Ocular syphilis: when love blinds-Epidemiological, Clinical, Biological and Evolutionary Aspects of 14 Patients-22 Eyes Cohort (2008-2015)
Received: 15-Feb-2018 / Accepted Date: 20-Feb-2018 / Published Date: 23-Feb-2018 DOI: 10.4172/2476-213X.1000124
Abstract
Introduction: Ocular Syphilis is a rare and poorly described pathology. Syphilis renewal of activity leads ophthalmologists, internal medicine and infectious diseases experts to consider this diagnosis in front of various symptoms, among which ocular proteiform involvement.
Objective: Describe epidemiological, clinical, paraclinical and evolutionary aspects of syphilis with ocular involvement. Seek out any differences between HIV-positive and HIV-negative populations.
Material and methods: Retrospective, descriptive, non-comparative study about all the cases of ocular syphilis diagnosed in a tertiary academic hospital in Nancy, France, from 2008 to 2015.
Results: 14 patients among which 10 HIV-free and 4 HIV co-infected patients, representing 22 eyes, were diagnosed for ocular involvement, all in the secondary stage of syphilis. Mean age was 46.2 ± 6.2 years (Range: 39-61 years) and 86% were male (n=12). 65% reported having multiple sex partners (n=9), 14.3% using intravenous drug (n=2). 29% were known co-infected with HIV at syphilitic uveitis diagnosis (n=4). At the time of uveitis diagnosis, none of the HIV-positive patients were treated with HAART. Only one patient had a CD4 LT count less than 250 mm-3. Ocular involvement was inaugural of syphilis in 71.4% of cases (n=10). The average diagnosis time was 63.1 ± 70, 3 days (Median=33.5 days, Range: 4-207 days). 57.1% of cases had an extra-ocular involvement at initial presentation. Meningitis was identified in 92.1% of cases (n=11 of 12 tested). The average cephalospinal fluid (CSF) cellularity was 54 ± 73.4 cells/mm3 (Range: 4-260 cells/mm3). CSF was mostly lymphocytic in all cases, up to 72 ± 19.1% (Range: 16.1-89.3%), except for an HIV co-infected patient who presented a majority of neutrophils. 92.9% of patients showed a decrease in Best Corrected Visual Acuity (Mean=0.51 ± 0.61 LogMAR; about 4.8/10 on Monoyer scale). All patients presented with uveitis, bilateral in 57% cases (n=8) with anterior, intermediate and posterior involvement in respectively 43% (n=6), 50% (n=7) and 93% of cases (n=13). 36% of patients had panuveitis (n=5). No isolated anterior or intermediate uveitis was observed. 43% of patients had an isolated posterior uveitis (n=6). Macular edema and retinal vasculitis were reported in 21% (n=3) and 29% of patients (n=4). 21% of patients had optic nerve damage papillitis type (n=3). 2 patients showed scleritis and one patient was diagnosed after onset of hypopion revealing anterior uveitis. Median follow up time was 3 months ± 488 days (Range: 21-1825 days). Ocular inflammation and visual acuity improved after a neurosyphilis-like parenteral antibiotherapy regimen for all patients when diagnosis was made promptly (Mean=0.39 ± 0.71 LogMAR; about 7/10 on Monoyer scale). Comparison according to HIV co-infection status did not show any clinical presentation, severity or outcome significant differences. VDRL in cephalospinal fluid was more likely to be positive in HIV patients with ocular involvement.
Conclusion: Ocular syphilis should, because of its potential gravity, its accessibility to a simple and effective treatment be considered in range of the uveitis causes and in the assessment of any new syphilis whatever the patient’s status toward an HIV co-infection.
Keywords: Ocular syphilis; Inflammatory diseases; Autoimmune; Vasculitis
Abbreviations
BCVA: Best Corrected Visual Acuity; CHU: Centre Hospitalo-Universitaire; CMIA: ChemIluminescent Microparticle Assay; CRP: C-Reactive Protein; CSF: CephaloSpinal Fluid; ELISA: Enzyme Linked Immunosorbent Assay; HAART : Highly Active Antiretroviral Therapies; HIV: Human Immunodeficiency Virus; IDSA: Infectious Diseases Society of America; IOP: Intra-Ocular Pressure; IV: Intravenous; MRI: Magnetic Resonance Imaging ; OCT: Optical Coherence Tomography; RPR: Rapid Plasma Reagin; STI: Sexual Transmitted Infection; TPHA: Treponema Pallidum Hemagglutination Assay; UK: United Kingdom; USA: United States of America; VA: Visual Acuity; VDRL: Veneral Disease Research Laboratory
Introduction
Syphilis is a sexually transmitted infection (STI) by the spirochete Treponema pallidum . More marginal contamination modes are described, including congenital by transplacental contamination or after a non-venereal close contact with infected lesions. This is a systemic, chronic, non-immunizing affection with potential to affect the whole organism among which the eyes structures [1].
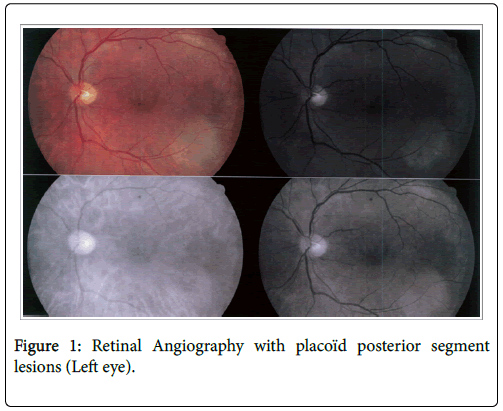
After a decline linked to the advent of penicillin, syphilis increases sharply in Western countries or so-called "developed" since the 2000’s. This resurgence affects without distinction hetero or homosexual subjects [2]. In France, its prevalence was multiplied by 6 between 1998 and 2001 [3]. In the UK, 50 cases per 1 million inhabitants were reported between 2009 and 2011 [4]. A similar trend is observed in the USA where its incidence has increased significantly between 2001 and 2009 [5]. A doubling of its prevalence has been observed in some regions of the US between 2000 and 2013 (5.3 cases/100,000 populations vs. 2.1 cases/100,000 inhabitants) where the homosexual male population aged 20-24 was particularly affected, accounting for 91.9% of reported cases of early syphilis in 2013 [6]. This comeback seems related to several phenomena: end of its mandatory reporting (since 2000 in France), its non-immunizing character source of reinfections but also the laxness in protection measures against STIs since the advent of Highly Active Antiretroviral Therapies (HAART) [3,7]. Outbreaks have been reported in several European countries [8]. diagnosis of syphilis relies on combination of a clinical context and serological tests [9]. Its natural history allows to distinguish early stages (primary, secondary or early latent), late stage and neurosyphilis [10]. Ophthalmic involvement is rare, occurring in 5-10% of syphilis cases, most often during secondary or tertiary stage [11,12]. It can also be seen in congenital cases [13]. Frequency of eye lesions can reach 10% in patients with secondary syphilis [14]. Ocular involvement of syphilis is nonspecific and usually bilateral [15-18]. All ocular structures can be reached but uveitis is the most common case [19]. Episcleritis, scleritis and keratitis have also been described [20]. Sometimes subclinical, ocular syphilis requires, not staying unknown, certain detailed ophtalmoscopic investigations with fundus photography possibly completed by retinal angiography (Figure 1). Ophthalmic involvement is often considered classifying for the diagnosis of neurosyphilis for most writers despite the lack of large consensus. The close anatomical relationship between ocular structures and central nervous system is behind this consideration [21]. Perspective of a full recovery, if diagnosis and appropriate therapeutic management are made promptly, must lead to increased vigilance from different medical experts to deal with this great imitator [22]. Main objective of this work is to describe epidemiological, clinical, paraclinical and evolutionary aspects of syphilis with ocular involvement encountered in our care structure. Secondary objective is to seek out the existence of any differences between HIV-positive and HIV-negative populations.
Material And Methods
Studied population
We conducted a retrospective, descriptive study, involving a case series of patients treated at the university hospital center (CHU) in Nancy, France, in internal medicine, infectious and tropical diseases, and ophthalmology departments after a diagnosis of syphilitic ocular involvement between 2008 and 2015.
Ophthalmologic diagnosis was held on the basis of compatible clinical history, association of ophthalmologic abnormalities documented in a specialized consultation and seropositive syphilis testing. Syphilis serological screening tests and diagnosis confirmation relied on combination of a non-treponemal test: VDRL (Veneral disease research laboratory), RPR (Rapid Plasma Reagin) and specific treponemal tests: TPHA (Treponema pallidum Hemagglutination Assay), ELISA (Enzyme Linked Immunosorbent Assay) or CMIA (Chemiluminescent Microparticle Assay). Differential diagnosis of ocular inflammatory diseases were excluded after a record including a chest X-ray, blood tests (Complete blood count, C-reactive protein (CRP), hepatic and renal assessments, angiotensin converting enzyme, HLA typing B27, Quantiferon, toxoplasmosis and HIV serologies). The data collection stayed confidential throughout its duration. It was made with the agreement of the competent administrative authorities at the Nancy CHU.
Data collection
Data from all patients diagnosed and followed in our structure for ocular involvement related to syphilis were retrospectively collected from the ophthalmology, internal medicine and infectious diseases departments. Epidemiological characteristics were recorded for each patient on parameters such as age, gender, existence of HIV coinfection, reckless behavior for STI transmission, sexual orientation, past history of STIs including syphilis. Clinical information collected during the initial consultation allowed identification of suggestive signs for systemic inflammatory, autoimmune, infectious or neoplasic disease. Delays between onset of symptoms and seeking care as well as from hospital admission to definitive diagnosis were calculated when possible. Bilateral ophthalmological specialized examination included a determination of the best corrected visual acuity (BCVA) according to Monoyer scale transcribed in LogMAR, intraocular pressure (IOP) in mmHg, a biomicroscopic examination with anterior segment evaluation and dilated fundus. Tyndall and/or hyalitis were quoted. Additional tests were performed if a posterior segment infringement was highlighted: fluorescein angiography with indocyanine green looking for chorioretinitis or vasculitis, with optical coherence tomography (OCT) in order to assess and allow follow up of any papular or macular edema. Ophthalmologic findings were expressed for each eye. When available, results of further cephalospinal analysis (CSF) and brain imaging by MRI were collected. Therapeutic modalities were identified in all patients in terms of antibiotic therapy and eventually systemic corticotherapy. Evolution after treatment: assessment of primary endpoint was based on measuring the best recovered (VA) during a specialized consultation. Other ocular and extra-ocular abnormalities observed during multidisciplinary followup were also traced. Serological monitoring of non-treponemal tests was compiled to establish serological healing. In case of relapse, search for an explanatory factor was specified when necessary.
Statistical analysis
Statistical analysis was performed thanks to the XLSTAT software (Version 2015.4.01.20219 Addinsoft Copyright 1995 to 2015). Quantitative and categorical variables were compared using respectively the Student's, Chi2 or Fisher's exact tests. Regression analyzes on existence of possible confounding factors could not be achieved due to the insufficient samples size.
Results
Clinical characteristics of the cohort at the initial presentation: 14 cases (22 eyes) met inclusion criteria and were analyzed in this study (Table 1). The average age at initial presentation was 46.2 ± 6.2 years (Median=44, Range: 39-61). 86% of cases were male (n=12). 65% have reported having multiple sex partners (n=9), 14.3% have reported using intravenous drug (n=2). 29% were known co-infected with HIV at syphilitic uveitis diagnosis (n=4). HIV seropositivity discovery was secondary to ocular syphilis diagnosis in 1 patient whereas it was already known in three other cases. Average duration of HIV seropositivity was 7.5 ± 5.8 years (Median=8.9, Range: 0-12.3). Half of HIV co-infected patients had a CD4 LT count above 500 mm-3. Only one patient had a CD4 LT count under 250 mm-3 (LT CD4=42 mm-3). Average HIV viral load was 45,285 ± 89,810 copies.mL1 (Median=449.5, Range: 240-180,000). At the time of uveitis diagnosis, none of the HIV-positive patients were treated with HAART. This was a voluntary medical decision in 3 cases, a dropout treatment in one case.
| Demographics | Non HIV (n=10) | HIV (n=4) | Total (n=14) | p-value | ||
| Age, y | Average | 47.3 ± 6.8 | 44 ± 4.1 | 46.2 ± 6.2 | NS | |
| Gender, % (n) | Female | 20% (n=2) | 0% (n=0) | 14.3% (n=2) | NS | |
| Male | 80% (n=8) | 100% (n=4) | 85.7% (n=12) | |||
| Drug Use | 20% (n=2) | 0% (n=0) | 14.3% (n=2) | NS | ||
| Sexual orientation, % (n) | Heterosexual | 40% (n=4) | 50% (n=2) | 42.9% (n=6) | 0.14 | |
| Homosexual | 0% (n=0) | 50% (n=2) | 14.3% (n=2) | |||
| Bisexual | 40% (n=4) | 0% (n=0) | 28.6% (n=4) | |||
| Unknown | 20% (n=2) | 0% (n=0) | 14.3% (n=2) | |||
| Multiple sex partners, % (n) | 50% (n=5) | 100% (n=4) | 64.3% (n=9) | 0.08 | ||
| Clinical characteristics | Past history of syphilis infection, % (n) | 0% (n=0) | 50% (n=2) | 14.3% (n=2) | 0.07 | |
| STI story, % (n) | 20% (n=2) | 75% (n=3) | 35.7% (n=5) | 0.09 | ||
| Uveitis duration prior to presentation, days | Average | 61.6 | 67 | 63.1 | NS | |
| Median | 30 | 39 | 33.5 | |||
| Time to hospital diagnosis, days | Average | 29.8 | 4.5 | 22.6 | 0.16 | |
| Median | 10.5 | 3 | 8.5 | |||
| Inaugural ocular manifestation, % (n) | 80% (n=8) | 50% (n=2) | 71.4% (n=10) | 0.26 | ||
| Extra-ocular involvement, % (n) | 70% (n=7) | 25% (n=1) | 57.1% (n=8) | 0.12 | ||
| Bilateral involvement, % (n) | 50% (n=5) | 75% (n=3) | 100% (n=14) | NS | ||
| Onset, % (n) | Sudden | 50% (n=5) | 75% (n=3) | 57.1% (n=8) | NS | |
| Insidious | 50% (n=5) | 25% (n=1) | 42.9% (n=6) | |||
| Biological findings | WBC count (blood), G/L | Average | 9.6 | 6.25 | 9.04 | 0.18 |
| Range | (4.31-16.4) | (4.81-7.7) | (4.31-16.4) | |||
| CRP level, mg/L | Average | 13.7 | 14.75 | 14 | NS | |
| Median | 3 | 3.5 | 3 | |||
| Positive TPHA/ELISA/CMIA, % (n) | 100% (n=10) | 100% (n=4) | 100% (n=14) | |||
| Positive VDRL/RPR, % (n) | 100% (n=10) | 100% (n=4) | 38.5% (n=5) | 0.01 | ||
| Positive VDRL (CSF), % (n) | 20% (n=2) | 100% (n=3/3) | 57.1% (n=8) | NS | ||
| CSF abnormalities | Meningitis, % (n) | 88.9% (a) | 100% (b) | 92.1% (c) | NS | |
| WBC count (CSF), mm-3 | Average | 31.8 | 122.3 | 54.4 | NS | |
| Lymphocyte proportion on WBC count, % | 77% | 57% | 72% | NS | ||
| Proteins (CSF), g/L | Average | 0.56 | 0.78 | 0.61 | NS | |
| Glucose (CSF)/Glucose (Blood) | 0.73 | 0.77 | 0.74 |
Table 1: Demographics, clinical and biological findings (n=Patients), (8 on 9 CSF tested, 3 on 3 CSF tested, 11 on 12 CSF tested; NS: insignificant).
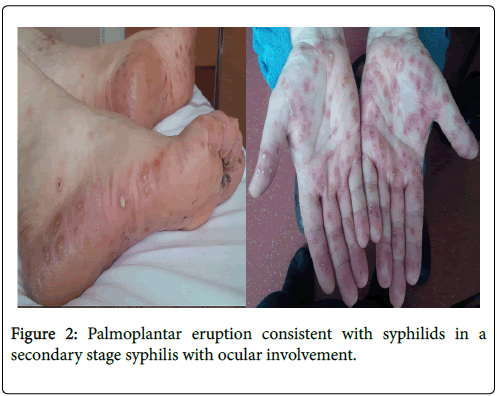
The average diagnosis time was 63.1 ± 70.3 days (Median=33.5. Range: 4-207). The average diagnosis time from first hospital evaluation was 22.6 ± 43.3 days (Median=8.5. Range: 1-173). First medical consultation was achieved at ophthalmology practice in 42.9% of cases (n=6), hospital ophthalmology department in 35.7% of cases (n=5), infectious diseases department in 14.3% of cases (n=2) and emergency’s department for one patient. All patients had positive blood treponemal (TPHA, ELISA, CMIA) and non-treponemal (VDRL, RPR) tests. Diagnosis of syphilis was made in the secondary stage for all patients. Ocular involvement was inaugural of syphilis in 71.4% of cases (n=10). 57.1% of cases (n=8) had an extra-ocular involvement at initial presentation: first of all syphilides (n=4), then syphilitic roseola (n=3) or genital wart stigmas (n=2) (Figure 2).
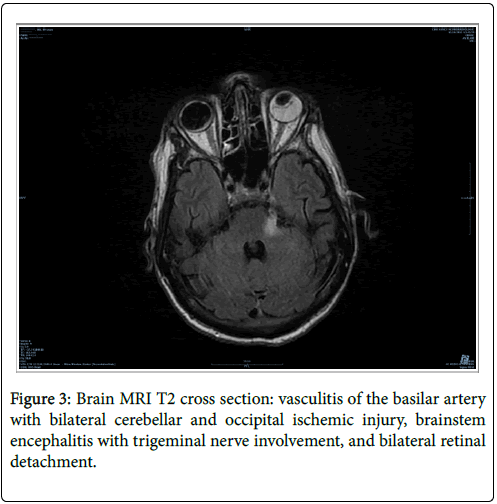
One patient had a hemiparesis and a trigeminal nerve involvement within the framework of brainstem encephalitis. Meningitis was identified in 92.1% of cases (n=11 of 12 CSF tested). The average CSF cellularity was 54 ± 73.4 cells/mm3 (Median=26.5. Range: 4-260). CSF was mostly lymphocytic in all cases, up to 72 ± 19.1% (Median=73.3, Range: 16.1-89.3), except for an HIV co-infected patient who presented a majority of neutrophils. The average CSF protein level was 0.61 ± 0.06 g/L (Median=0.56, Range: 0.3-1.3). 38.5% of tested patients had a non-treponemal test (VDRL) positive in the CSF (n=5). The average CRP level at presentation was 14 ± 6.2 mg/L (Median=3, Range: 1-80). 57.1% of patients underwent a brain MRI imaging (n=8). This proved abnormalities in a single HIV co-infected patient (Figure 3).
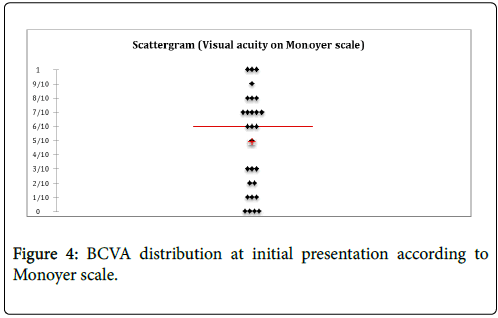
Ocular damage characteristics: 92.9% of patients (n=13) showed a decrease in AV at the time of diagnosis (Figure 4). The average BCVA at diagnosis was 0.51 ± 0.61 LogMAR (About 4.8/10 on Monoyer scale). Over 50% of eyes (n=16) had an initial VA lower or equal to 0.2 LogMAR (6.3/10 on Monoyer scale).
One patient showed bilaterally no light perception. Another patient could only count fingers at one meter. Average IOP was 14.2 ± 7 mmHg (Median=14.5, Range: 8-19).
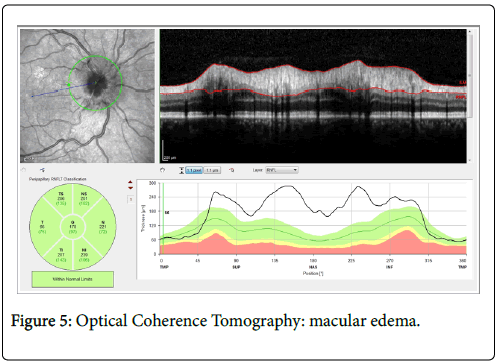
All patients showed ocular involvement with uveitis. It was bilateral in 57% cases (n=8). No isolated anterior or intermediate uveitis was observed. 43% of patients (n=6) had an isolated posterior uveitis. Anterior involvement was identified in 43% of patients (n=6), intermediate in half cases (n=7) and posterior in 93% of cases (n=13). 36% of patients had panuveitis (n=5). In case of anterior segment inflammation, Tyndall was also qualified. 18 eyes were free of anterior inflammation. 6 had a Tyndall quoted with 1 cross, one with 2 crosses and 3 eyes had a 3 crosses graded inflammation. In case of intermediate involvement, vitreous body inflammation was also graded. 16 eyes were free of inflammation at this level, 29% of eyes were quoted with 1 cross (n=8), 11% with 2 crosses (n=3) and one eye was graded with 3 crosses. 21% of patients had macular edema (n=3) (Figure 5).
21% of patients (n=3) had optic nerve damage papillitis type. retinal vasculitis was demonstrated in 29% of cases (n=4).
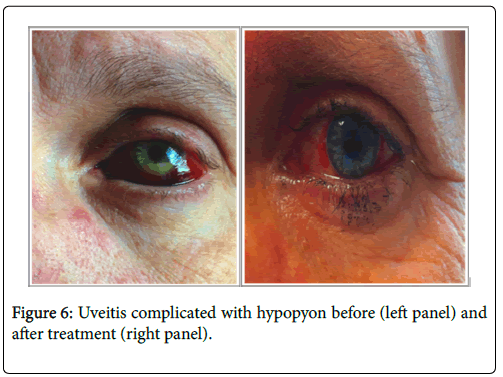
2 patients showed scleritis and one patient was diagnosed after onset of hypopion revealing anterior uveitis (Figure 6).
Therapeutic management
Every patient diagnosed with syphilitic uveitis received parenteral (IV) antibiotic therapy. 79% of them were initially treated with Penicillin G 6 × 4MUI a day (n=11/14). 14% were initially treated with Ceftriaxone 2 g a day (n=2/14). 64% of patients had their first antibiotic plan modified in 64% of cases (n=9/14). Drug switch was mainly justified to keep ambulatory treatment continuation with daily intravenous single dose of Ceftriaxone. The average duration of antibiotic therapy was 16 days. 57% of patients were treated for a total period of 14 days (n=8/14), 36% for 21 days (n=5/14). One patient in the situation of a relapse has not been treated parenterally (Doxycycline given orally for 14 days). 57% of patients received corticosteroid therapy (n=8/14), whatever considering its temporality respect to antibiotic therapy.
Follow-up
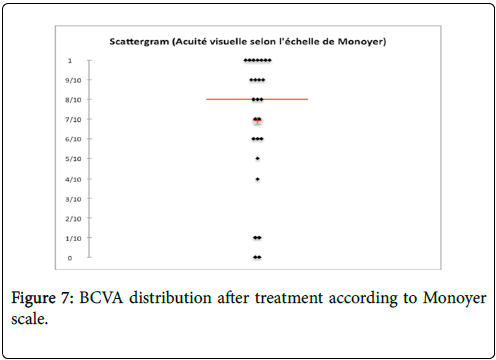
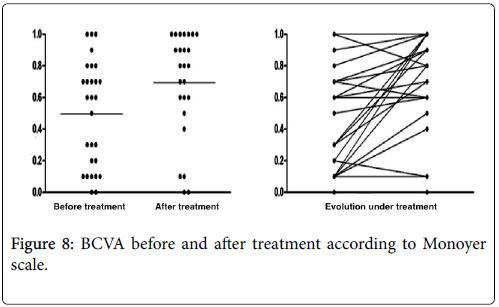
Every patients diagnosed with ocular syphilis had an ophthalmologic control. Its frequency was individualized and adapted to severity of the case. Initiated in a hospital environment, it could sometimes be continued in town consultation. Meanwhile, regular revaluations were performed by the internist physician and/or infectious disease practitionner. They ensured tolerance of treatment (One Herxheimer reaction) and its effectiveness including monitoring the decay of serological titles of nontreponemal tests (RPR, VDRL). The average follow-up time was 508 ± 295 days (Median=90. Range: 21-1825). The average BCVA obtained after treatment was 0.39 ± 0.71 LogMAR (Mean=4/10, Median=7/10, Range: “Seeing shake hands by one meter–10/10 on Monoyer scale”) (Figure 7). The average BCVA improvement after treatment was at least 2/10 for each affected eye (p=0.03) (Figure 8). For one patient, severe lesions at initial presentation remained irreversible.
The average IOP after treatment was 17 ± 4 mmHg (Median=18, Range: 8-22). The anterior chamber inflammation was resolved for every patient initially involved. A hyalitis, with diffuse macular edema characterized by retinal thickening in OCT, persisted for one patient that had a 3 months medical follow up, without evolutionary nature. For another patient, a persistent evolutive retinal neovascularization after anti-infective treatment required administration of two intravitreal injections of bevacizumab (AVASTIN) spaced from four months. Vascular abnormalities amended after. Visual field examination highlighted for one patient, a sequel superolateral notch type with nasal blind spot’s enlargment. Serological cure was obtained in 86% of patients (n=12). Four patients presented during their follow up a reappearance of active syphilis suggestive signs. For two of them, we diagnosed recurrence of uveitis respectively 3 months and 4 years after inaugural episode. The analysis of CSF did not show any hypercellularity and CSF non treponemal test (VDRL) remained negative. For two other patients, syphilitic recurrence was not followed by any ocular nor neurological impairment. With every relapsing patients, anamnesis predicted reinfection. Two patients, for whom the non-treponemal test had not been divided by four to 12 months, showed symptoms for syphilitic reinfection. A new analysis of CSF was performed without highlighting any hypercellularity and CSF nontreponemal test (VDRL) remained negative.
Compared cases according to HIV co-infection status, 31% of patients (n=5), including 3 HIV co-infected, had already submitted an STI. One patient with HIV co-infection, was known for a past syphilis history seven years ago. There was no significant difference between HIV free and HIV co-infected patients in terms of time to diagnosis, revelation mode nor extra-ocular syphilitic involvement. Cytological and biochemical CSF abnormalities were not significantly different. Nevertheless, co-infected patients with HIV were more likely to have positive non-treponemal test (VDRL) on CSF (100% vs. 20%, p=0.01). Regarding ocular involvement, severity of VA decline at diagnosis was not significantly different between the two groups. Any disparity was highlighted in terms of various ocular segments infringement (Table 2).
| HIV co-infected (n=20) | HIV free (n=8) | p-value | ||
|---|---|---|---|---|
| Initial visual acuity (LogMAR) | Average | 0.45 | 0.58 | 0.55 |
| Median | 0.55 | 0.35 | ||
| Range | 0-2.3 | 0-1.7 | ||
| Anterior uveitis | % (n) | 50% | 35% | 0.58 |
| Posterior uveitis | % (n) | 87% | 70% | NS |
| Panuveitis | % (n) | 50% | 30% | NS |
| Optic nevritis | % (n) | 0% | 30% | 0.5 |
| Intraocular pression (mmHg) | Average | 14.9 | 13.9 | 0.37 |
| Median | 15.5 | 14 | ||
| Range | Sep-19 | 08-Oct | ||
| Vasculitis | % (n) | 25% | 30% | NS |
| Keratitis | % (n) | 0% | 20% | NS |
| Papillar oedema | % (n) | 33% | 43% | 0.68 |
| Macular involvement | % (n) | 33% | 18% | 0.61 |
Table 2: Comparison of initial ocular damage according to HIV status (n=eyes), (NS: insignificant).
Discussion
Epidemiologically, if the male population is often affected in our serie, it seems that there is no preferential involvement between homoor bisexual populations compared to heterosexual populations. This element is in step with the recent STI’s epidemiology that affects any population whatever sexual its orientation. Multiplying sexual partners is a riskier behavior of contamination, but not in a significant way, more likely for HIV co-infected patients. Clinically, as in the serie described by Puech et al. [17], ocular involvement was associated in more of half the cases to extra-ophthalmic lesions. Mostly syphilides or roseola, these remained usually unnoticed or neglected by the patient before the ophthalmological diagnosis. Ocular involvement resulted in a decrease of VA for all our patients and was inaugural of the disease in more than 50% of the cases.
Median time from symptoms onset to seeking care is relatively long in our serie (33.5 days) compared to those reported by Marty et al. (13.5 days) [23] but twice shorter than in Moradi et al. (60 days) [22]. Median time to diagnosis from first hospital consultation was less than 10 days. Despite a longer course before treatment introduction, infringement severity based on VA drop was not higher in our serie than the one quoted above (initial median VA 4.8/10 vs. 2/10 on Monoyer scale).
If ophthalmological lesions spectrum was quite wide and protean, every patients with ocular involvement exhibited uveitis. Dominance of this entity in syphilitic ocular involvement is widely described in the literature. Reach of the posterior segment was almost systematic, isolated or associated with lesions of the anterior and/or intermediary segments. Such topographical distribution diverges from descriptions made previously in the literature. It’s used to be reported prevalence of anterior lesions (71%) [24], then panuveitis (27-50%) [11,24] and posterior impairment (8%) [24,25]. Furthermore, the existence of an anterior uveitis alone is strongly correlated with the existence of HIV co-infection [26]. Uveal involvement was bilateral in about 50% of cases, as described by Ormerod et al. [15]. Other series including Moradi et al. one found a higher proportion of bilateral involvement (74.3%) [22].
Intraocular inflammation was relatively heterogeneous between our patients, without significant differences according to HIV co-infection status. If ocular syphilis characteristics remain nonspecific, some patterns are more readily described as evocative and allow earlier etiological orientation. Superficial retinal precipitates [27], less dense infiltrate than necrotizing herpetic retinitis or also posterior placoid chorioretinitis [28] are distinctive patterns that might evocate the ophthalmologic diagnosis.
More atypical presentations like scleritis in two patients and hypopyon in a patient have been identified. Syphilis represents according to Akpek et al. 0.4% of scleritis etiologies [29]. Biologically, ophtalmic syphilis was generally not followed by major systemic inflammation on the basis of the observed rate of C-reactive protein. Every patient had treponemal and non-treponemal tests positive blood tests.
In our study, CSF analysis allowed detection of meningitis in 90% of cases. Cytological and biochemical analyzes found mostly lymphocytic profile with normal glycorrhachia/glycemia ratio. CSF non-treponemal test (VDRL) was positive for all patients with meningeal involvement in the HIV co-infected group against only 20% of HIV-negative patients. A larger CSF disruption in HIV co-infected patients patients has already been reported (up to 85% of cases with posterior uveal damage) [15]. However, CSF abnormalities are common in patients with early syphilis and undetermined significance in the absence of neurological or ophthalmological clinical signs associated [30]. Further, definition of neurosyphilis is not consensual. It is established by some as a combination of aseptic lymphocytic meningitis, elevated cephalospinal fluid protein and a non-treponemal test positive in the CSF [31]. For some others it can be characterized by the simple achievement of neurological structures of the posterior segment (retina, optic nerve) associated with positive syphilis serology on blood [21]. Finally, some consider combination of lymphocytic meningitis with a positive syphilis serology blood as sufficient [32]. For IDSA, diagnosis of neurosyphilis is categorical when neurological clinical signs combined with a nontreponemal positive test in the CSF. Subjects with suggestive neurological symptoms with nontreponemal test negative in the CSF will be considered as suffering from neurosyphilis and treated as such if they exhibit cytological and/or biochemical CSF abnormalities [5]. Direct detection of Treponema pallidum genome (polymerase 1 gene) in ocular fluids as described by Muller et al. [33] was not performed in our patients. Brain imaging with MRI performed for about half the patients was contributory in one patient with a central neurological deficiency byconfirming existence of brainstem encephalitis. In Marty et al. study MRI performed consistently found abnormalities type pachymeningitis, cortico-subcortical atrophy and nonspecific white matter hyperintensities [23]. The used antibiotic regimens used were all based on IV administration, with a large proportion of first-line use of IV penicillin G (doses 24 MUI a day in 6 injections). An early relay by 2 g IV ceftriaxone daily usually allowed completing the ambulatory use due to its long half-life and its good penetration into the central nervous system. Minimum treatment duration observed was for 14 days, extended to 21 days for some patients.
More than half of the patients received corticosteroids. Corticotherapy was initiated mostly after, or in a simultaneous way, with the anti-infective therapy. Administration schema was various (IV Bolus on short period or progressive decay) as treatment durations. It did not show any long term pejorative outcome in patients who received prior corticosteroid before anti-infective treatment. Used topically in anterior uveitis and interstitial keratitis [31,34], systemic corticosteroid therapy failed to show additional benefit to this day in management of syphilitic uveitis. Its use remains significant in the presence of scleritis, optic neuropathy, retinal vasculitis or posterior ocular structures involvement, in association with antibiotic treatment [24]. A good tolerance to treatment was generally showed; only one case of Herxheimer reaction has been diagnosed. Treatment effectiveness on visual prognosis remains excellent with correction of VA for most patients except two. This overall favorable trend is consistent with results reported in the literature in most series. In two failure cases, patients had at initial presentation advanced lesions with major retinal detachment and macular edema. No improvement was observed during treatment (Respectively 6 and one month follow-up).
For one patient, persistent retinal neovascularization led to start indication anti-angiogenic treatment with bevacizumab (AVASTIN) intravitreally. Vascular proliferation decreased after two injections spaced from four months. Persistent ophthalmologic manifestations after well conduced anti-infective treatment raise questions about their mechanism. Chronicized infection has already been described, involving mainly immunological phenomena depending on the Th2 lymphocyte pathway. On the follow-up period, 4 patients had a new syphilitic episode. Ocular syphilitic recurrence was associated for two of them. Their CSF analysis did not reflect meningitis stigmas. CSF non-treponemal test (VDRL) remained negative. Most likely etiology of theses recurrences after initial healing was attributed to syphilis reinfection. Regarding HIV co-infection status, we did not find any significant differences in terms of initial severity, type of ocular involvement or recovery prognosis under treatment. CSF nontreponemal test in case of ocular syphilis with meningitis was consistently positive in HIV co-infected patients compared with HIVfree patients.
Conclusion
Ocular syphilis, even if it is rare, should because of its potential gravity and of its accessibility to a simple and effective treatment be considered in range of the uveitis causes and in the assessment of any new syphilis whatever the patient’s status toward an HIV co-infection.
References
- Pilly E (2014) Infectious and tropical diseases. In: College of Academics of Infectious and Tropical Diseases. Vivactis Plus Ed pp: 421-424.
- Fonollosa A, Giralt J, PelegrÃn L, Sánchez-Dalmau B, Segura A, et al. (2009) Ocular syphilis-back again: understanding recent increases in the incidence of ocular syphilitic disease. Ocul Immunol Inflamm 17: 207‑212.
- Herida M, Michel A, Goulet V, Janier M, Sednaoui P, et al. (2005) The epidemiology of sexually transmitted infections in France. Med Mal Infect 35: 281-289.
- Jones NP (2015) The Manchester Uveitis Clinic: the first 3000 patients--epidemiology and casemix. Ocul Immunol Inflamm 23: 118‑126.
- Workowski KA, Bolan GA (2015) Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64: 1‑137.
- Patton ME, Su JR, Nelson R, Weinstock H, Centers for Disease Control and Prevention (CDC) (2014) Primary and secondary syphilis-United States, 2005-2013. MMWR Morb Mortal Wkly Rep 63: 402‑406.
- Butler NJ, Thorne JE (2012) Current status of HIV infection and ocular disease. Curr Opin Ophthalmol 23: 517‑522.
- Doris JP, Saha K, Jones NP, Sukthankar A (2006) Ocular syphilis: the new epidemic. Eye (Lond) 20: 703‑705.
- Maves RC, Cachay ER, Young MA, Fierer J (2008) Secondary syphilis with ocular manifestations in older adults. Clin Infect Dis 46: e142‑145.
- Margo CE, Hamed LM (1992) Ocular syphilis. Surv Ophthalmol 37: 203‑220.
- Tamesis RR, Foster CS (1990) Ocular syphilis. Ophthalmology 97: 1281‑1287.
- Baglivo E, Kapetanios A, Safran AB (2003) Fluorescein and indocyanine green angiographic features in acute syphilitic macular placoid chorioretinitis. Can J Ophthalmol 38: 401‑405.
- Davis JL (2014) Ocular syphilis: Curr Opin Ophthalmol 25: 513‑518.
- Kiss S, Damico FM, Young LH (2005) Ocular manifestations and treatment of syphilis. Semin Ophthalmol 20: 161‑167
- Ormerod LD, Puklin JE, Sobel JD (2001) Syphilitic posterior uveitis: correlative findings and significance. Clin Infect Dis 32: 1661‑1673.
- Tucker JD, Li JZ, Robbins GK, Davis BT, Lobo A-M, et al. (2011) Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect 87: 4‑8.
- Puech C, Gennai S, Pavese P, Pelloux I, Maurin M, et al. (2010) Ocular manifestations of syphilis: recent cases over a 2.5-year period. Graefes Arch Clin Exp Ophthalmol 248: 1623‑1629.
- Yap SC, Tan YL, Chio MTW, Teoh SC (2014) Syphilitic uveitis in a Singaporean population. Ocul Immunol Inflamm 22: 9‑14.
- Hong MC, Sheu SJ, Wu TT, Chuang CT (2007) Ocular uveitis as the initial presentation of syphilis. J Chin Med Assoc 70: 274‑280.
- Fénolland JR, Bonnel S, Rambaud C, Froussart-Maille F, Rigal-Sastourné JC (2016) Syphilitic Scleritis. Ocul Immunol Inflamm 24: 93-95
- Browning DJ (2000) Posterior segment manifestations of active ocular syphilis, their response to a neurosyphilis regimen of penicillin therapy, and the influence of human immunodeficiency virus status on response. Ophthalmology 107: 2015‑2023.
- Moradi A, Salek S, Daniel E, Gangaputra S, Ostheimer TA, et al. (2015) Clinical Features and Incidence Rates of Ocular Complications in Patients With Ocular Syphilis. Am J Ophthalmol 159: 334‑343.e1.
- Marty AS, Cornut PL, Janin-Manificat H, Perard L, Debats F, et al. (2015) Caractéristiques cliniques et paracliniques des uvéites syphilitiques. J Fr Ophtalmol 38: 220‑228.
- Barile GR, Flynn TE (1997) Syphilis exposure in patients with uveitis. Ophthalmology 104: 1605‑1609.
- Mora P, Borruat FX, Guex-Crosier Y (2005) Indocyanine green angiography anomalies in ocular syphilis. Retina 25: 171‑181
- Amaratunge BC, Camuglia JE, Hall AJ (2010) Syphilitic uveitis: a review of clinical manifestations and treatment outcomes of syphilitic uveitis in human immunodeficiency virus-positive and negative patients. Clin Experiment Ophthalmol 38: 68‑74
- Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, et al. (2009) Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology 116: 1195‑200.
- Gorovoy IR, Desai S (2013) Syphilitic posterior placoid chorioretinitis. Sex Transm Dis 40: 852‑853.
- Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA (2004) Evaluation of patients with scleritis for systemic disease. Ophthalmology 111: 501‑506
- Lukehart SA, Hook EW, Baker-Zander SA, Collier AC, Critchlow CW, et al. (1988) Invasion of the Central Nervous System by Treponema pallidum: Implications for Diagnosis and Treatment. Ann Intern Med 109: 855‑862.
- Brezin A (2010) French Society of Ophthalmology. Les Uvéites: 9782294711077 Elsevier / masson.
- Brown DL, Frank JE (2003) Diagnosis and management of syphilis. Am Fam Physician 68: 283‑290.
- Müller M, Ewert I, Hansmann F, Tiemann C, Hagedorn HJ, et al. (2007) Detection of Treponema pallidum in the vitreous by PCR. Br J Ophthalmol 91: 592‑595.
- Solebo AL, Westcott M (2007) Corticosteroids in ocular syphilis. Ophthalmology 114: 1593.
Citation: Garci R (2018) Epidemiological, Clinical, Biological and Evolutionary Aspects of 14 Patients-22 Eyes Cohort (2008-2015). J Clin Infect Dis Pract 3: 124. DOI: 10.4172/2476-213X.1000124
Copyright: © 2018 Gaci R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 7801
- [From(publication date): 0-2018 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 6849
- PDF downloads: 952