Novel Benzo[d]thiazole with Dimethylpyrazol Derivatives: Design, Synthesis and Biological Evaluation
Received: 16-May-2019 / Accepted Date: 07-Jun-2019 / Published Date: 14-Jun-2019 DOI: 10.4172/2476-2067.1000141
Abstract
A series of new benzothiazole derivatives containing dimethylpyrazole were synthesized and evaluated for their anticonvulsant activity, neurotoxicity and cytotoxicity by using the maximal electroshock (MES), rotarod neurotoxicity (TOX) and MTT colorimetric assay. Among the compounds studied, four compounds (6a, 6b, 6g and 6m) showed better anticonvulsant than the others. All the synthetic compounds showed lower neurotoxicity and little cytotoxicity, so that the compounds with better activities also had higher protective index.In particular, the compound 6g, 2-(3,5- dimethyl-1H-pyrazol-1-yl)-6-((2-fluorobenzyl)oxy)benzo[d]thiazole showed better activity with an ED50 value of 160.4 mg/kg and higher protective index (PI) values of 2.74 in the MES test than the standard drugs used as positive controls in this study. Then the compound 6g demonstrated antagonistic activity against seizures induced by pentylenetetrazol, which proved 6g maybe exert activity through effecting GABAergic neurotransmission.
Keywords: Benzothiazole; Dimethylpyrazol; Maximal electroshock; MTT assay; GABAergic
Introduction
Epilepsy is a chronic noncommunicable disease of the brain that affects people of all ages, which affects approximately 50 million people [1]. Currently, 40% of patients in high-income countries and more than 70% of patients in developing countries do not get the treatment they need, because of the high expense or low availability of the appropriate drugs [2,3]. Therefore, there is a pressing need to develop more effective antiepileptic drugs (AEDs) endowed with an improved safety profile.
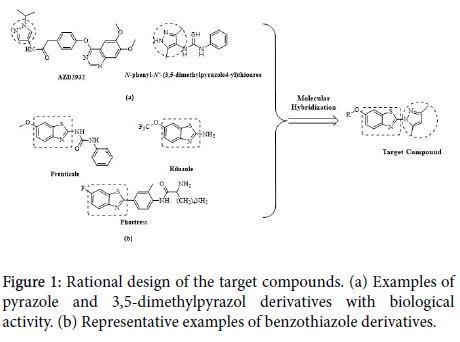
On the basis of related materials, pyrazoles, especially 3, 5- dimethylpyrazol, occupy a distinct niche in heterocyclic chemistry and represent a key motif in medicinal chemistry because of their capability to exhibit an array of properties and bioactivities, including fungicidal [4] anticancer [5] anti-inflammatory [6] anticonvulsant [7] and nitrification inhibitor [8] activities. Furthermore, we also demonstrated that the benzothiazole nucleus is a unique scaffold for further molecular exploration to synthesize novel compounds. A literature survey revealed that benzothiazole analogs are associated with diverse pharmacological effects [9-12] including anticonvulsant activity [13,14] For these reasons and in continuation to our efforts directed toward the synthesis of new heterocyclic compounds with anticonvulsant biological activities, in this study, we combined both biological components 3, 5-dimethylpyrazol and benzothiazole, to obtain a series of 2-(3,5-dimethyl-1H-pyrazol-1-yl)-6- alkoxybenzo[d]thiazole or 2-(3,5-dimethyl-1H-pyrazol-1-yl)-6- benzylbenzo[d]thiazole (The design thought and the structure of target compounds were shown in Figure 1). Their anticonvulsant activities were evaluated using the maximal electroshock (MES) test and subcutaneous pentylenetetrazole (scPTZ), their neurotoxicity (TOX) was evaluated with the rotarod test in mice.
Experimental Procedures
General information
Melting points were determined in open capillary tubes and were uncorrected. IR spectra were recorded (in KBr) on IR Prestige-21. 1HNMR and 13C-NMR spectra were measured on an AV-300 (Bruker, Switzerland), and all chemical shifts were given in ppm relative to tetramethylsilane. Mass spectra were measured on an AXIMA CFR Plus MALDI-TOF (Shimadzu, Japan). Elemental analyses were performed on a 204Q CHN (Perkin Elmer, USA).The chemicals were purchased from Aldrich Chemical Corporation. All other chemicals were of analytical grade.
Synthesis method
Synthesis of 6-methoxybenzo[d]thiazol-2-amine(2): A mixture of 4- methoxyaniline (50 mmol, 6.15 g) and 19.0 g ammonium sulfocyanate (5 e.q., 250mmol) in 50 mL acetic acid was stirred for 30 min at room temperature. Then, 3.0 mL acetic acid solution, containing 0.055 mol bromine, was dropwise added with stirring and cooling in ice bath. And after that, the reaction continued at room temperature for 5 h. The mixture was added into 500 mL water and adjusted with ammonia solution to pH 9, the product precipitate out. Then it was filtered to obtain a yellow-white solid (compound 2) with 69% yield.
Synthesis of 2-hydrazinyl-6-methoxybenzo[d]thiazole(3): 20mmol compound 2 (3.60 g) and 0.6 mL 98% H2SO4 solution (water solutions) in 20 mL of glycol was refluxed for 0.5 h at 80°C. Then 10ml of hydrazine hydrate was added into the mixture and heated at 140°C for 5 h. After cooling to room temperature, the mixture was added into 50 mL of ice-water. The precipitate formed was filtered and washed with water to obtain a light green needle-like solid. The yield was 60%.
Synthesis of 2-(3, 5-dimethyl-1H-pyrazol-1-yl)-6- methoxybenzo[d]thiazole(4): A mixture of compound 3 (10 mmol) and equimolar amounts of acetylacetone were added into a flask with 30 mL dioxane as solvent and refluxed reacted for 1 h at 100°C. After removing the solvent under reduced pressure, the crude product was obtained. Then crude product was purified by silica gel column chromatography with CH2Cl2 to get compound 4 (72.3% yield) as a white solid. Melting point and spectral data of compound 4 was given: M.p. 124-126°C. 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J=8.9 Hz, 1H, Ar-H), 7.31 (d, J=2.3 Hz, 1H, Ar-H), 7.05 (d, J=8.9 Hz, 1H, Ar-H), 6.04 (s, 1H, pyrazole-H), 3.90 (s, 3H, -OCH3), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 159.17 (s), 157.11 (s), 151.80 (s), 145.81 (s), 142.17 (s), 134.13 (s), 122.83 (s), 114.85 (s), 109.97 (s), 104.30 (s), 55.76 (s), 13.82 (s), 13.62 (s). IR (KBr) cm-1: 1604.77 (C=N), 1577.77 (C=C). MS-EI m/z 260 (M+H+). Anal. Calcd. for C13H13N3OS: C, 60.21; H, 5.05; N, 16.20. Found: C, 60.32; H, 5.25; N, 16.07.
Synthesis of 2-(3, 5-dimethyl-1H-pyrazol-1-yl)benzo[d]thiazol-6- ol(5): 2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-methoxybenzo[d]thiazole (compound 4) (1.5 g, 5.8 mmol) was dissolved in 50 mL dichloromethane. BBr3 (29.0 mmol) was added dropwise to the solution and then the mixture was stirred at room temperature. After 4 h the mixture was added slowly 20 mL ice cold water and continue to stir for 0.5 h. The resulting white precipitate was obtained by filtration with 38.8% yield. Melting point and spectral data of compound 5 was given: M.p. 186-188°C. 1H NMR (300 MHz, CDCl3) δ 7.73 (d, J=8.7 Hz, 1H), 7.28 (s, 1H), 6.96 (d, J=8.7 Hz, 1H), 6.05 (s, 1H), 5.30 (s, 1H), 2.77 (s, 3H), 2.32 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 160.53 (s), 157.77 (s), 153.05 (s), 146.74 (s), 146.19 (s), 134.41 (s), 123.03 (s), 115.12 (s), 110.02 (s), 107.00 (s), 90.41 (s), 13.71 (s), 13.56 (s). IR (KBr) cm-1: 1602.81 (C=N), 1579.77 (C=C). MS-EI m/z 246 (M+H+).
General procedure for the synthesis of 2-(3, 5-dimethyl-1Hpyrazol- 1-yl)-6-alkoxybenzo[d]thiazole (6a-6o): Compound 5 (3.0 mmol), potassium carbonate (3.6 mmol) were dissolved in 50 mL acetonitrile and refluxed for 30 min, then appropriate alkyl bromide or benzyl chloride derivatives (3.3 mmol) and a catalytic amount of benzyltriethylamine chloride (TEBA) were added into the mixture. The reaction mixture was heated at reflux temperature for 12-48 h. After removing the solvent under reduced pressure, 50 mL water was poured into the mixture and stirred for 0.5 h to remove the excess K2CO3. The remaining precipitate was filtered to obtain a white solid. The yield, melting point and spectral data of each compound were given below.
6-butoxy-2-(3,5-dimethyl-1H-pyrazol-1-yl)benzo[d]thiazole 6a: yield: 76.52%. m.p. 92-94°C. 1H NMR (300 MHz, CDCl3) δ 7.75 (d, J=8.9 Hz, 1H, Ar-H), 7.30 (s, 1H, Ar-H), 7.04 (d, J=8.7 Hz, 1H, Ar-H), 6.04 (s, 1H, pyrazole-H), 4.04 (t, J=6.4 Hz, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3), 1.94-1.74 (m, 2H, -CH2-), 1.59-1.45 (m, 2H, -CH2-), 1.02 (t, J=7.1 Hz, 3H, -CH3). 13C NMR (75 MHz, CDCl3) δ 156.69 (s), 151.77 (s), 145.68 (s), 142.16 (s), 134.10 (s), 122.78 (s), 115.31 (s), 109.93 (s), 105.04 (s), 68.34 (s), 31.34 (s), 19.26 (s), 13.86 (s), 13.82 (s), 13.62 (s). IR (KBr) cm-1: 1604.77 (C=N), 1577.77 (C=C). MS-EI m/z 302 (M+H+). Anal. Calcd. for C16H19N3OS: C, 63.76; H, 6.35; N, 13.94. Found: C, 63.91; H, 6.43; N, 13.85.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(pentyloxy)benzo[d]thiazole 6b: yield: 61.28%. m.p. 89-91°C. 1H NMR (300 MHz, CDCl3) δ 7.75 (d, J=8.9 Hz, 1H, Ar-H), 7.30 (d, J=2.5 Hz, 1H, Ar-H), 7.04 (d, J=8.9 Hz, 1H, Ar-H), 6.04 (s, 1H, pyrazole-H), 4.03 (t, J=6.6 Hz, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3), 1.83 (dd, J=14.4, 6.8 Hz, 2H, -CH2-), 1.54-1.33 (m, 4H, -(CH2)2-), 0.96 (t, J=7.1 Hz, 3H, -CH3). 13C NMR (75 MHz, CDCl3) δ 158.99 (s), 156.63 (s), 151.70 (s), 145.61 (s), 142.09 (s), 134.04 (s), 122.72 (s), 115.24 (s), 109.87 (s), 104.97 (s), 68.58 (s), 28.93 (s), 28.15 (s), 22.41 (s), 13.97 (s), 13.75 (s), 13.55 (s). IR (KBr) cm-1: 1604.77 (C=N), 1577.77 (C=C). MS-EI m/z 316 (M+H+). Anal. Calcd. for C17H21N3OS: C, 64.73; H, 6.71; N, 13.32. Found: C, 64.82; H, 6.85; N, 13.18.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(hexyloxy)benzo[d]thiazole 6c: yield: 67.71%. m.p. 78-80°C. 1 H NMR (300 MHz, CDCl3) δ 7.75 (d, J=9.0 Hz, 1H, Ar-H), 7.30 (d, J=2.7 Hz, 1H, Ar-H), 7.04 (d, J=8.8 Hz, 1H, Ar-H), 6.04 (s, 1H, pyrazole-H), 4.03 (t, J=6.5 Hz, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3), 2.00-1.74 (m, 2H, -CH2-), 1.82-1.40 (m, 2H, -CH2-), 1.46-1.19 (m, 4H, -(CH2)2-), 0.94 (t, J=7.1 Hz, 3H, -CH3). 13C NMR (75 MHz, CDCl3) δ 159.00 (s), 156.63 (s), 151.70 (s), 145.62 (s), 142.08 (s), 134.04 (s), 122.72 (s), 115.24 (s), 109.87 (s), 104.97 (s), 68.59 (s), 31.53 (s), 29.20 (s), 25.68 (s), 22.55 (s), 13.98 (s), 13.75 (s), 13.56 (s). IR (KBr) cm-1: 1608.63(C=N), 1577.77 (C=C). MS-EI m/z 330 (M+H+). Anal. Calcd. for C18H23N3OS: C, 65.62; H, 7.04; N, 12.75. Found: C, 65.78; H, 7.15; N, 12.66.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(heptyloxy)benzo[d]thiazole 6d: yield: 70.32%. m.p. 85-87°C. 1H NMR (300 MHz, CDCl3) δ 7.75 (d, J=8.9 Hz, 1H, Ar-H), 7.30 (d, J=2.5 Hz, 1H, Ar-H), 7.04 (dd, J=8.9, 2.5 Hz, 1H, Ar-H), 6.04 (s, 1H, pyrazole-H), 4.03 (t, J=6.5 Hz, 2H, - OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3), 1.95-1.74 (m, 2H, -CH2-), 1.55-1.27 (m, 8H, -(CH2)4-), 0.92 (t, J=6.6 Hz, 3H, -CH3). 13C NMR (75 MHz, CDCl3) δ 159.00 (s), 156.63 (s), 151.69 (s), 145.61 (s), 142.07 (s), 134.03 (s), 122.72 (s), 115.24 (s), 109.86 (s), 104.96 (s), 68.59 (s), 31.73 (s), 29.13 (d, J=17.1 Hz), 25.96 (s), 22.56 (s), 14.04 (s), 14.04 (s), 13.75 (s), 13.56 (s). IR (KBr) cm-1: 1608.63 (C=N), 1577.77 (C=C). MS-EI m/z 344 (M+H+). Anal. Calcd. for C19H25N3OS: C, 66.44; H, 7.34; N, 12.23. Found: C, 66.61; H, 7.51; N, 12.10.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(octyloxy)benzo[d]thiazole 6e: yield: 62.78%. m.p. 80-82°C. 1H NMR (300 MHz, CDCl3) δ 7.75 (d, J=8.9 Hz, 1H, Ar-H), 7.30 (d, J=2.5 Hz, 1H, Ar-H), 7.04 (dd, J=8.9, 2.5 Hz, 1H, Ar-H), 6.04 (s, 1H, pyrazole-H), 4.03 (t, J=6.5 Hz, 2H, - OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3), 1.96-1.73 (m, 2H, -CH2-), 1.60-1.12 (m, 10H, -(CH2)5-), 0.90 (t, J=7.0 Hz, 3H, -CH3). 13C NMR (75 MHz, CDCl3) δ 159.00 (s), 156.63 (s), 151.70 (s), 145.61 (s), 142.08 (s), 134.03 (s), 122.72 (s), 115.24 (s), 109.86 (s), 104.97 (s), 68.60 (s), 31.76 (s), 29.24 (s), 26.00 (s), 22.61 (s), 14.05 (s), 14.05 (s), 13.75 (s), 13.56 (s). IR (KBr) cm-1: 1608.63 (C=N), 1577.77 (C=C). MS-EI m/z 358 (M+H+). Anal. Calcd. for C20H27N3OS: C, 67.19; H, 7.61; N, 11.75. Found: C, 67.30; H, 7.72; N, 11.68.
6-(benzyloxy)-2-(3,5-dimethyl-1H-pyrazol-1-yl)benzo[d]thiazole 6f: yield: 56.18%. m.p. 117-119°C. 1H NMR (300 MHz, CDCl3) δ 7.77 (d, J=8.9 Hz, 1H, Ar-H), 7.58-7.34 (m, 6H, Ar-H), 7.12 (dd, J=8.9, 2.5 Hz, 1H, Ar-H), 6.05 (s, 1H, pyrazole-H), 5.15 (s, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.33 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 159.27 (s), 156.19 (s), 151.77 (s), 145.97 (s), 142.11 (s), 136.64 (s), 134.04 (s), 128.55 (s), 128.00 (s), 127.44 (s), 122.80 (s), 115.45 (s), 109.93 (s), 105.57 (s), 70.57 (s), 13.76 (s), 13.56 (s). IR (KBr) cm-1: 1604.77 (C=N), 1579.70 (C=C). MS-EI m/z 336 (M+H+). Anal. Calcd. for C19H17N3OS: C, 68.04; H, 5.11; N, 12.53. Found: C, 68.19; H, 5.02; N, 12.57.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-((2-fluorobenzyl)oxy)benzo[d] thiazole 6g: yield: 61.18%. m.p. 126-128°C. 1H NMR (300 MHz, CDCl3) δ 7.78 (d, J=8.9 Hz, 1H, Ar-H), 7.56 (t, J=7.3 Hz, 1H, Ar-H), 7.42 (d, J=2.4 Hz, 1H, Ar-H), 7.36 (d, J=5.9 Hz, 1H, Ar-H), 7.22 (d, J=7.5 Hz, 1H, Ar-H), 7.18-7.05 (m, 2H, Ar-H), 6.05 (s, 1H, pyrazole- H), 5.22 (s, 2H, -OCH2-), 2.78 (s, 3H, pyrazole-CH3), 2.33 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 162.02 (s), 159.37 (s), 158.74 (s), 155.93 (s), 151.79 (s), 146.12 (s), 142.12 (s), 134.05 (s), 129.68 (dd, J=7.9, 6.1 Hz), 124.20 (d, J=3.5 Hz), 122.81 (s), 115.31 (t, J=10.6 Hz), 109.94 (s), 105.66 (s), 64.34 (s), 13.74 (s), 13.54 (s). IR (KBr) cm-1: 1604.77 (C=N); 1577.97 (C=C). MS-EI m/z 354 (M+H+). Anal. Calcd. for C19H16FN3OS: C, 64.57; H, 4.56; N, 11.89. Found: C, 64.69; H, 4.62; N, 11.67.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-((3-fluorobenzyl)oxy)benzo[d] thiazole 6h: yield: 63.36%. m.p. 101-103°C. 1H NMR (300 MHz, CDCl3) δ 7.78 (d, J=8.9 Hz, 1H, Ar-H), 7.38 (s, 2H, Ar-H), 7.28-7.15 (m, 2H, Ar-H), 7.11 (d, J=8.9 Hz, 1H, Ar-H), 7.05 (s, 1H, Ar-H), 6.05 (s, 1H, pyrazole-H), 5.14 (s, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 164.54 (s), 161.27 (s), 159.39 (s), 155.86 (s), 151.80 (s), 146.14 (s), 142.12 (s), 139.26 (d, J=7.3 Hz), 134.07 (s), 130.08 (dd, J=8.7, 3.9 Hz), 123.04-122.13 (m), 115.33 (s), 115.13 (d, J=28.9 Hz), 114.66 (s), 114.27 (s), 113.98 (s), 109.94 (s), 105.65 (s), 69.72 (s), 13.71 (s), 13.51 (s). IR (KBr) cm-1: 1604.77 (C=N), 1597.77 (C=C). MS-EI m/z 354 (M+H+). Anal. Calcd. for C19H16FN3OS: C, 64.57; H, 4.56; N, 11.89. Found: C, 64.73; H, 4.60; N, 11.71.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-((4-fluorobenzyl)oxy)benzo[d] thiazole 6i: yield: 72.10%. m.p. 97-99 °C. 1H NMR (300 MHz, CDCl3) δ 7.77 (d, J=8.9 Hz, 1H, Ar-H), 7.45 (dd, J=8.4, 5.5 Hz, 2H, Ar-H), 7.38 (d, J=2.5 Hz, 1H, Ar-H), 7.22-7.04 (m, 3H, Ar-H), 6.05 (s, 1H, pyrazole-H), 5.10 (s, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 164.09 (s), 160.82 (s), 159.34 (s), 155.99 (s), 151.79 (s), 146.06 (s), 142.11 (s), 134.05 (s), 132.40 (s), 129.27 (d, J=8.1 Hz), 122.81 (s), 115.47 (d, J=14.5 Hz), 109.95 (s), 105.59 (s), 69.89 (s), 13.72 (s), 13.52 (s). IR (KBr) cm-1: 1604.77 (C=N); 1573.91 (C=C). MS-EI m/z 354 (M+H+). Anal. Calcd. for C19H16FN3OS: C, 64.57; H, 4.56; N, 11.89. Found: C, 64.77; H, 4.59; N, 11.66.
6-((2-chlorobenzyl)oxy)-2-(3,5-dimethyl-1H-pyrazol-1-yl)benzo[d] thiazole 6j: yield: 52.52%. m.p. 108-110°C. 1H NMR (300 MHz, CDCl3) δ 7.79 (dd, J=8.8, 2.6 Hz, 1H, Ar-H), 7.67-7.58 (m, 1H, Ar-H), 7.49-7.39 (m, 2H, Ar-H), 7.32 (dd, J=6.3, 3.1 Hz, 2H, Ar-H), 7.21-7.10 (m, 1H, Ar-H), 6.05 (s, 1H, pyrazole-H), 5.25 (s, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.33 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 159.41 (s), 155.89 (s), 151.78 (s), 146.17 (s), 142.12 (s), 134.24 (d, J=22.1 Hz), 132.56 (s), 129.32 (s), 129.13-129.04 (m), 128.85 (d, J=21.1 Hz), 126.89 (s), 122.83 (s), 115.30 (s), 109.93 (s), 105.77 (s), 67.74 (s), 13.73 (s), 13.53 (s). IR (KBr) cm-1: 1602.82 (C=N), 1573.91 (C=C). MS-EI m/z 370 (M+H+). Anal. Calcd. for C19H16ClN3OS: C, 61.70; H, 4.36; N, 11.36. Found: C, 61.81; H, 4.26; N, 11.54.
6-((3-chlorobenzyl)oxy)-2-(3,5-dimethyl-1H-pyrazol-1-yl)benzo[d] thiazole 6k: yield: 78.96%. m.p. 112-114°C. 1H NMR (300 MHz, CDCl3) δ 7.86-7.73 (m, 1H, Ar-H), 7.56-7.47 (m, 1H, Ar-H), 7.46-7.30 (m, 4H, Ar-H), 7.18-7.05 (m, 1H, Ar-H), 6.06 (s, 1H, pyrazole-H), 5.13 (s, 2H, -OCH2-), 2.78 (s, 3H, pyrazole-CH3), 2.33 (s, 3H, pyrazole- CH3). 13C NMR (75 MHz, CDCl3) δ 159.52 (s), 155.95 (s), 151.92 (s), 146.27 (s), 142.24 (s), 138.83 (s), 134.57 (s), 134.18 (s), 129.91 (s), 128.20 (s), 127.45 (s), 125.38 (s), 122.96 (s), 115.42 (s), 110.08 (s), 105.75 (s), 69.80 (s), 13.86 (s), 13.66 (s). IR (KBr) cm-1: 1602.85 (C=N), 1573.91(C=C). MS-EI m/z 370 (M+H+). Anal. Calcd. for C19H16ClN3OS: C, 61.70; H, 4.36; N, 11.36. Found: C, 61.85; H, 4.19; N, 11.41.
6-((4-chlorobenzyl)oxy)-2-(3,5-dimethyl-1H-pyrazol-1-yl)benzo[d] thiazole 6l: yield: 70.62%. m.p. 110-112°C. 1H NMR (300 MHz, CDCl3) δ 7.77 (d, J=8.9 Hz, 1H, Ar-H), 7.42 (d, J=9.8 Hz, 4H, Ar-H), 7.37 (d, J=2.5 Hz, 1H, Ar-H), 7.10 (dd, J=8.9, 2.5 Hz, 1H, Ar-H), 6.05 (s, 1H, pyrazole-H), 5.11 (s, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.32 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 159.49 (s), 156.02 (s), 151.93 (s), 146.22 (s), 142.24 (s), 135.25 (s), 134.17 (s), 133.88 (s), 128.81 (s), 122.95 (s), 115.45 (s), 110.09 (s), 105.73 (s), 69.87 (s), 13.86 (s), 13.66 (s). IR (KBr) cm-1: 1604.77 (C=N); 1573.91 (C=C). MS-EI m/z 370 (M+H+). Anal. Calcd. for C19H16ClN3OS: C, 61.70; H, 4.36; N, 11.36. Found: C, 61.79; H, 4.30; N, 11.45.
6-((2,6-dichlorobenzyl)oxy)-2-(3,5-dimethyl-1H-pyrazol-1- yl)benzo[d] thiazole 6m: yield: 52.80%. m.p. 128-130°C. 1H NMR (300 MHz, CDCl3) δ 7.79 (dd, J=8.9, 1.9 Hz, 1H, Ar-H), 7.47 (d, J=2.3 Hz, 1H, Ar-H), 7.44-7.24 (m, 3H, Ar-H), 7.21-7.11 (m, 1H, Ar-H), 6.05 (s, 1H, pyrazole-H), 5.36 (s, 2H, -OCH2-), 2.78 (s, 3H, pyrazole-CH3), 2.33 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 159.59 (s), 156.37 (s), 151.87 (s), 146.47 (s), 142.25 (s), 137.07 (s), 134.14 (s), 132.06 (s), 130.77 (s), 130.46 (s), 128.50 (s), 122.91 (s), 115.79 (s), 115.38 (s), 110.01 (s), 106.21 (s), 66.13 (s), 13.76 (s), 13.59 (s). IR (KBr) cm-1: 1602.85 (C=N); 1573.91 (C=C). MS-EI m/z 404 (M+H+). Anal. Calcd. for C19H15Cl2N3OS: C, 56.44; H, 3.74; N, 10.39. Found: C, 56.63; H, 3.90; N, 10.08.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-((4-(trifluoromethyl)benzyl) oxy)benzo[d]thiazole 6n: yield: 50.12%. m.p. 142-144°C. 1H NMR (300 MHz, CDCl3) δ 7.79 (d, J=9.0 Hz, 1H, Ar-H), 7.76 (s, 1H, Ar-H), 7.67 (d, J=7.3 Hz, 1H, Ar-H), 7.62 (s, 1H, Ar-H), 7.56 (d, J=7.5 Hz, 1H, Ar-H), 7.40 (d, J=2.4 Hz, 1H, Ar-H), 7.13 (dd, J=9.0, 2.4 Hz, 1H, Ar- H), 6.05 (s, 1H, pyrazole-H), 5.19 (s, 2H, -OCH2-), 2.78 (s, 3H, pyrazole-CH3), 2.33 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 159.57 (s), 155.89 (s), 151.97 (s), 146.34 (s), 142.26 (s), 137.79 (s), 134.19 (s), 131.23 (s), 130.61 (s), 129.11 (s), 127.40 (s), 124.87 (dd, J=7.4, 3.7 Hz), 124.10 (dd, J=7.4, 3.6 Hz), 122.99 (s), 115.38 (s), 110.10 (s), 105.74 (s), 69.82 (s), 13.84 (s), 13.64 (s). IR (KBr) cm-1: 1604.77 (C=N), 1573.91 (C=C). MS-EI m/z 404 (M+H+). Anal. Calcd. for C20H16F3N3OS: C, 59.55; H, 4.00; N, 10.42. Found: C, 59.72; H, 3.88; N, 10.58.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-((4-methylbenzyl)oxy) benzo[d]thiazole 6o: yield: 72.52%. m.p. 110-112°C. 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J=8.9 Hz, 1H, Ar-H, Ar-H), 7.37 (d, J=6.8 Hz, 3H, Ar-H), 7.23 (d, J=8.0 Hz, 2H, Ar-H), 7.11 (dd, J=8.8, 2.2 Hz, 1H, Ar-H), 6.05 (s, 1H, pyrazole-H), 5.10 (s, 2H, -OCH2-), 2.77 (s, 3H, pyrazole-CH3), 2.39 (s, 3H, Ar-CH3), 2.32 (s, 3H, pyrazole-CH3). 13C NMR (75 MHz, CDCl3) δ 159.34 (s), 156.40 (s), 151.85 (s), 146.04 (s), 142.22 (s), 137.89 (s), 134.15 (s), 133.72 (s), 129.34 (s), 127.70 (s), 122.89 (s), 115.61 (s), 110.03 (s), 105.67 (s), 70.64 (s), 21.26 (s), 13.87 (s), 13.68 (s). IR (KBr) cm-1: 1606.70 (C=N), 1577.77 (C=C). MS-EI m/z 350 (M+H+). Anal. Calcd. for C20H19N3OS: C, 68.74; H, 5.48; N, 12.02. Found: C, 68.85; H, 5.56; N, 11.93.
Pharmacology
All compounds were evaluated for anticonvulsant activities with KunMing mice in the 18-22 g weight range purchased from the Laboratory of Animal Research, Bengbu Medical College. The animals were maintained on a 12 h light/dark cycle and allowed free access to food and water, except during the time they were removed from their cages for testing. The experimental substances were dissolved in DMSO with 30% PEG 400 and administered intraperitoneally (i.p.) in a volume of 0.1 ml/20g body weight. The test method with reference to the Antiepileptic Drug Development (ADD) program [15,16].
MES screening test: Seizures were elicited with a 60-Hz alternating current of 50 mA intensity applied via corneal electrodes for 0.2 s. Protection against the spread of MES-induced seizures was defined as the abolition of the hind leg, and tonic maximal extension component of the seizure. the MES test was performed at thirty minutes after compound administration.
Neurotoxicity screening test: The neurotoxicity of the compounds was measured in mice using the rota-rod test. Mice were tested on a knurled plastic rod (diameter, 3.2 cm) rotating at 6 rpm for 1 min, at 30 min after compound administration. Neurotoxicity was measured by the inability of the animal to maintain equilibrium on the rod for at least 1 minute in each of the trials.
Measurement of cell viability by MTT assay: MDA-MB-231 cells (human breast cancer cells) were cultured in minimum essential medium (MEM, also obtained from Gibco) supplemented with 10% fetal bovine serum (FBS, Millipore, USA) and maintained at 37°C in a humidified incubator with 5% CO2. The cells were obtained from Shanghai Cell Bank, Chinese Academy of Sciences (CAS). The compounds were dissolved in dimethyl sulfoxide (DMSO, BIOSHARP, Hefei, China). The blank group was treated with DMSO only under identical conditions.
The 231 cells were seeded at 1 × 105 cells/ml in 96-well plates containing 100 μl of DMEM medium with 10% FBS and incubated overnight. Compounds 4 and 6a-6o was dissolved in DMSO, and the cells were pretreated with 20 μM concentration of the compounds for 24 h. Then the media was removed and cells were cultured with MTT solution (5 mg/ml) [3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide] (Sigma, St. Louis, MO, USA) for 4 h. The viable cells converted MTT to formazan, which generated a blue purple color after dissolving in 100 μl of DMSO. The absorbance at 570 nm was measured by Multiskan GO.
ScPTZ seizures screening test: At 1.5 h after the administration of the test compound, 85 mg/kg PTZ, which 100% of the mice showed tonic-clonic seizure and deaths, dissolved in saline was administered sc. The number of animals in each group tested was five. The animals placed in individual cages and observed for 1 h. The number of tonicclonic seizure and deaths in mice were noted.
Results and Discussion
Chemistry
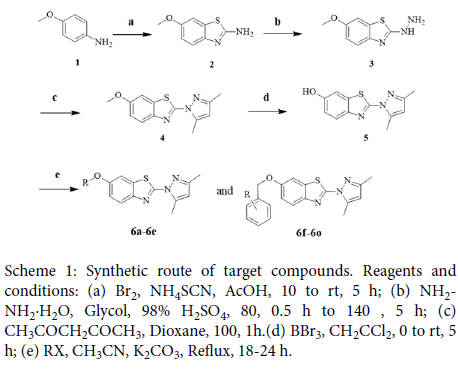
All the target compounds were synthesized according to Scheme 1. The starting material, 4-methoxyaniline (compound 1), was reacted with ammonium sulfocyanate by liquid bromine catalyzed to obtain 6- methoxy-1,3-benzothiazol-2-amine (compound 2). Then compound 2 was treated with hydrazine hydrate in the presence of sulfuric acid (98% water solutions) to produce 2-hydrazinyl-6- methoxybenzo[d]thiazole (compound 3) [17]. 2-(3,5-dimethyl-1Hpyrazol- 1-yl)-6-methoxybenzo[d]thiazole (compounds 4) was synthesized from compound 3 and acetylacetone at 100 ºC in dioxane. Then compound 4 was reacted with hydrobromic acid (48% water solutions) to obtain compound 5 [18] Finally, compound 5 was converted to the target compounds 6a-6o by reacting with appropriate alkanol and substituted phenol in acetonitrile under reflux conditions [19]. Their chemical structures were characterized using 1H NMR, 13C NMR, IR and EI-MS. A detailed overview of their physical and analytical data has been provided in the experimental part.
Pharmacology and structure-activity relationship
The anticonvulsant activity evaluation of all the compounds were determined using the MES test, which is a mechanism-independent animal seizure model that enables the identification of compounds preventing seizure spread [20]. It should be noted that the MES model remains the most useful tool for the identification of new anticonvulsants, despite significant advances in epilepsy research in the past several years [21]. The MES seizure model was used for preliminary screening of compounds 4, 5 and 6a-6o. They were administered to mice intraperitoneally (i.p.) at the fixed dose of 100 mg/kg and the anticonvulsant protection was observed at two posttreatment times: 0.5 and 4 h. The method applied here allowed the determination of the number of animals (in a group consisting of three mice) protected against electrically induced seizures as well as the estimation of the time course of anticonvulsant activity including quick-acting (0.5 h) or long-acting properties (4 h). The results are presented in Table 1. The preliminary pharmacological screening revealed that four compounds (6a, 6b, 6g and 6m) showed about 33% anticonvulsant protection in the 0.5 h period at the dose of 100 mg/kg, but none of them had activity in the 4 h period at the same dose. None of all the compounds presented neurotoxicity at the dose of 300 mg/kg.
| Compound | R | MESa (100 mg/kg) | MES (300 mg/kg) | TOXb (300 mg/kg) | |||
|---|---|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h | 4 h | ||
| 4 | -CH3 | -c | - | 0/3d | 0/3 | 0/3 | 0/3 |
| 5 | H | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6a | n-C4H9 | 1/3 | 0/3 | 2/3 | 0/3 | 0/3 | 0/3 |
| 6b | n-C5H11 | 1/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 |
| 6c | n-C6H13 | - | - | 1/3 | 0/3 | 0/3 | 0/3 |
| 6d | n-C7H15 | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6e | n-C8H17 | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6f | -CH2C6H4 | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6g | -CH2C6H4 (o-F) | 1/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 |
| 6h | -CH2C6H4 (m-F) | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6i | -CH2C6H4 (p-F) | - | - | 1/3 | 0/3 | 0/3 | 0/3 |
| 6j | -CH2C6H4 (o-Cl) | - | - | 1/3 | 0/3 | 0/3 | 0/3 |
| 6k | -CH2C6H4 (m-Cl) | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6l | -CH2C6H4 (p-Cl) | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6m | -CH2C6H3 (2,6-2Cl) | 1/3 | 0/3 | 2/3 | 1/3 | 0/3 | 0/3 |
| 6n | -CH2C6H4 (p-CF3) | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| 6o | -CH2C6H4 (p-CH3) | - | - | 0/3 | 0/3 | 0/3 | 0/3 |
| blank | - | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
b Neurotoxicity test
c Not tests
d n1/n2: the animals protected/the animals tested.
Table 1: Anticonvulsant activities screening (MES test) in mice at the dose of 100 mg/kg, 300 mg/kg and neurotoxicity screening at the dose of 300 mg/kg.
On the basis of the preliminary screening results, compounds 6a, 6b, 6g and 6m were subjected to the next phase of trials regarding the quantification of their anticonvulsant activity in mice. The results of the quantitative tests are reported in Table 2, along with the data from sodium valproate as the positive drug control group. As shown in Table 2, all of the three compounds showed similar or stronger anticonvulsant activities than sodium valproate (the ED50 was 216.9 mg/kg). Especially, compound 6g showed better activities (the ED50 was 160.4 mg/kg) and higher safety (the PI was 2.74) than other compounds, including the positive drug.
| Compound | ED50e MES (mg/kg) | TD50f (mg/kg) | PIg |
|---|---|---|---|
| 6a | 214.7 (135.4-340.4) h | 409.1 (357.9-467.6) | 1.91 |
| 6b | 190.9 (124.1-293.8) | 435.2 (333.1-568.6) | 2.28 |
| 6g | 160.4 (113.0-227.6) | 440.1 (372.9-519.4) | 2.74 |
| 6m | 214.4 (128.96-356.4) | 432.1 (330.7-564.6) | 2.02 |
| VPA j | 216.9 (207.5-226.3) | 372.9 (356.0-389.8) | 1.72 |
fTD50: median toxic dose eliciting minimal neurological toxicity in 50% of animals.
gPI: protective index (TD50/ED50)
h95% confidence intervals given in parentheses.
Table 2: Quantitative Pharmacological Parameters ED50, TD50, and PI Values in Mice.
The following structure-activity relationships (SAR) were obtained, while analyzing the preliminary screening of the synthesized compounds. Among the six alkyl chain-substituted derivatives, 6a and 6b showed better activities. And 6b was also a little better than 6a. However, with the increase in length, the activities of the compounds did not increase. Compound 6f, substituted with a benzyloxy group at the 6-position of the benzothiazole core, showed no activity at 100 mg/kg. But when the F, Cl and so on groups were subsequently added onto the benzyloxy group, some compounds showed activities. Substituent position on the phenyl ring also influenced anticonvulsant activity in the 6-fluorobenzyl derivatives as o-F > p-F > m-F. However, o-Cl showed activity at the dose of 300 mg/kg and all the 6- chlorobenzyl derivatives showed no activity at the dose of 100 mg/kg. Only compound 6m, with two added chlorine atoms, showed activity in the 0.5 h period. When the substituent was changed to the electrodonating substituent (6o), the anticonvulsant activity disappeared.
In vitro cytotoxicity
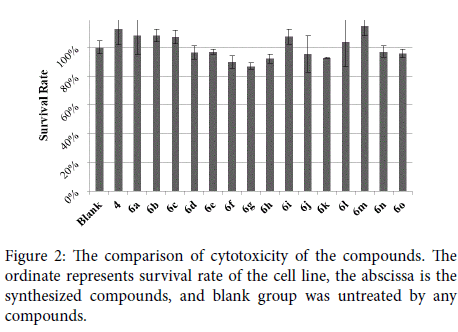
As we known that clinically antiepileptic drug normally had a narrow therapeutic index. All of the synthesized compounds (6a-6o) were evaluated for their cytotoxicity against cell lines to demonstrate the safety. Form Figure 2, we could see that the synthesized compounds showed little or no cytotoxicity to the cell line at 20μM. The survival rate of the cells treated with target compounds exceeded 85%. Hence it could be deduce that this series of compounds was also safety to the cell line. Further study towards safety of these compounds would be tested at the next step.
Chemical-induced seizure tests speculated on the mechanism
The scPTZ model employs chemically (pentylenetetrazol) induced myoclonic seizures and allows the identification of agents raising the seizure threshold. This test is related to human generalized absence seizures [22]. Thus, the compounds, which showed anticonvulsant activities in the preliminary screening at 300 mg/kg, were infused to stomach of mice to observe the effect on the convulsion of the pentylenetetrazol model. As shown in Table 3, the results were similar to the MES model, compounds 6b, 6c and 6g showed better activity against PTZ in varying degrees. Especially, compound 6g (o-F) showed about 80% anticonvulsant protection in the 1.5 h period at the dose of 100 mg/kg. And although the compounds 6a, 6j and 6m had weaker effective against scPTZ model, they were able to resist PTZ-induced lethality to some extent in mice. Research shows, PTZ has been reported to produce seizures by inhibiting γ-aminobutyric acid (GABA) neurotransmission [22,23]. GABA is the main inhibitory neurotransmitter in the brain, and is widely implicated in epilepsy. Inhibition of GABAergic neurotransmission or activity has been shown to promote and facilitate seizures [24]. Further study towards determining the mechanisms of action of these compounds is currently underway in our laboratory.
| Compound | R | Doses | Clonic seizures | Lethality |
|---|---|---|---|---|
| (mg/kg) | (%) | (%) | ||
| blank | - | 100 | 100 | 100 |
| 6a | n-C4H9 | 100 | 60 | 40 |
| 6b | n-C5H11 | 100 | 40 | 40 |
| 6c | n-C6H13 | 100 | 40 | 20 |
| 6g | -CH2C6H4 (o-F) | 100 | 20 | 20 |
| 6i | -CH2C6H4 (p-F) | 100 | 60 | 40 |
| 6j | -CH2C6H4 (o-Cl) | 100 | 100 | 60 |
| 6m | -CH2C6H3 (2,6-2Cl) | 100 | 60 | 20 |
Table 3: Effects of the target compounds on s.c. pentylenetetrazolinduced seizures in mice.
Conclusion
In the present study, we described the synthesis and anticonvulsant activity evaluation of 2-(3,5-dimethyl-1H-pyrazol-1-yl)-6- alkoxybenzo[d]thiazole (6a-6e) or 2-(3,5-dimethyl-1H-pyrazol-1- yl)-6-benzylbenzo[d]thiazole (6f-6o). All the compounds were tested for their anticonvulsant activity and neurotoxicity using the MES and rotarod tests at the dose of 100 mg/kg. Among them, compounds 6g showed the most active in this study in the MES assay and least neurotoxicity, and that made 6g had higher safety than marketed drugs sodium valproate. In addition, all the synthetic compounds, including 6g, had shown little cytotoxicity in the MTT test. It was well to be reminded that compound 6g demonstrated antagonistic activity against seizures induced by pentylenetetrazol. It suggested that compound 6g maybe exert anticonvulsant activity through effecting GABAergic neurotransmission in the brain.
Acknowledgments
This work was supported by Education Department of Anhui Natural Science Research Project (KJ2018A1006) and Natural Science Fund projects of Bengbu Medical College (BYKY1715ZD, BYKY1878) under Grant.
References
- Nieuwenhuyse BV, Raedt R, Sprengers M, Dauwe I, Gadeyne S (2015) The systemic kainic acid rat model of temporal lobe epilepsy: Long-term EEG monitoring. Brain Research 1627: 1-11.
- Wang ZH, Wang H, Guo YZ, Wei ZW, Ma L (2012) Synthesis, structure and fungicidal activity of organotin 1-aryl-3,5-dimethylpyrazole-4-carboxylates. Chinese Journal of Inorganic Chemistry 28: 1926-1934.
- Reddy VG, Reddy TS, Nayak VL, Prasad, B, Reddy AP (2016) Design, synthesis and biological evaluation of N-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-1,3-diphenyl-1H-pyrazole-4-carboxamides as CDK1/Cdc2 inhibitors. Eur J Med Chem 122: 164-177.
- Hu DK, Zhao DS, He M, Jin HW, Tang YM, et al. (2018) Synthesis and bioactivity of 3,5-dimethylpyrazole derivatives as potential PDE4 inhibitors. Bioorg Med Chem Lett 28: 3276-3280.
- Kaymakcioglu BK, Rollas S, Aricioglu FK (2003) In vivo metabolism of N-phenyl-N'-(3,5-dimethylpyrazole-4-yl) thiourea in rats. Eur J Drug Metab Pharmacokinet 28: 273-278.
- Åžahan S, Åžahin U, BaÅŸaran M, Uzun O, GuneÅŸ A (2017) Determination of 3, 5-dimethylpyrazolium glyceroborate nitrification inhibitor in nitrogen fertilizer samples: HPLC-DAD method development and validation for 3, 5-dimethylpyrazole. J Chromatogr B Analyt Technol Biomed Life Sci 1069: 277-281.
- Agarwal S, Agarwal DK, Gautam N, Agarwal K, Gautam DC (2014) Synthesis and in vitro antimicrobial evaluation of benzothiazole incorporated thiazolidin-4-ones derivatives. J Korean Chem Soc 58: 33-38.
- Singh MK, Tilak R, Nath G, Awasthi SK, Agarwal A (2013) Design; synthesis and antimicrobial activity of novel benzothiazole analogs. Eur J Med Chem 63: 635-644.
- Stone EL, Citossi F, Singh R, Kaur B, Gaskell M (2015) Antitumour benzothiazoles. Part 32: DNA adducts and double strand breaks correlate with activity; synthesis of 5F203 hydrogels for local delivery. Bioorg Med Chem 23: 6891-6899.
- Ma J, Bao G,Wang L, Li W, Xu B, et al. (2015) Design; synthesis; biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. European Journal of Medicinal Chemistry 96: 173-186.
- Ugale VG, Patel HM, Wadodkar SG, Bari SB, Shirkhedkar AA, et al. (2012) Quinazolino-benzothiazoles: fused pharmacophores as anticonvulsant agents. European Journal of Medicinal Chemistry 53: 107-113.
- Siddiqui N, Rana A, Khan SA, Bhat MA, Haque SE (2007) Synthesis of benzothiazole semicarbazones as novel anticonvulsants -the role of hydrophobic domain. Bioorganic & Medicinal Chemistry Letters 17: 4178-4182.
- Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA (1978) Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 19: 409-428.
- Porter RJ, Cereghino JJ, Gladding GD, Hessie BJ, Kupferberg HJ, et al. (1984) Antiepileptic Drug Development Program. Cleve Clin Q 51: 293-305.
- Deng XQ, Song MX, Wei CX, Li FN, Quan ZS (2010) Synthesis and anticonvulsant activity of 7-alkoxy-triazolo-[3, 4-b]benzo[d]thiazoles. Med Chem 6: 313-320.
- Liu DC, Deng XQ, Wang SB, Quan ZS (2014) Synthesis and anticonvulsant activity evaluation of 7-alkoxy[1, 2, 4]triazolo[3, 4-b]benzothiazol-3(2H)-ones. Arch Pharm 347: 268-275.
- Chen YL, Hung HM, Lu CM, Li KC, Tzeng CC (2004) Synthesis and anticancer evaluation of certain indolo[2,3-b]quinoline derivatives. Bioorg Med Chem 12: 6539-6546.
- Rogawski MA (2006) Molecular targets versus models for new antiepileptic drug discovery. Epilepsy Res 68: 22-28.
- CastelBranco MM, Alves GL, Figueiredo IV, Falcao AC, Caramona MM (2009) The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find. Exp Clin Pharmacol 31: 101-106.
- Loscher W (2011) Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20: 359-368.
- Okada R, Negishi N, Nagaya H, (1989) The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol-induced seizures. Brain Res 480: 383-387.
- Gale K (1992) GABA and epilepsy: basic concepts from preclinical research. Epilepsia 33: S3-S12.
Citation: Liu D, Cheng X, Wang Y, Wu C (2019) Novel Benzo[d]thiazole with Dimethylpyrazol Derivatives: Design, Synthesis and Biological Evaluation. Toxicol Open Access 5: 141. DOI: 10.4172/2476-2067.1000141
Copyright: © 2019 Liu D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4219
- [From(publication date): 0-2019 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 3272
- PDF downloads: 947