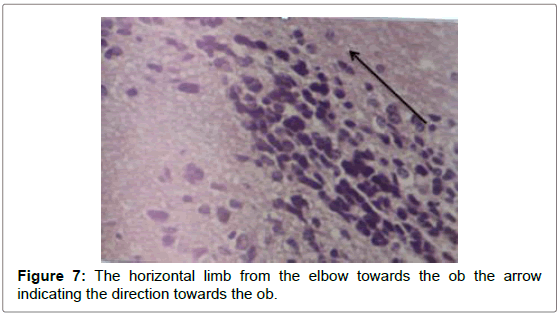
Sagittal view through the RMS revealed strip of cells extending from anterior tip of lateral ventricle along a shallow sigmoidal shape pathway, proceeds rostrally around the striatum from the rostrodorsal to the rostroventral aspects of the striatum and then curving up dorsally to continue horizontally till reaching the olfactory bulb where it would fan out to terminate the RMS. Gross structure of the RMS, in this investigation, coincided with a previous results of. Moreover, “and for a descriptive purposes”, the sigmoidal shape stream had been divided in all previous studies into 3 distinct anatomical parts vertical elbow and horizontal limbs by Carlos Lois et al. [16-18] via studying the stream by two-photon time lapse microscopy; however, in this study we named the part of the stream by which it emerged from the SVZ by infundibulum which was a short funnel-shaped part emerging from the anterior tip of the ventral horn of the lateral ventricle and roofed by corpus callosum, and shortly it would join the vertical part underneath the corpus callosum. We segregated such short part from the vertical limb as we found it had different shape with different proportion between the cells, in addition, the neuroblasts in this part had less elongated nuclei than in the vertical limb. The vertical limb descended vertically in between the corpus callosum dorsally and the striatum ventrally, the elbow that’s the shallow turn-over connecting the vertical with the horizontal limb, then the horizontal limb at which the cells entered the core of the OB.
The stream was regular and organized along its whole length and limited by the corpus callosum at its emergence from the lateral ventricle. The corpus callosum was nicely roofing the infundibulum and the vertical limb till the elbow; this might suggest a role for corpus callosum in keeping the stream highly organized and directed towards its target.
Conclusion
In this study we proved that the antidoublecortin antibody along with the H&E are easy reproducible way to demonstrate and describe the chain of neuroblasts along the RMS from the SVZ to the OB. The neuroblasts produced in a neurogenic niche along the lateral wall of the lateral ventricle, had a unique behavior after their production with a tendency to arrange themselves in chains towards the OB through the RMS. The chain of neuroblasts take the shape of shallow sigmoidal stream, here in this study this stream described by both H&E and antidoublecortin and seen with LM. The RMS delineated here as four limbs: infundibulum, vertical limb, elbow and horizontal limb, instead of three limbs previously described. The new described part is the first part the infundibulum which is a funnel shape starter of the chain emerged from SVZ. Shortly thereafter it joins the second part the vertical limb.
References
- Ming GL, Song H (2011) Adult Neurogenesis in the Mammalian Brain Significant Answers and Significant Questions. Review. Neuron 70: 687-702.
- Alvarez-Buylla A, Lim DA (2004) For the long run maintaining germinal niches in the adult brain. Neuron 41: 683-686.
- Kempermann G, Wiskott L, Gage F (2004) Functional significance of adult neurogenesis. Current Opinion in Neurobiology 14: 186-191.
- Braun SMG, Jessberger S (2014) Adult neurogenesis mechanisms and functional significance Development. 141: 1983-1986.
- Decimo I, Bifari F, Krampera M, Fumagalli G (2012) Neural Stem Cell Niches in Health and Diseases. Curr Pharm Des 18: 1755-1783.
- Oyarce K, Nualart F (2014) Unconventional Neurogenic Niches and Neurogenesis Modulation by Vitamins. Stem Cell Research & Therapy 4: 1-11.
- Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, et al. (2013) Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. PNAS 110: 1045-1054.
- Lledo PM, Alonso M, Grubb MS (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7: 179-193.
- Sawada M, Sawamoto K (2013) Mechanisms of Neurogenesis in the Normal and Injured Adult Brain. Review, Keio J Med 62: 13-28.
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A (2008) Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of adult the brain. Cell StemCell 3: 265-278.
- Imura T, Kornblum HI, Sofroniew M (2003) The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci 23: 2824-2832.
- Kahle MP, Bix GJ (2012) Structural and Chemical Influences on Neuronal Migration in the Adult Rostral Migratory Stream. J Cell Sci Ther 3: 1-9.
- Carleton A, Petreanu L, Lansford R, Alvarez-Buylla AA, Lledo P (2003) Becoming a new neuron in the adult olfactory bulb. Nature Neuroscience 6: 507-518.
- Luna L (1968) In Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. Blakiston Division, McGraw-Hill.
- James R, Kim Y, Hockberger P, Szele F (2011) Subvntricular zone cell migration lessons from quantitive two photon microscopy. Frontiers in Neuroscience 5: 1-11.
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A (1996) Chain Migration of Neuronal Precursors. Science Magazine 271: 978-981.
- Alonso M, Perez OI, Grubb M, Bourgeois JP, Charneau P, et al. (2003) Turning astrocyte from the rostral migratory stream into neurons. The Journal of Neuroscience 28: 11089-11102.
- Martinez-Molina N, Kim Y, Hockberger P, Szele F (2011) Rostral Migratory Stream Neuroblasts Turn and Change their Direction in Stereotypic Patterns. Cell Adhesion and Migration 5: 83-95.
Citation: Saadoon ZZ, Al-Khateeb HM (2017) New Description of the Rostral Migratory Stream in Adult Mice Brains Delineating the Starter of the Stream as an Infundibulum: A New Described Limb, by H&E and Antidoublecortin Antibody. J Cell Sci Apo 1: 101.
Copyright: ©2017 Saadoon ZZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 6130
- [From(publication date): 0-2017 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 5127
- PDF downloads: 1003