Nontyphoidal Salmonella infection Associated with Risk of Retinal Disorders
Received: 30-Apr-2022 / Manuscript No. jcidp-22-62355 / Editor assigned: 02-May-2022 / PreQC No. jcidp-22-62355 / Reviewed: 16-May-2022 / QC No. jcidp-22-62355 / Revised: 23-May-2022 / Manuscript No. jcidp-22-62355 (R) / Published Date: 30-May-2022 DOI: 10.4172/2476-213X.1000148
Abstract
Objective: The aim of this study was to investigate the relationship between nontyphoidal salmonellosis (NTS) and new-onset retinal diseases.
Methods: We conducted a 13-year nationwide, population-based, retrospective cohort study to examine the association between the history of NTS and risk of retinal diseases by using the Longitudinal Health Insurance Database (LHID) of Taiwan. The NTS cohort included 636 patients with newly diagnosed NTS who were older than 30 years old and recruited between 2000 and 2012. Each patient was propensity score matching with 1:4 person without NTS (N=2544) from the LHID. To determine the occurrence of retinal diseases, the study population was followed up until the end of 2013. Cumulative incidence, hazard ratios (HRs), and 95% confidence intervals (CIs) were calculated after adjusting for age, gender, and medical comorbidities, Cox regression was adopted to assess hazard ratios (HRs) and 95% confidence intervals (CIs), with the unexposed group as reference.
Results: The adjusted HR for newly diagnosed retinal diseases in the NTS cohort was 1.15 (95% CI= 1.05 to 1.25), compared with the non- NTS cohort. Stratified subgroup analysis revealed that the HRs of retinal diseases were 1.64 (95% CI = 1.04 to 2.58) for NTS among aged older than 61 years old. Risk of the retinal diseases was also significant in patients with hypertension (aHR= 2.10, 95% C.I. =1.38, 3.2), diabetes mellitus (aHR= 2.05, 95%C.I. = 1.15, 3.65), and hyperlipidemia (aHR= 2.05, 95% C.I. =1.22, 3.45).
Conclusions: Old (aged older than 61 years old) patients with NTS had an increased 64% risk of retinal diseases than those without NTS.
Keywords
Nontyphoid Salmonellosis; Retinal diseases, Cohort study
Implications for Practice
Retinal diseases are identified as an important cause of ocular morbidity and visual impairment globally. In this Taiwan nationwide cohort study, there was a 1.15- fold increase risk of developing newonset retinal diseases for incident nontyphoid salmonellosis (NTS) patients, compared with the matched controls. Furthermore, Stratified analysis showed that the associated risk of the investigated outcome was significant aged than 61 years old (aHR 1.64, 95% CI = 1.04 to 2.58), in patients with hypertension (aHR= 2.10, 95% C.I. =1.38, 3.2), diabetes mellitus (aHR= 2.05, 95%C.I. = 1.15, 3.65), and hyperlipidemia (aHR= 2.05, 95% C.I. =1.22, 3.45).
Introduction
Retinal disease is a leading cause of blindness worldwide. It is a highly prevalent ocular disorder which has affected all ethnic groups [1-3]. According to the previous report, population-based studies have estimated a prevalence of 5.35% to 21.02% at aged more than 40 years [4- 6]. The retina is a crucial organ for vision, and its complex structure with several layers needed to turn the light stimulus into a chemical signal ultimately sent to the brain. Different retinal disorder such as retinal vascular occlusion, retinal detachment, and macula degeneration are important causes of blindness and accounted for most common retinal problems [7,8]. Risk factors for retinal diseases might include aging, smoking, obesity, diabetes, hypertension, atherosclerosis, trauma, and family history of retinal diseases [9,10]. Chronic inflammatory events have recently been identified as plausible causes of atherosclerosis, which may predispose to some retinal disorders.
Salmonellosis is a common bacterial infection that affects the intestinal tract and an estimated 93.8 million cases of gastroenteritis occurring globally each year [10,11]. Salmonella may adhere to damaged endothelium persistly and cause secondary retina vascular lesions. Previous case report indicates that immune-mediated retinitis and macular neurosensory detachment is well-recognized sequelae of typhoid fever [12]. Cases of retinal necrosis, detachment, and phthisis with biopsy revealing Salmonella typhi in vitreous cultures are reported [13,14]. Furthermore, stellate maculopathy due to Salmonella typhi had been reported [15].
As Salmonella can multiply in various host cells including macrophages and endothelial cells, the pathogens tend to cause a chronic infection [16,17]. Nontyphoidal salmonellosis (NTS) is caused by orally ingested salmonellae bacterium. There has been no reported study to link retinal disorders before. Because retinal disorder is mostly asymptomatic in many cases, timely precautions and early detection is vital to prevent irreversible blindness. Therefore, identification of their risk factors could help. We hypothesized that NTS could reside in reticular endothelial systems and may be associated with subsequent risk of retinal disorder. This study was conducted to explore the interrelations by using Taiwan’s National Health Insurance Research Database (NHIRD), among the largest medical database around the world.
Materials and Methods
The study was approved by the Institutional Review Board of China Medical University (IRB permit number: CMUH104-REC2- 115(AR-4)).
Data source
This study was conducted using data from the National Health Insurance Research Database (NHIRD), including the claims data from Taiwan’s National Health Insurance (NHI) program, which was established in 1995 and covers over 99.99% of Taiwan’s 23 million citizens. Insurance benefits include all types of medical visits, eg.Inpatient, ambulatory, emergency, dental, and traditional Chinese medicine services. Quarterly expert reviews on random samples of claims data, with a sampling rate of 1 in 50–100, are performed by the Bureau of NHI to ensure accuracy of diagnosis. The NHI records diseases on the basis of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Before releasing the database for research, the original identification numbers were anonymized to protect patients’ privacy. The informed consent was waived because of a de-identified procedure for research purposes and anonymously analyzed nature.
Sampled participants
We identified patients newly diagnosed with NTS (ICD-9-CM code 003) between 2000 and 2012 from among those with at least three ambulatory care visits or one inpatient care visit. The index date was defined as the date of the first diagnosis of NTS. We excluded patients with history of retinal disorder before the index date. For creating the comparison cohort (non- NTS group), we randomly selected insured people without NTS from the same period to maintain the same index date. The non-NTS cohort were frequency matched according to age and gender with the NTS cohort at a ratio of 1:4. A propensity score matching to balance the heterogeneity of baseline characteristics is used to reduce potential selection bias. The propensity score is a probability calculated by logistic regression. We corrected risk factors including age, gender, and history of medical comorbidities in both cohorts. The medical comorbidities in the logistic regression models included diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), asthma (ICD- 9-CM code 493), chronic obstructive pulmonary disease (ICD-9-CM code 491, 492 and 496), sleep apnea (ICD-9-CM code 780.51, 780.53 780.57 and 327.23), cancer (ICD-9-CM code 140-208), chronic liver diseases (ICD-9-CM code 571.4), chronic kidney disease (ICD-9-CM code 585), retinal diseases such as retinal vascular occlusion(ICD- 9-CM code362.3), retinal detach (ICD-9-CM code362.4), macula degeneration(ICD-9-CM code362.5), other retinal vascular change, (ICD-9-CM code362.1), other proliferative retinopathy (ICD-9- CM code362.2), other retinal disorder(ICD-9-CM code362.8), and unspecified retinal disorder (ICD-9-CM code 362.9), was identified as the study end point. Both cohorts were monitored from the index year until being diagnosed with any kind of retinal disorder diseases, loss to follow-up, death, and withdrawal from insurance or at the end of 31 December 2013.
Statistical Analysis
We analyzed the data of demographic characteristics (distributions of categorical age, gender, and comorbidities) of the NTS and non- NTS group by the Chi-square (χ2) test or Student’s t-test as appropriate. We calculated the incidence rate by the number of occurrences and personyears. We utilized hazard ratios (HRs) and 95% confidence intervals (CIs) of the two groups for estimate in univariate and multivariate Cox proportional hazard regression models. The Kaplan-Meier curve was plotted to describe the cumulative incidence between the two groups; then the log rank test was used. SAS statistical software package, version 9.1 (SAS Institute, Cary, NC), was used for our statistical analysis and the significance level was set at 0.05.
Results
Demographics of the Study Population
There were 636 subjects with NTS and 2544 subjects without NTS in this study, with similar distributions of age, sex and medical comorbidities after propensity score matching. The baseline characteristics of two groups.
The follow-up times (person-years) in the NTS and comparison groups were 4276and 18762 person-years, respectively.
The results of univariate Cox regression analysis and multivariate Cox regression analysis. At the end of this study, the overall incidence rates of retinal disorder in the NTS cohort and non- NTS cohort were 10.5 and 6.88 per 1,000 person-years, respectively.After adjusting for age, gender, and medical comorbidities, the NTS cohort exhibited a higher risk of retinal diseases compared with the non- NTS cohort [aHR=1.15, 95% CI= 1.05 to 1.25]. Among all relevant variables, male, patients aged ≥60 years, and patients with the comorbidities of diabetes, hypertension, and hyperlipidemia exhibited a high risk of retinal diseases.
Demonstrates the association of risks of incidental retinal diseases and NTS among different subgroups. In age-subgroup analysis, only the HRs of retinal diseases were 1.64 (95% CI = 1.04 to 2.58) for NTS among aged older than 61 years old.
Among comorbidities, hypertension patients with NTS had a significant higher risk (2.10, 95% C.I. 1.38, 3.2) in developing retinal diseases compared to those non- NTS.
Risk of the retinal diseases was also significant in patients with diabetes mellitus (aHR= 2.05, 95%C.I. = 1.15, 3.65), and hyperlipidemia (aHR= 2.05, 95% C.I. =1.22, 3.45) shows the risk of retinal disease in different follow up periods
The incidence density rates of retinal diseases were the highest and most significant in the 1-5 years after index date (the aHRs=1.83, 95% CI = 1.08, 3.10).
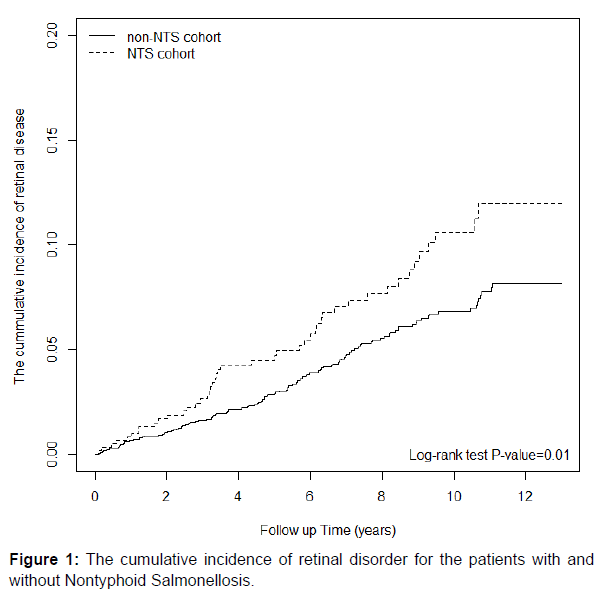
The Kaplan Meier analysis revealed that the cumulative incidence of retinal diseases was significantly higher in the NTS cohort than in the non-salmonella cohort (log-rank test, P < 0.01; (Figure 1).
Discussion
In this 13-year cohort study using population-based data from the NHIRD of Taiwan, we first found that NTS patients were associated with an increased incidence of retinal diseases. Furthermore, the subgroup for NTS among aged older than 61 years old was also significant in patients with hypertension, diabetes mellitus and hyperlipidemia. Our study investigated in a Taiwanese patient population and the findings could help us to estimate the burden and underlying risk factors for retinal disorders.
The most frequent bacterial diseases that affect the retina are syphilis and tuberculosis [18]. The ability of the syphilis retinal-specific autoimmune cells to persist in the eye for longer duration results in persistent inflammation and prolongation of the disease process [19]. Tuberculosis of the retina most commonly results from the direct extension from the underlying uvea but may also be caused by hematogenous spread. Retinal lesions may take the form of either focal tubercles or diffuse retinitis [20]. Salmonella can rarely affect the eye either by direct infection or rarely by immune-mediated mechanism. As Salmonella can multiply in various host cells including macrophages and endothelial cells, the pathogens tend to cause a chronic infection a low grade chronic inflammatory reaction may appear as possible mechanism [21].
Typhoid fever affecting the eye has been reported as early as 1893. Practically every layer of the eye can be affected. The mechanism of Typhoid retinitis is via a direct invasion by the organism or via an immune-complex mediated hypersensitivity reaction [22]. The pathogenicity of Salmonella is multifactorial and attributed in part to production of cholera toxin–like and Escherichia coli heat-labile enterotoxin–like toxins [23].
Limitations
This study had several limitations. First, not all NTS patients were included. The patients coded with NTS mostly due to positive stool cultures, so the result is probably trustworthy despite being underestimated. Furthermore, some NTS patients may have been included in the comparison group because mild clinical symptoms do not submit claims for medical services. If NTS is associated truly with subsequent retinal disease, patient-misclassification would suppress the estimated HRs to the null. Therefore, our study results are reliable [24]. Second, the lack of data regarding retinal disease subtypes. We don’t know the stratification of retinal disease subtypes. Third, the study involved only Taiwanese population, so this data is not sure to be applicable to other ethnical populations except Han people. Regardless of these limitations, we believe that the current results provide novel and important information for nontyphoidal salmonellosis (NTS) patients.
In conclusion, this is a novel study demonstrating that an infection with nontyphoid Salmonellosis may be an additional risk factor for retinal disorders, especially in the subgroup of older than 61 years and hypertension, diabetes mellitus and hyperlipidemia patients. Therefore, we propose that findings of should be provided to the physician taking care of NTS. Further studies are needed to characterize retinal disease findings.
Funding Sources Statement
Partly funded by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW108-TDU-B-212-133004).The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
References
- World Health Organization. Blindness and vision impairment prevention
- Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR et al. (2013) Vision Loss Expert Group. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health.1:339-349.
- Resnikoff S, Pascolini D, Etyaale D (2004) Global data on visual impairment in the year
- Thapa SS, Thapa R, Paudyal I (2013) Prevalence and pattern of vitreo-retinal disorders in
- Hatef E, Fotouhi A, Hadhemi H, Mohammad K, Jalali KH (2008) Prevalence of retinal diseases and their pattern in Tehran: The Tehran eye study. Retina 28: 755-762.
- Nirmalan PK, Katz J, Robin A (2004) Prevalence of vitreoretinal disorders in a rural population of southern India. Arch Ophthalmol 122:581–586.
- Rai BB, Morley MG, Bernstein PS, Maddess T (2020) Pattern of vitreo-retinal diseases at the national referral hospital in Bhutan: a retrospective, hospital-based study. BMC Ophthalmol.20:51.
- zChauhan A, Chaudhary KP, Rajput GC (2014) Pattern of retinal diseases in hilly terrain of Himachal Pradesh, India. Int Eye Sci 14:2114–2118.
- Saunier V, Merle BMJ, Delyfer MN (2018) Incidence of and risk factors associated with age-related macular degeneration: four-year follow-up from the ALIENOR study. JAMA Ophthalmol 136:473-481
- .Hallak JA, de Sisternes L, Osborne A, Yaspan B, Rubin DL et al.(2019) Imaging, Genetic, and Demographic Factors Associated With Conversion to Neovascular Age-Related Macular Degeneration: Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol.137:738-744
- Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M et al.(2010) Hoekstra RM, International Collaboration on Enteric Disease 'Burden of Illness S. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882-889.
- Hohmann EL (2001) Nontyphoidal salmonellosis.Clin Infect Dis 32:263-269
- Relhan N, Pathengay A, Albini T, Priya K, Jalali S et al. (2014) A case of vasculitis, retinitis and macular neurosensory detachment presenting post typhoid fever. J Ophthalmic Inflamm Infect 18:4-23.
- Sinha MK, Jalali S, Nalamada S.Semin (2012) Review of endogenous endophthalmitis caused by Salmonella species including delayed onset Salmonella typhi endophthalmitis. Ophthalmol 27:94-98
- Fusco R, Magli A, Guacci P (1986) stellate maculopathy due to Salmonella typhi. Ophthalmologica 192:154-158.
- Ellis MJ, Tsai CN, Johnson JW, French S, Elhenawy W et al. (2019) A macrophage-based screen identifies antibacterial compounds selective for intracellular Salmonella Typhimurium. Nat Commun 10:197
- Stapels DAC, Hill PWS, Westermann AJ, Fisher RA, Thurston TL et al. (2018) Salmonella persisters undermine host immune defenses during antibiotic treatment. Science 362:1156-1160
- .Pirani V, Pelliccioni P, De Turris S, Rosati A, Franceschi A et al. (2019) The Eye as a Window to Systemic Infectious Diseases: Old Enemies, New Imaging. J Clin Med 8:1392
- .Fonollosa A, Giralt J, Pelegrin L (2009) Ocular syphilis–back again: understanding recent increases in the incidence of ocular syphilitic disease. Ocul Immunol Inflamm 17:207-212.
- Albert DM, Raven ML (2016) Ocular Tuberculosis. Microbiol Spectr. 4:10.1128/microbiolspec.TNMI7-0001-2016
- Boumart Z, Velge P, Wiedemann A (2014) Multiple invasion mechanisms and different intracellular Behaviors: a new vision of Salmonella–host cell interaction. FEMS Microbiol Lett 361:1-7
- .Mahendradas P, Kawali A, Luthra S, Srinivasan S, Curi AL et al. (2020) Post-fever retinitis - Newer concepts. Indian J Ophthalmol. 68:1775-1786
- Rachitskaya AV, Flynn HW, Davis JL (2012) Endogenous Endophthalmitis Caused by Salmonella Serotype B in an Immunocompetent 12-Year-Old Child. Arch Ophthalmol. 130:802-804.
- .Chang R, Wei JC, Lin MC, Hung YM, Hung CH (2020) Risk of subsequent ischemic stroke in patients with nontyphoidal salmonellosis: A nationwide population-based cohort study. J Infect. 81:396-402.
Indexed at, Google Scholar, Crossref
2002. Bull World Health Organ.82:844–851
Indexed at, Google Scholar, Crossref
Nepal: the Bhaktapur Glaucoma Study. BMC Ophthalmol 13:9.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at Google Scholar
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Chen YS, Yip HT, Hung YM, Chang R (2022) Nontyphoidal Salmonella Infection Associated with Risk of Retinal Disorders. J Clin Infect Dis Pract, 7: 148. DOI: 10.4172/2476-213X.1000148
Copyright: © 2022 Chen YS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4569
- [From(publication date): 0-2022 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 3852
- PDF downloads: 717