Pharmacological Activity of a Few Transition Metal Complexes: A Short Review
Received: 24-Apr-2016 / Accepted Date: 01-Jul-2016 / Published Date: 07-Jul-2016 DOI: 10.4172/2572-0406.1000108
Abstract
Transition metals have an important place within medicinal inorganic chemistry. Transition metals exhibit different oxidation states and can interact with a number of negatively charged molecules. This activity of transition metals led to the recent development of drugs which are based on metals and are considered to be potential candidates for pharmacological and therapeutic applications. This review focuses on research undertaken over the past few decades which has sought to possess preclinical pharmacological screenings like anti-microbial, anti-inflammatory and anti-tumor action of synthetic transition metal complexes. It concentrates primarily on a limited number of first row transition metal complexes particularly V(IV), Co(II), Ni(II), Cu(II) and Zn(II) complexes and traces the pharmacological applications of these coordination compounds. In the first part, the nitrogen, oxygen and sulfur donor ligands chelating to transition metals used in metallodrugs are described. The second part describes the pre-clinical screenings viz., anti-microbial, anti-inflammatory and anti-tumor responses of the above coordination compounds incorporating these nitrogen, oxygen and sulfur donor ligands. This survey encourages further research in this field for future applications.
Keywords: Transition metal complexes; Pharmacological applications; Anti-microbial; Anti-inflammatory; Anti-tumor responses
9758Introduction
Metal ions play important roles in biological processes and the field of knowledge concerned with the application of inorganic chemistry to therapy or diagnosis of disease is medicinal inorganic chemistry [1]. Among the natural sciences, medicinal inorganic chemistry is still considered a rather young discipline by many, but this is contrary to the historically proven use of metals in pharmaceutical potions, which traces back to the ancient civilizations of Mesopotamia, Egypt, India, and China [2-4]. The introduction of metal ions or metal ion binding components into a biological system for the treatment of diseases is one of the main subdivisions in the field of bioinorganic chemistry [5].
Nowadays, the bioinorganic chemists target the heterocyclic ligands and their metal complexes to study their pharmacology as the main focus of research [6]. A wide range of biological activities [7-9] such as antibacterial, antifungal, antitumor and antiviral activities are exhibited by the nitrogen-containing organic compounds and their metal complexes. Transition metal complexes offer two distinct advantages as DNA-binding agents [10]. First and foremost, transition metal centers are particularly attractive moieties for reversible recognition of nucleic acids research because they exhibit well-defined coordination geometries. Besides, they often show distinct electrochemical or photophysical properties, thereby increasing the functionality of the binding agent [11]. In fact, these smart features have fuelled the complexes to be used in a broad spectrum of applications, from fluorescent markers to DNA footprinting agents, to electrochemical probes [12].
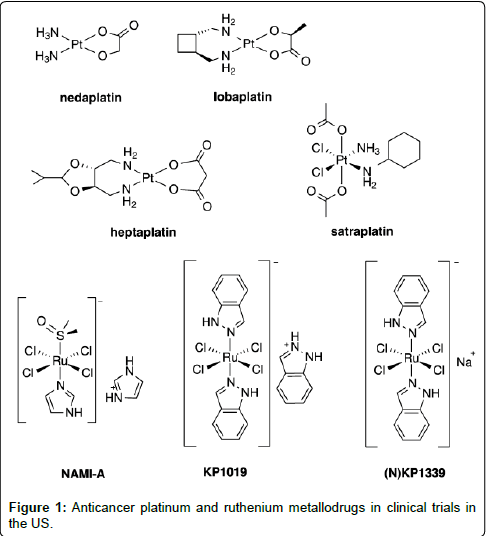
Among the metal ions regarded as coordination centers of potential anticancer agents, platinum and ruthenium ions (Figure 1) are commonly explored [13,14]. However, there is an emerging curiosity in the synthesis of cheaper first-row coordination compounds as efficient DNA binders with potential cytotoxic activity [15-20]. Hence, herein the attention is focused primarily on the research concerning with a few pharmacological activities of the cheaper and easily available first-row transition metal coordination compounds V(IV), Co(II), Ni(II), Cu(II) and Zn(II) complexes. Moreover, these metal ions are the essential elements present in the biological intracellular environment of living organisms [21-24]. They are most abundantly found trace elements present in biological systems together with iron and most of the metalloproteins have these elements [23,25,26]. These metal ions are nowadays present in several inorganic pharmaceuticals used as drugs against a variety of diseases, ranging from antibacterial and antifungal to anticancer applications [27-30]. Another fact for targeting these particular metal ions is their less toxic nature which can be further decreased when coordinated with the ligands. Though there are innumerable ligands available, the chosen amino acids, N-heterocycles (1,10 Phenanthroline, Bipyridine) and pyrazolones each have an added benefit to their properties which is a major advantage in designing an ideal drug. The amino acids are the building blocks of the human body and when the environment of the drug is similar to that of the functions carried out inside the body, the chance of succeeding in designing a less toxic and cheap drug with high activity is more. In addition to that, the N-heterocycles and pyrazolones as ligands affect the environment of the complex in such a way that their lipophilicity increases which is a major factor in designing a drug.
The chosen ligands and their characteristic are discussed in detail in the further sections. The literature survey reveals that numerous review articles and books have been published on medicinal inorganic chemistry [31,32] in the field of metallodrugs [33-39] and especially on anticancer treatments [40-43]. We present an overview of the field today and explore a few pharmacological effects of the above transition metal complexes viz., in vivo anti-inflammatory response and convulsive response as well as anti-tumor and microbial activity.
Bioactive Chelating Agents
ONS donors
Schiff bases, the condensation products of carbonyl compounds and primary amines were first described by Hugo Schiff (1864). The Schiff base compounds containing the azomethine group having the general formula RHC=N–R’, where R and R’=Alkyl, cyclo alkyl, aryl, or heterocyclic groups may be differently substituted (Figure 2). The Schiff bases are considered as ‘privileged ligands’ because of their capability to stabilize different metals in various oxidation states and their metal chelates are widely studied owing to the synthetic flexibility, sensitivity and selectivity toward various metal ions [44,45]. In azomethine derivative, the C=N linkage is essential for biological activity. Several azomethines were reported to possess important biological activities [46-49]. In many cases, beside the N-donor atoms, there are several other donor atoms such as O and S in the backbones of the ligands such that they can be coordinated to transition metal ions in the various modes to form the stable metal complexes [50]. These ligands are not only used in the development of coordination chemistry but also find applications in catalysis, optical and bioinorganic chemistry [51].
Aliphatic Schiff bases are relatively unstable [52,53] and easily polymerizable compared to aromatic Schiff bases because the latter possesses effective conjugation and hence they are more stable. Generally, in condensation reactions, aldehydes react faster than ketones leading to the formation of Schiff bases because the reaction centres of aldehydes are sterically less hindered than ketones. Moreover, the additional carbon of ketone contributes electron density to the azomethine carbon and makes the ketone less electrophilic compared to the aldehyde.
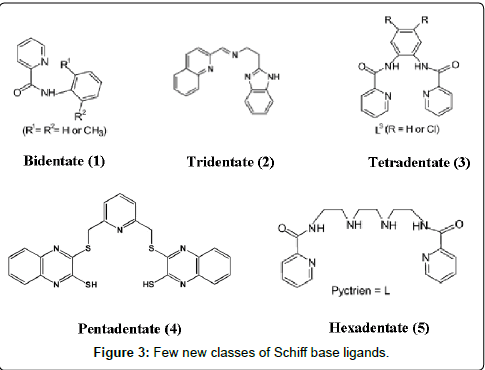
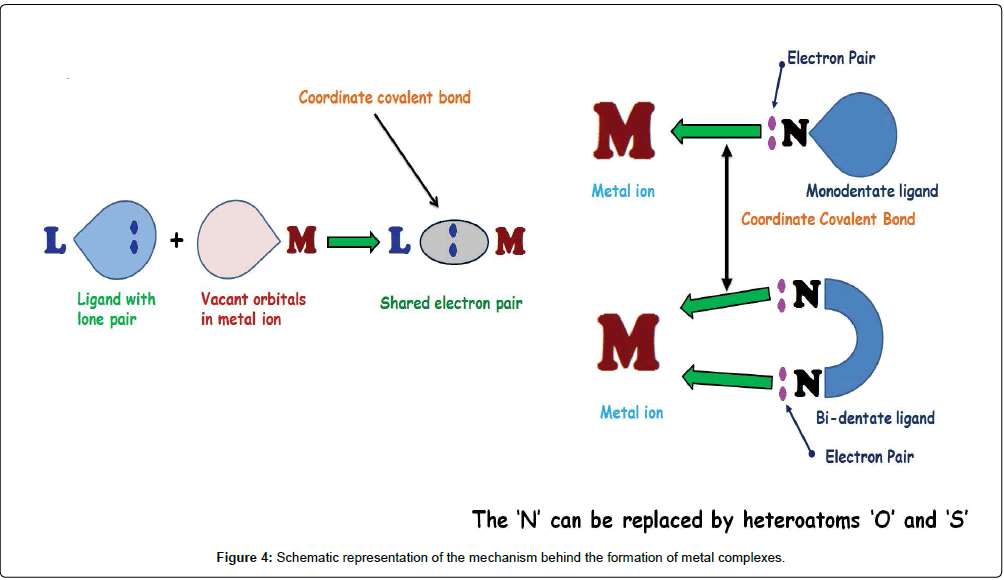
Generally, Schiff bases are classified into bidentate (1), tridentate (2), tetradentate (3) or polydentate (4, 5) ligands (Figure 3) which are able to form very stable complexes with transition metal ions. If they contain additional functional groups like –OH, –NH2 or –SH, the resulting Schiff bases can serve as mixed-donor ligands which can participate in bi-, tri-, tetra- and higher coordination modes. Therefore, the suitable choice of metals in a pharmacologically active organic scaffold changes the pharmacological activity. The literature survey indicates that the pharmacological activity depends on the nature of metal ion, organic scaffold and specific DNA binding site [54]. The mechanism involved in the formation of metal complexes through monodentate and bidentate ligands is represented in a schematic diagram (Figure 4).
Chelation and its relationship with different biological processes invent a promising research for designing novel therapeutic methodologies used for procurement of global issue “bacterial/ fungal resistance”. Metal chelation is an excellent way to increase the lipophilic character of the organic moiety. In fact, on coordination, ligands might improve their bioactivity profiles while some inactive ligands may acquire pharmacological properties and consequently they have become an important class of structure-selective binding agents for nucleic acids.
Schiff bases have a chelating structure and are in demand since they can be prepared directly and are moderate electron donors with easily tunable electronic and steric effects thus being versatile. Their derivatives represent one of the modest classes of biologically active agents which have been deeply studied during a search on new potential agents which are widely used for synthesis purpose by the chemists [55]. These ligands have received much attention mainly because of their wide range of applications due to their antimicrobial [56], anti-tuberculosis [57], antitumour activity [58], anticonvulsant [59], anti-inflammatory [60], anti-HIV [61], anthelmintic [62] and cardiovascular [63] activities and anti-carcinogenic properties [64].
Recently Tabassum and his associates [65] have synthesized new copper(II) Schiff base complexes based SOD mimics with various nitrogen heteroatomic rings such as imidazole, pyrazole, 1,10-phenanthroline, 2,2’-bipyridine, pyridine etc. which possess remarkable DNA binding tendency. These complexes have been evaluated for their DNA nuclease, antimicrobial and antitumor activities. They exhibited good results in the development of excellent chemotherapeutic potentials.
Bis[N-(p-tolyl)imino]acenaphthene mixed-ligand Cu(II) complexes and novel cisplatin-based Cu(II) and Zn(II) complexes having Knoevenagel condensate Schiff base ligands are studied against Ehrlich ascites carcinoma (EAC) cells in Swiss albino mice [66,67]. These compounds increased the life span, reduced average tumour weight and inhibited tumour cell growth of EAC cell-bearing mice. They also repaired the depleted haematological parameters such as haemoglobin content, red blood cells (RBC) and white blood cells (WBC) counts towards normal. They are compared by running parallel experiments with 5-FU (a standard anticancer drug). The macrophages which have some protection action against EAC cells are found to be developed in mice when treated with the compounds alone. Hence, these compounds can be regarded as promising antitumor agents.
Amino acids
Schiff bases derived from amino acids are found to be very effective metal chelators. Their metal complexes are models for a number of important biological systems [68]. They are the key intermediates in a number of metabolic reactions involving amino acids such as decarboxylation, transamination, racemization and C-C bond cleavage. They are catalyzed by enzymes [69]. The reduced Schiff bases having various amino acid derivatives are excellent multidentate ligands for creating interesting multidimensional network structures. The added reactive functional groups on the amino acid side chain of the ligands can lead to the formation of surprising and remarkable structures [70,71]. Another fact to be considered is that synthetic ligands with longer chains are preferred for pharmacological purposes as opposed to original compound because the length of chain helps in inter/ intrastrand crosslinking with DNA with lesser torsion making it a thermodynamically preferred process.
Amino acids can act as coordinating agents through their amino (NH2) and carboxylate (COO–) groups [72]. For sulfur-containing amino acids, the SH group confers a more versatile coordination activity toward heavy metal ions. The –SH (sulfhydryl), –NH2 (amino) and –COO– (carboxylate) groups are the possible coordination sites for the complexation processes. Sequestration of toxic heavy metal ions and obtaining safer drugs or antidotes for metal poisoning [73] by complexation is a very promising field.
As ligands, amino acids also act as ambidentate so that they can bind through (S,N), (N,O) or (S,O) donor atoms. Due to the acidobasic behaviour, amino acids are considered as ampholytes which means that with the negatively charged –COO- group or positively charged -NH3+ + can behave outwardly as acids or as bases. In an organism, amino acids can be both free and bound. These bound amino acids may be permanently or temporarily incorporated into the proteins or other possible biochemical functional structures. Free amino acids comprise other amino acids generated by modifying the known biogenic amino acids. These are known as biogenic amines, including histamine, dopamine, noradrenaline, ornithine, taurine, or growth factors sarcosine, spermine and spermidine. Determination of free amino acids or biogenic amines is insufficient to understand the metal biochemistry. It needs to deal directly with their own interactions between the metal and amino acids.
The uncontrolled move of metal ions in the body leads to the formation of reactive oxygen species such as superoxide anion radical (O2 -.), singlet oxygen (1O2), hydrogen peroxide (H2O2), and the highly reactive hydroxyl radical (•OH). Thus they can oxidize the biochemical molecules thereby disturbing the homeostasis in an organism. The first-line defence against these free radicals is always a compound that is able to mitigate these effects or completely scavenge these species without harming the body or the disruption of homeostasis. This is done by chemical reduction using hydrogen transfer.
Several Schiff base metal complexes which are the derivatives of salicylaldehyde and amino acid [74-76] and reduced salicylidene amino acid [77,78] were reported and some of them have been proven to be efficient DNA cleavers [79-81] and as novel tumor chemotherapeutic and tumor radio-imaging agents [82].
3d1-vanadium(IV) complexes of N-salicylidene-L-methionine and N-salicylidene-L-tryptophan having phenanthroline bases, synthesized by Chakravarty and his co-workers [83] can photocleave DNA in red light [84-86]. L-arginine and L-lysine derived Schiff base ligands are chosen to enhance the binding of DNA and photocleavage activity since these two amino acids have photoactive pendant cationic guanidinium and amine moieties that enhance the DNA binding propensity with better selectivity [85].
Recently, Singh et al. [86] have prepared Co(II), Ni(II) and Cu(II) complexes of 2-nitrobenzaldehyde–glycine and 2-nitrobenzaldehyde– methionine and studied their coordination properties. They evaluated the antimicrobial potential of these complexes against the growth of bacteria in vitro. They are found to be efficient antimicrobial agents.
Pyrazolones
Pyrazolones are a class of organic compounds that have been studied extensively due to their pharmaceutical properties [87]. Pyrazolone is a five-membered lactam ring which contains two nitrogens and a ketone in the same molecule and is an active moiety in pharmacological activity such as anti-flammatory agents [88], analgesics and arthritis treatment [89,90]. It is an active moiety as the pharmaceutical ingredient, especially in non-steroidal antiinflammatory drugs (NSAID) and is used in the treatment of arthritis and other musculoskeletal and joint disorders. Anticancer activity has also been reported [91]. Furthermore, the applications of pyrazolones have extended outside the pharmaceutical field such as in the solvent extraction of metal ions [92], for analytical purposes [93] and as ligands in complexes with catalytic activity [94]. It is well known that from a coordination chemistry viewpoint, the only atoms available for coordination are the nitrogen atoms of the pyrazole ring and the oxygen atom of the carbonyl group. If the nitrogens are blocked by substitution such as in antipyrine, coordination can only be achieved through the oxygen atom. Recently, there is a great deal of interest in the synthesis and characterization of transition metal compounds having pyrazolone precursor. It is well known from the literature that 4-aminoantipyrine (pyrazolone derivative) is a remarkable reagent due to its variety of applications. It was used as an antipyretic but replaced due to the possibility of agranulocytosis as the side effect. Jayabalakrishnan and his associates [95] have recently synthesized a Schiff base complex of copper(I) with 5-dimethyl-2-phenyl-4-[(pyridin-2-ylmethylene)- amino]-1,2-dihydro-pyrazol-3-one and investigated its DNA binding propensity, nuclease, radical-scavenging and cytotoxic activities which reveal that it can act as effective anti-cancer agent.
Nitrogen heterocycles
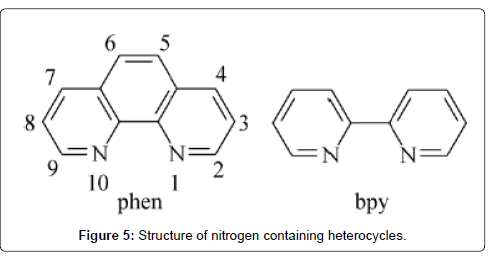
They are one of the most important classes of ligands in coordination chemistry [96-99]. The ability to readily fit in more than two donor atoms or two or more aromatic nitrogen-containing heterocycles into one molecule, has afforded access to numerous chelating and bridging ligands [96-102]. In the latter case, ligands possessing more than one coordination site can link a number of metal atoms. These bridging ligands have attracted in recent years because they enable the formation of multinuclear metallosupramolecular assemblies [98,103,104] or coordination polymers [105-112] with desirable structures and properties. Within such bridging ligands, the additional stabilising effect of chelation to multiple metal atoms can be achieved through the incorporation of bidentate donor groups that form either a five- or sixmembered chelate ring with each coordinated metal centre (Figure 5). Bridging ligands with the prospective to chelate at both metal centres result in complexes with greater stability and potentially enhance the metal-metal interactions.
1,10-phenanthroline: 1,10-phenanthroline (phen) is a classic nitrogen heterocycle and chelating bidentate ligand for metal ions. It has played a vital role in the progress of coordination chemistry [113-115] and still it continues to be of considerable interest as versatile starting material for organic, inorganic and supramolecular chemistry. Phen is a rigid planar, hydrophobic, electron-poor heteroaromatic system whose nitrogen atoms are beautifully placed to act cooperatively in cation binding. These structural features determine its coordination ability towards metal ions. Nevertheless, phen displays noticeable coordination ability for transition metal cations. Phen easily forms octahedral complexes with first-row transition metal cations in aqueous solution of the type [M(phen)(H2O)4]2+, [M(phen)2(H2O)2]2+ and [M(phen)3]2+. Also, phen is an appropriate ligand for DNA binding due to its heteroaromatic planar and hydrophobic structure. The DNA binders are able to recognize specific base sequences or luminescent probes. However, phen has also been used to design non-metal-based compounds which are able to interact with DNA and to perform specific functions from simple DNA damage to stabilization of particular DNA structures [116].
In addition, 1,10-phenanthroline has shown retardation of growth of a Sarcoma-37 tumor [117] and it inhibits the cell proliferation of Ehrlich ascites [118]. This chelating agent may show better antitumor activity if the hydrophilic groups of the chelating agent are masked by metal ions to form neutral chelate compounds. This will be more permeable through the cell membrane [119,120] and eventually behave as carriers of antitumor agents.
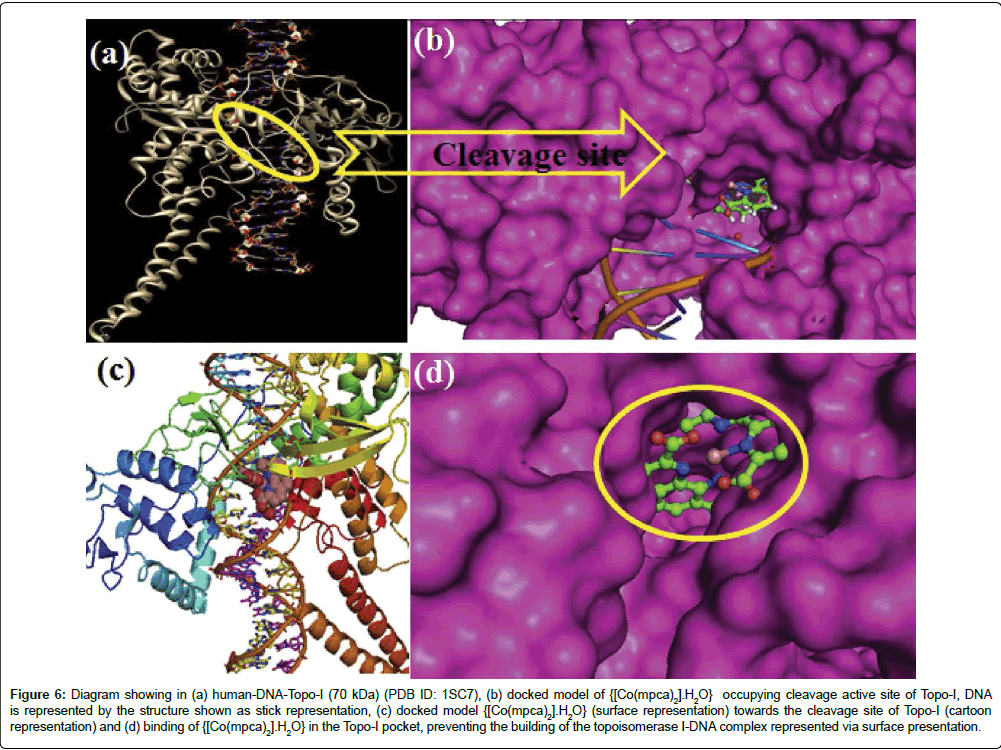
Ahmad et al. [121] have synthesized a Co(II) complex, [Co(mpca)2].H2O, which is capable of recognizing a specific sequence in DNA minor groove to inhibit the expression of Topo-I (Figure 6) and thereby controlling the multiplication of tumor affected cells. This has been made possible by joining an element of specific recognition, 2,9-dimethyl-1,10-phenanthroline with Co(II) metallic center under a hydrothermal condition in the presence of Na2MoO4 catalyst. Raman and his coworkers have done a lot of works using 1,10-phenanthroline. Recently, they synthesized a series of bio-active coordination compounds containing 1,10-phenanthroline as co-ligand [122]. They studied the DNA binding properties and cleavage of transition metal(II) complexes. Antiproliferative activity has been carried out recently by his group targeting protein kinase and DNA molecules by diimine-phthalate complexes [123]. Their synthesized complexes have a good affinity in targeting the DNA and cyclin dependent kinase-2 molecules.
Figure 6: Diagram showing in (a) human-DNA-Topo-I (70 kDa) (PDB ID: 1SC7), (b) docked model of {[Co(mpca)2].H2O} occupying cleavage active site of Topo-I, DNA is represented by the structure shown as stick representation, (c) docked model {[Co(mpca)2].H2O} (surface representation) towards the cleavage site of Topo-I (cartoon representation) and (d) binding of {[Co(mpca)2].H2O} in the Topo-I pocket, preventing the building of the topoisomerase I-DNA complex represented via surface presentation.
Bipyridine: 2,2′-bipyridine is a chelating component. For more than several decades, it has been used as bridging ligand and so it finds applications in different fields particularly in coordination chemistry. It forms a 5-membered chelate ring which is stable upon coordination of a metal. The 2,2′-bipyridyl moiety has been extensively used as a chelating donor site within such bridging ligands due to its robust redox stability and relative ease of functionalization. This allows ligands containing multiple 2,2′-bipyridine units to be synthesised and the interconnection of metal centres to be achieved with well-defined spatial arrangements. The synthesis of such ligands and their use in coordination chemistry has been reviewed elsewhere [99]. While bridging ligands that contain donor sets capable of forming five-membered chelate rings such as those derived from 2,2′-bipyridine (bpy), have been extensively studied over many years [99]. Related bridging ligands that comprise at least two di-2,2′-pyridylmethyl or amino arms have attracted a significant level of attention only recently. This has been directed towards studying metal–metal interactions in supramolecular chemistry to study anion–interactions as sensors in new coordination materials and as structural and functional enzyme active site models. The 2,2′-bipyridyl-containing ligands, particularly the biaryl bond which experiences restricted rotation, are planar and more readily able to facilitate metal–metal interactions through the conjugated system of the ligand [124]. Pothi et al. synthesized a series of Schiff base Cu(II) and Zn(II) metal complexes having polypyridyl ligands and explored their DNA interactions [125].
Biological Significance of Metal Complexes
The use of metals and metal-containing compounds in medicine dates back to millennia which provide an empirical evidence for the effectiveness of such metal-based therapeutics. Transition metal chelates that play a key role in bio-inorganic chemistry and redox enzyme systems serve as the basis of models for active sites in biologically important compounds. They have varied coordination geometry, versatile redox, spectral and magnetic properties which are appropriate for designing non-porphyrinic metal-based PDT agents that could photocleave DNA in visible light.
The relationship between active metals and cancer is a multifaceted issue which combines the expertise of bioinorganic chemists, pathologists, pharmacologists and oncologists. Redox-active metals generally form reactive oxygen species (ROS) and this ROS can be used to induce DNA cleavage. The earliest report of the medicinal use of metals or metal complexes dates back to the sixteenth century [126]. The main application is the anti-tumour action of certain heavy metals which bind to DNA and distorting DNA causing cell death. For example, today the cis-platin is one of the most potent and widely used anticancer drugs in use. However, it cures only limited spectrum of cancers and acquired resistance [127]. To defeat these limitations of cis-platin, less toxic and more effective metallodrugs have been developed like oxaliplatin and carboplatin.
DNA-metal Complex Interactions
DNA is the storage site of cellular information that is accessed continuously for storing and dispensing information required for existence. Thus, it acts as the main intracellular target for those who thrive to develop a new drug for innumerable diseases, especially cancer. Added to the fact, small molecules that can bind and react with specific DNA sites provide a means to access and manipulate this cellular information creating the desired results. There are many binding modes by which the small molecules bind to the DNA which are covalent and non-covalent binding. Cisplatin binds covalently with the DNA thereby restricting its replication. Among the non-covalent binding modes, intercalation, groove binding and external electrostatic binding, intercalation is the most important one because it invariably leads to cellular degradation [128]. Humungous reports are available throughout the literature regarding the interactions of V(IV), Ni(II), Co(II), Cu(II) and Zn(II) complexes with DNA and still now many research groups have actively involved in this field [129-137]. Among the research groups, most of them are concentrating only copper Schiff-base complexes. For, copper is found in all living organisms and is a vital trace element in redox chemistry, growth and development. It is significant for the function of several enzymes and proteins involved in energy metabolism, respiration and DNA synthesis, particularly cytochrome oxidase, superoxide dismutase (SOD), ascorbate oxidase and tyrosinase. Copper is found to bind DNA with high affinity than any other divalent cation, thus promoting DNA oxidation.
Acquaye et al. synthesized two new copper Schiff-base complexes and carried out DNA interactions with CT-DNA. The resultant Kb values are 1.52 × 105 M−1 and 5.00 × 105 M−1 respectively for the complexes [138]. Yang and his colleagues have synthesized and characterised two novel Schiff base copper(II) complexes derived from kaempferol and polyamines such as ethylenediamine and diethylenetriamine. They evaluated the DNA interactions with CT DNA and predicted the mode of interactions to be intercalation [139]. An extensive DNA-metal complex interaction has been carried out by Lin and his colleagues by synthesizing two new benzimidazole based copper complexes. The studies showed that the complexes exhibited partial intercalation towards the DNA [140]. The novel copper complexes synthesized by Gup and Gokce are found to bind significantly to calf thymus DNA by both groove binding and intercalation modes and effectively cleave pBR322 DNA [141]. Xu et al. synthesized three novel structurally associated copper(II) complexes which displayed enhanced intercalation into CT DNA [142].
Pharmacological Actions
Chemotherapy using chemical agents is one of the effective methods for the treatment of various cancers. With the increasing number of compounds synthesized as potential anticancer drugs, effective screening methods are needed for classification of these compounds according to their anticancer activities. For preliminary screening, the in vivo methods are usually more accurate but rather expensive and time-consuming whereas the in vitro methods are simpler and more rapid but with lower accuracy. In general, for large scale preliminary screening, the in vitro methods are more effective for refined screening on a smaller scale, naturally, the in vivo methods with test animals must be used and the clinical experimental tests are also required. The key advances in the cancer chemotherapy are shown in Figure 7.
Medicinal inorganic chemistry presents additional opportunities for the design of therapeutic agents, not accessible to organic compounds [143-145]. The broad range of coordination numbers and geometries, available redox states, thermodynamic and kinetic characteristics and intrinsic properties of the cationic metal ion and ligand itself offer the medicinal chemist a large variety of reactivities to be exploited.
The increasing number of multi-drug resistant microbial pathogens hardened the treatment of infectious diseases that posed as an important and challenging problem. Despite the availability of a large number of antibiotics and chemotherapeutics, a substantial need for the development of new class of potent antimicrobial agents arose on account of the emergence of old and new antibiotic resistant bacterial strains. Thus, the heterocyclic compounds play a significant role in designing a new class of structural entities of medicinal importance with new mechanisms of action [146]. The diverse pharmacological properties possessed by heterocyclic compounds are the well known antimalarial, antimicrobial, anti-inflammatory, anticancer, analgesic and anticonvulsant.
The search for novel antimicrobial and analgesic agents devoid of side effects continues to be an active area of research in medicinal chemistry. Although new and more expensive drugs have been developed, their cost is beyond the common man′s reach. Accordingly, the pressing need for new, more effective, cheaper and safe antimicrobial agents arose and has been emphasized.
Antioxidants are molecules capable of inhibiting the oxidation of other molecules and thereby preventing the cell death that occurs due to the release of free radicals. Free radicals such as DPPH•, NO• O2 •-, OH•, hydrogen peroxide and lipid peroxide radicals have been implicated in a variety of diseases such as asthma, cancer, cardiovascular, diabetes, gastrointestinal inflammation, periodontal disease and other inflammatory processes [147,148]. Hydroxyl is one of the key groups to enhance the antioxidant activity due to its easy conversion to phenoxy radical through hydrogen transfer mechanism [149].
The superoxide dismutases (SODs) known as metalloenzymes are able to keep the concentration of superoxide radicals in controllable low limits and thus, they can protect cells against an oxidative damage [150]. Only recently, it has been found that reactive oxygen species, such as the superoxide radical or hydrogen peroxide, are important regulators of cell death [151]. Particularly, H2O2 is implicated as a mediator of the apoptosis in cells [152]. The cellular damage caused by H2O2 is due to the hydroxyl radical production that results from the reaction of H2O2 with transition metal ions [153].
Anti-microbial agents
A review by Turel focuses on the crisis of decrease in quinolone drug absorption when consumed simultaneously with magnesium or aluminium antacids. He reviewed selected crystal structures of quinolone-metal compounds and their anti-microbial activities. The reason for such behaviour is proposed to be the chelate bonding of the quinolone to the metal [154]. The complex [Cu(cx)2].2H2O (where cx = cinoxacin) was screened for activity against several bacteria [minimal inhibitory concentration (MIC) values] showing the same antimicrobial activity as the free ligand [155].
Using a series of Gram positive and Gram negative bacterial strains, Scozzafava and his co-workers [156] tested zinc and copper ciprofloxacin complexes which showed moderated antimicrobial effects. Very recently, Psomas research group synthesized few quinolone cobalt(II) complexes and tested their antimicrobial activity using oxolinic acid (Hoxo) in the absence or presence of the Lewis bases 2,2′-bipyridine (bipy), 2,2′-bipyridylamine (bipyam), 1,10-phenanthroline (phen), pyridine (py) or 4-benzylpyridine (4bzpy). Their antimicrobial activity showed that the activity was similar to the free Hoxo [157].
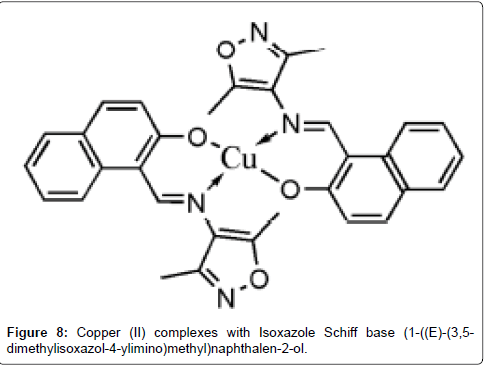
Kumar et al. [158] synthesized copper(II) complexes with isoxazole Schiff bases, [Cu(L1)2], [Cu(L2)2] and [Cu(L3)2] where, L1 = [(1-((E)-(3,5-dimethylisoxazol-4-ylimino)methyl)naphthalen-2-ol, C16H14N2O2), L2= [2-((E)-(3,5-dimethylisoxazol-4-ylimino) methyl)-4- methoxyphenol, C13H14N2O3 and L3 = (2-((E)-(3,5-dimethylisoxazol-4- ylimino) methyl)-4-bromophenol, C12H11BrN2O2]. Their antimicrobial activities were screened and the results were favourable for Cu(II) complex of ligand L1 which possessed good anti-microbial activity against the other ligands and standard (Figure 8).
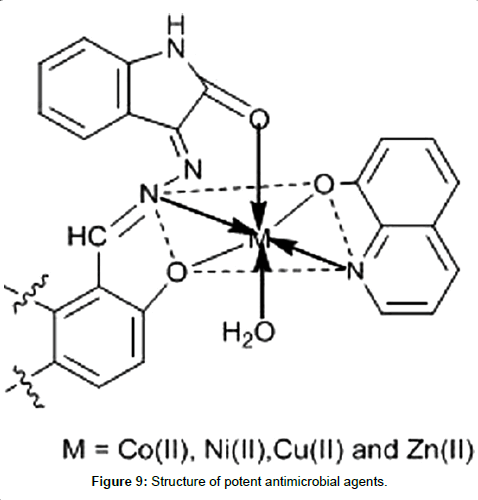
Devi et al. [159] have synthesized a series of mixed ligand complexes of Co(II), Ni(II), Cu(II) and Zn(II) using various uninegative tridentate ligands derived from isatin monohydrazone with 2-hydroxynapthaldehyde/substituted salicylaldehyde and heterocyclic nitrogen base 8-hydroxyquinoline and characterized them by elemental analysis, conductometric studies, magnetic susceptibility and spectroscopic techniques (Figure 9). They tested in vitro antimicrobial activity against various pathogenic Gram positive bacteria, Gram negative bacteria and fungi using different concentrations (25, 50, 100, 200 μg/mL) of ligands and their complexes. The results indicate that complexes exhibited enhanced activity as compared to free ligands and copper(II) complex was found to be the most potent antimicrobial agent.
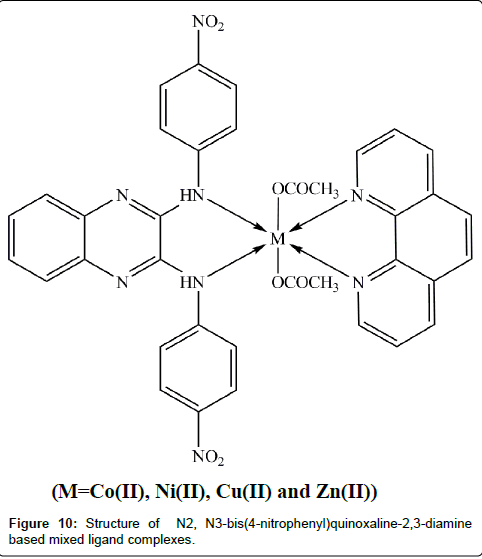
A new Co(II), Ni(II), Cu(II) and Zn(II) mixed ligand complexes (Figure 10) from N2, N3-bis(4-nitrophenyl)quinoxaline-2,3-diamine and 1,10-phenanthroline have been synthesized by Dhanaraj et al. The compounds have been characterized by elemental analyses, magnetic susceptibility, molar conductance, UV–Vis., IR, 1H NMR, mass and ESR spectra [160]. The complexes were screened for antimicrobial activity against various bacterial and fungal species viz., E. coli, K. pneumoniae, P. aeruginosa, S. aureus, A. niger and C. albicans by disc diffusion method. The Cu(II) complex exhibited the highest zone of inhibition against the bacterial species K. pneumoniae (12 mm) and P. aeruginosa (11 mm). The Ni(II) mixed ligand complex exhibited a higher zone of inhibition against E. coli (12 mm) and Zn(II) complex exhibited a higher zone of inhibition against S. aureus (12 mm). From the above, it was concluded that among the four mixed ligand metal complexes, Cu(II) mixed ligand metal complex showed higher antibacterial activity. In the case of antifungal activity, Co(II) complex showed the higher zone of inhibition against the fungal species C. albicans (13 mm) and Ni(II) complex showed higher activity against A. niger (8 mm). Overall, the antimicrobial activity of the complexes is in the following order: Cu (II)>Co(II)>Ni(II)>Zn(II). The superior activity of the metal complexes may possibly be as a result of increased lipophilic nature of the complexes attributed to chelation and heteroatoms present in the ligand moiety [161].
Anti-tumor agents
Although there were multiple reports in recently published papers about the ternary copper(II) complexes, that are synthesized by the combination of a bidentate N-donor heterocyclic ligand (phen, bpy or their substituted derivatives) and other synthetic co-ligands (i.e., salicylic acid [162], tetracycline derivatives [163], terpyridine [164], or imidazolidine-2-thione [165]), with remarkable in vitro cytotoxicity towards the human cancer cell lines, none of these dealt with the directed synthesis of mixed ligand copper(II) coordination compounds containing flavonoid-inspired co-ligands.
Lately, Reedijk et al. have found that efficient self-activated DNA cleavage and cytotoxic effects toward L1210 murine leukemia and A2780 human ovarian carcinoma cell lines can be brought out by the complex [Cu(pyrimol)Cl], synthesized by them [166]. Sadler and his co-workers have observed [167] that cytotoxic and antiviral activities are exhibited by their synthesized mixed ligand bis(salicylato) copper(II) complexes with diimines as co-ligands. Palaniandavar and his co-workers reported [168-170] the role of hydrophobicity of ligands in many ternary copper(II) complexes which exhibited strong DNA binding and cleavage and induced apoptosis in cancer cells. Kumbhar and his co-workers have investigated the cytotoxicity of certain mixed ligand Cu(II) complexes against HeLa (cervical) cancer cell lines [171].
Generally, the molecules that are approved for clinical use are those which damage DNA, inhibit nucleic acid precursor biosynthesis thereby blocking DNA synthesis indirectly, or disrupt hormonal stimulation of cell growth as anticancer agents [172]. Sigman et al. reported [173] a facile approach for investigating the interaction of nucleic acids and oligonucleotides with proteins that is provided by the oxidation of DNA and RNA. Burstyn and his coworker have found that copper(II) complexes of macrocyclic triamines promote the hydrolytic cleavage of plasmid DNA [174]. So, the copper(II) complexes possessing high nucleobase affinity and biologically accessible redox potentials are considered as potential reagents for cleavage of DNA both oxidatively [175] and hydrolytically [176]. Such metal complexes would permit targeting of specific DNA sites by matching the shape, symmetry and functionality of the complexes to those of the DNA target. Marin- Hernandez et al. [177] indicated that some mixed chelate transition metal-based drugs had more potent antitumor activity than cisplatin in in vivo and in vitro studies of a variety of tumor cells. However, human cancer cell lines are a useful model to study cell growth inhibition of tumor cells by natural compounds or newly synthesized compounds.
Sinha group have synthesized a monoanionic tetradentate- N2O2 Schiff base 2-[{[2-(dimethylamino)ethyl]imino}methyl]-6- methoxyphenol [178-181] and two of its analogous are mononuclear Co(II) derivatives, [Co(LH)2(NCS)]NO3 and [Co(LH)2(N3)]NO3. Interestingly, the tetradentate ligand LH behaves either in a bidentate- NO or terdentate-N2O fashion to coordinate the metal ions. The anticancer efficiency (in vitro) of these Co(II) derivatives has been investigated using various human cancer cells like human colorectal carcinoma cells (COLO 205 cells), human hepatocellular carcinoma cells (PLC5 cells), human lung carcinoma cells (A549 cells) and human fibroblasts cells (NIH 3T3). The biological effects of both Co(II) derivatives on the viability on NIH 3T3 cells indicate that these complexes induce a decrease in cell-population of human fibroblast cells with apoptosis. The human fibroblasts cells (NIH 3T3) are treated with [Co(LH)2(N3)]NO3 and the investigations reveal the apoptotic properties of the Co(II) complex and also suggest that a mitochondriamediated pathway is induced by this compound.
Recently, Stanojkovic et al. have reported [182] that the antiproliferative activity of zinc(II) complexes of 2-acetylpyridine1-(4- fluorophenyl)-piperazinylthiosemicarbazone is found to be noticeably stronger than that of cis-platin. The IC50 values range from 26-90 μM, against all cell lines tested, while for cis-platin the IC50 values range from 2-17 μM and for the zinc salt, ZnCl2, the IC50 values range from 81-93 μM. The highest activity is exhibited by [Zn(HAcPipPheF)(OAc)] complex against all four cancer cell lines whereas the highest selectivity is against K562 and MDA-MB-453 cancer cell lines. The tumor cell proliferation is achieved by arresting the cell cycle progression at the S phase by the compounds.
Recently, Stanojkovic et al. have reported [182] that the antiproliferative activity of zinc(II) complexes of 2-acetylpyridine1-(4- fluorophenyl)-piperazinylthiosemicarbazone is found to be noticeably stronger than that of cis-platin. The IC50 values range from 26-90 μM, against all cell lines tested, while for cis-platin the IC50 values range from 2-17 μM and for the zinc salt, ZnCl2, the IC50 values range from 81-93 μM. The highest activity is exhibited by [Zn(HAcPipPheF)(OAc)] complex against all four cancer cell lines whereas the highest selectivity is against K562 and MDA-MB-453 cancer cell lines. The tumor cell proliferation is achieved by arresting the cell cycle progression at the S phase by the compounds.
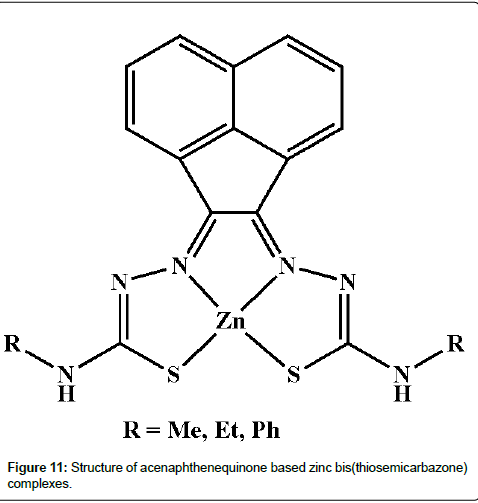
El-Asmy et al. have synthesized metal complexes having OO, ON, NS and ONS-donors and evaluated for anticancer activity against either Ehrlich ascites tumor cells (EACs) [183-186] or human cancer cell lines [187-189]. Recently, Pascu and his co-workers document that acenaphthenequinone based zinc bis(thiosemicarbazone) complexes (Figure 11) exhibit comparable cytotoxicity to cisplatin in the MCF-7 cell line and emit fluorescence as well [190].
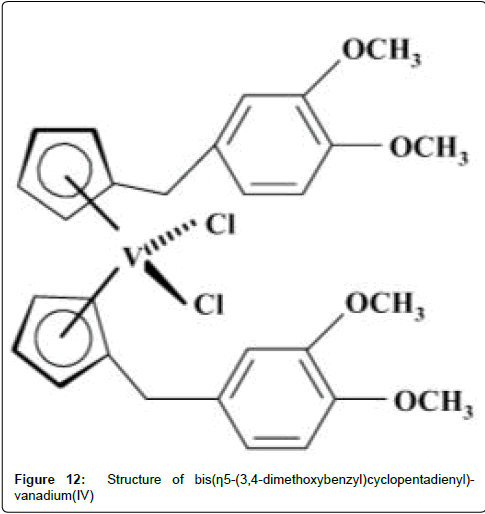
Harding and his co-worker have synthesized bis(η5-(3,4- dimethoxybenzyl) cyclopentadienyl)-vanadium(IV) dichloride complex. Further in vitro and in vivo work reveal that V(IV) organometallic compounds exhibit significant anti-tumor properties [191] with vanadocene dichloride being one of the most promising among metallocenes [192]. These results encourage further preclinical studies [193,194] and have since been extended [195] with the study of the cytotoxic properties of vanadocene (Figure 12) and various derivatives.
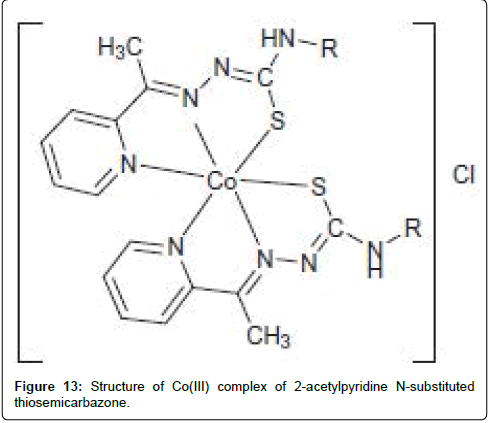
Manikandan et al. [196] have newly synthesized Co(II) complexes of 2-acetylpyridine N-substituted thiosemicarbazone with (PPh3)2 (Figure 13). The higher cytotoxic activity for the complex substituted benzene may be due to terminal phenyl substitution of the coordinated ligand. By comparing the cytotoxicity with that of the conventional standard cisplatin, they found that the complexes exhibited excellent activity in both the cancer cell lines. However, the cytotoxic activity of complexes against human breast cancer cell line (MCF-7) stood higher than that of skin carcinoma cell line (A431).
Anti-inflammatory agents
The protective response of an organism, when treated by a noxious stimulus is known as inflammation. Such inflammatory conditions lead to rheumatic diseases that cause major disability. It is a part of the complex biological response of vascular tissues to harmful stimuli such as pathogens, damaged cells and irritants.
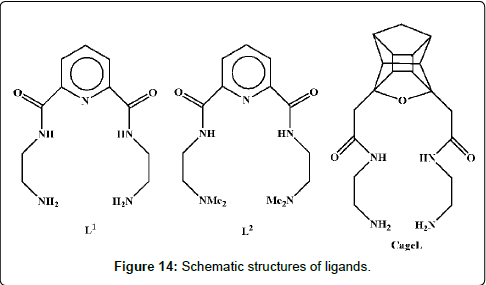
Recently, Odisitse and Jackson reveal that 3,5-diaminodiamido- 4-oxahexacyclododecane (cageL) (Figure 14) can survive in vivo through a demonstration by speciation calculations using blood plasma model and animal bio-distribution experiments, which is because they are stable in lipophilic conditions [197]. In pursuit of developing better copper(II)-based anti-inflammatory drugs which can be administered orally, intravenously or even transdermally, they have designed and synthesized two ligands, N,NO-di(aminoethylene)- 2,6-pyridinedicarbonylamine (L1) and bis-(N,Ndimethylethyl)-2,6- pyridinedicarboxamide (L2). L1 and L2 both have pyridyl groups which are found in most of the non-steroidal anti-inflammatory drugs (NSAIDs) [198].
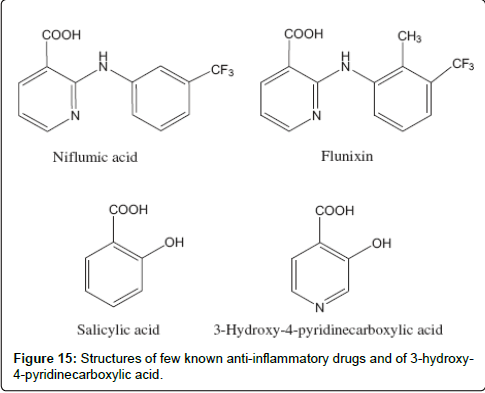
Pyridine derivatives are of high interest in several areas of medicinal chemistry, specifically, aminopyridinylmethanols and aminopyridinamines [199] have been considered as an analgesic as well as anti-inflammatory agents and for treating Alzheimer’s disease [200]. The pyridine ring characterizes niflumic acid and flunixin [201], two standard NSAIDs belonging to the class of fenamates (Scheme 15). These drugs are derived from N-aryl substituted anthranilic acid (2-amino-3-pyridinecarboxylic acid), and they are commonly used as analgesic, anti-inflammatory and anti-pyretic agents, like salicylates. Flunixin meglumine [202,203] is a substituted derivative of nicotinic acid (3-pyridinecarboxylic acid) which is structurally unique when compared to other NSAIDs (Figure 15).
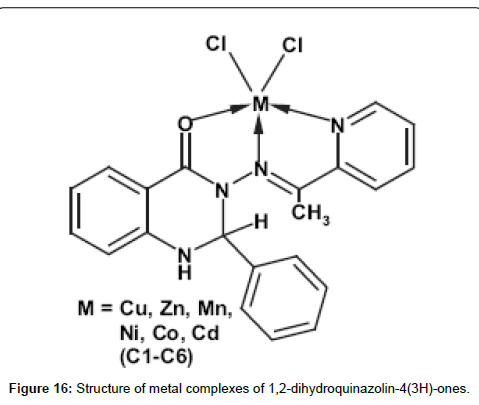
Hoonur group has studied about the 1,2-dihydroquinazolin- 4(3H)-ones based metal complexes along with their structure and biological activity [204-209] with a view to explore structure-activity relationships. Study of the reactivity of this free amino group with aromatic aldehydes resulted in the formation of biologically active 1,2-dihydroquinazolinone. Compounds have demonstrated dose dependent activity which is better even at lower dose level (3 mg/kg) while the reported analogous compounds have shown activity at higher dose level (such as 10, 20 and 50 mg/kg). The anti-inflammatory activity of these metal complexes is higher than their parent ligand perhaps due to their enhanced lipophilicities (Figure 16).
A class of quinoline based compounds has been explored and found to have the ability to inhibit platelet-activating factor (PAF) synthesis which also contributes to anti-inflammatory properties [210]. The role of copper in the pathology of inflammation emphasizes a lot of evidence [211]. The thorough investigation of copper complexes with different ligands together with anti-inflammatory drugs and of the copper containing enzyme super oxide dismutase (SOD) brought to light the various therapeutic value of copper which is concluded to be an exogenous anti-inflammatory agent [211].
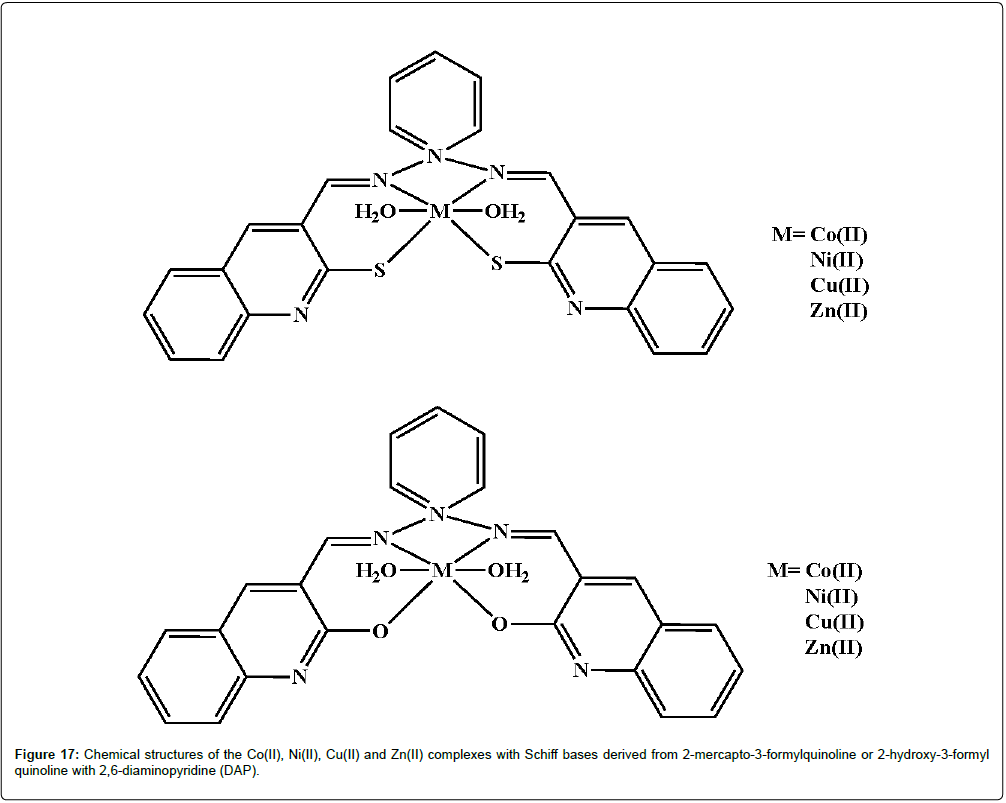
Naik and his coworkers have explored a series of Schiff bases derived from 2-mercapto-3-formyl quinoline/2-hydroxy-3-formyl quinoline with 2,6-diaminopyridine (DAP) and their corresponding Co(II), Ni(II), Cu(II) and Zn(II) complexes (Figure 17) for their anti-inflammatory activity [212]. The rats challenged by carrageenan when treated with the complexes significantly reduced the inflammatory edema. The complexes did not induce sedation, ataxia, tremors, convulsions, lacrimation or changes in motor activity in mice and caused no significant toxicity to the stomach, intestines and liver of mice. The Cu(II) complexes showed the highest biological activities amongst the compounds tested.
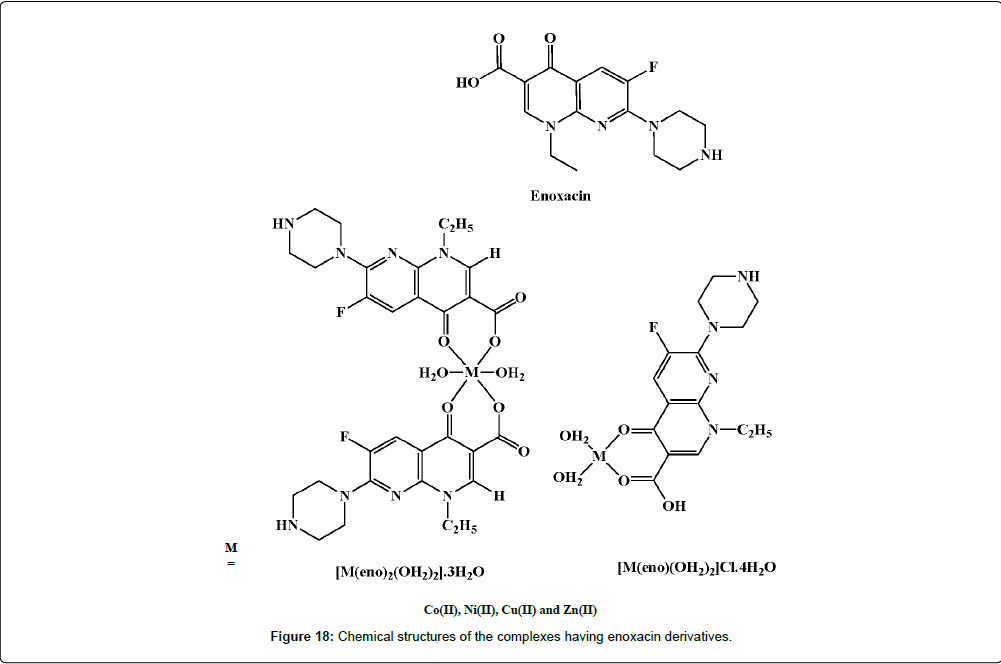
In 2009, Arayne and his group studied the anti-inflammatory properties of enoxacin and their Cu(II) and Ni(II) complexes (Figure 18) [213]. When enoxacin 36 is subjected to infrared spectroscopic analysis, it is inferred that the compound acts as a monoanionic bidentate ligand and is coordinated to the metal ions via its carboxyl and carbonyl groups. The levels of reactive free radicals released by activated phagocytic cells are measured for evaluating the antiinflammatory properties of the enoxacin complexes. The IC50 values of 15.3 and 18.7 μg.mL−1 of Cu(II) enoxacin complexes are found to be the most active against free radical release whereas enoxacin and its Ni(II) complexes are less effective (IC50>50 μg.mL−1). Nevertheless, the immunomodulatory effect of the enoxacin complexes that is governed by the molecular mechanism is not determined.
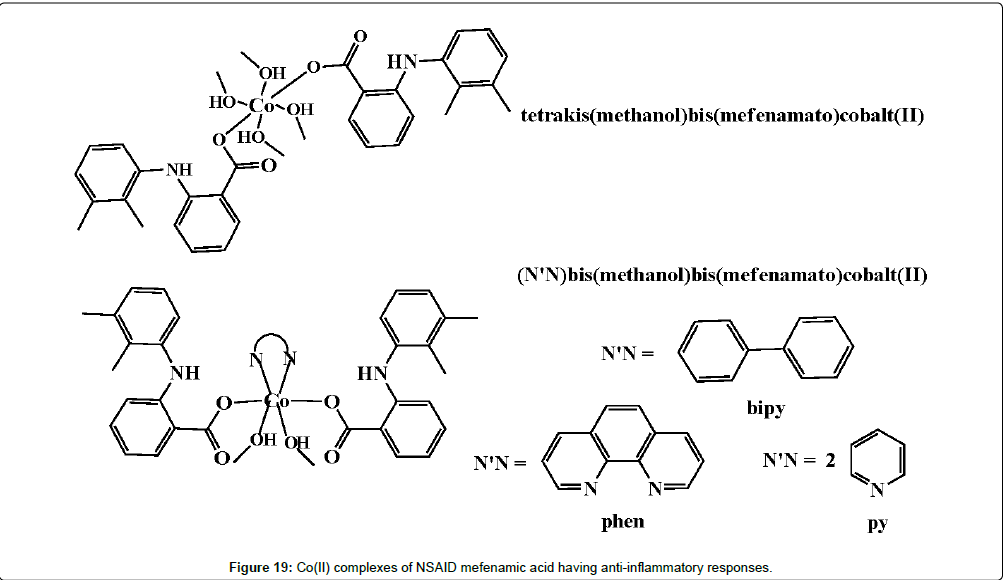
A series of potential anti-inflammatory agents that are Co(II) complexes and bearing the NSAID mefenamic acid ligand have also been investigated (Figure 19) [214-216]. Mefenamic acid is found to act as a deprotonated monodentate ligand. It is coordinated to the Co(II) ion through its carboxylato oxygen atom, forming octahedral [Co(mef)2(MeOH)4] or [Co(mef)2(MeOH)2(N^N)] (where mef = mefenamic acid and N^N = 2,2′-bipyridine, 1,10-phenanthroline or (pyridine)2) complexes which is in accordance with the physicochemical and spectroscopic data. In later studies, Cu(II) complexes of mefenamic acid, naproxen, diclofenac, diflunisal and flufenamic acid, Co(II) complexes of naproxen and tolfenamic acid, and Mn(II) complexes of tolfenamic acid have been reported by the research groups that showed anti-inflammatory activity.
Conclusions and Future Directions
In this work, the pharmacological effects of a few transition metal complexes have been reviewed. The application of bioinorganic chemistry to medicine is a rapidly developing field. Novel therapeutic and diagnostic metal complexes are now having an impact on medical practice. Advances in bioinorganic chemistry are important for improving the design of compounds to reduce toxic side-effects and understand their mechanisms of action. This review reveals that the pharmacologically interesting metals such as copper, cobalt, nickel and zinc could be a suitable strategy to develop novel therapeutic tools for the medical treatment.
Acknowledgements
The authors express their heartfelt thanks to the College Managing Board, Principal and Head of the Department of Chemistry, VHNSN College, Virudhunagar for their constant encouragement.
References
- Thompson KH (2011) Encyclopedia of Inorganic Chemistry. In: King RB (ed.), John Wiley & Sons Ltd., Chichester, UK, p: 1.
- Magner LN (2005) A History of Medicine (2nd edn.), Taylor & Francis Group, LLC: Boca Raton, FL, USA.
- Orvig C, Abrams MJ (1999) Medicinal inorganic chemistry: introduction. Chem Rev 99: 2201-2204.
- Thompson KH, Orvig C (2006) Concepts and Models in Bioinorganic Chemistry. In: Kraatz HB, Metzler-Nolte N(eds.), Wiley-VCH: Weinheim, Germany,p: 25.
- Thompson KH, Orvig C (2003) Boon and bane of metal ions in medicine. Science 300: 936-939.
- Naik AD, Annigeri SM, Gangadharmath UB, Revankar VK, Mahale VB, et al. (2002)Anchoring mercapto-triazoles on dicarbonyl backbone to assemble novel binucleating, acyclic SNONS compartmental ligands. Indian J Chem 41A: 2046-2053.
- Pedrares AS, Romero J, Vazquez JAG, Duran ML, Casanova I, et al. (2003) Electrochemical synthesis and structural characterisation of zinc, cadmium and mercury complexes of heterocyclic bidentate ligands (N, S). Dalton Trans 7: 1379-1388.
- El-Asmy AA, Khalifa ME, Hassanian MM (2004) Synthesis and characterization of transition metal complexes containing oxime, amido and thioamido groups. Indian J Chem 43A: 92-97.
- Ekegren JK, Roth P, Källström K, Tarnai T, Andersson PG (2003) Synthesis and evaluation of N,S-compounds as chiral ligands for transfer hydrogenation of acetophenone. Org Biomol Chem 1: 358-366.
- Zeglis BM, Pierre VC, Barton JK (2007) Metallo-intercalators and metallo-insertors. Chem Commun (Camb), pp: 4565-4579.
- Farrell N (2003) Comprehensive Coordination Chemistry II.In: McCleverty JA, Meyer TJ (eds.), Pergamon, Oxford, p: 809.
- Erkkila KE, Odom DT, Barton JK (1999) Recognition and reaction of metallointercalators with DNA. Chem Rev 99: 2777-2796.
- Ang WH, Dyson PJ (2006) Classical and Non-Classical Ruthenium-Based Anticancer Drugs: Towards Targeted Chemotherapy. Eur J Inorg Chem 2006: 4003-4018.
- Busto N, Valladolid J, Aliende C, Jalón FA, Manzano BR, et al. (2012) Preparation of organometallic ruthenium-arene-diaminotriazine complexes as binding agents to DNA. Chem Asian J 7: 788-801.
- Hannon MJ (2007) Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl Chem79: 2243-2261.
- Marzano C, Pellei M, Tisato F, Santini C (2009) Copper complexes as anticancer agents. Anticancer Agents Med Chem 9: 185-211.
- MejÃa Vázquez DMDC, Navarro S (2010)New Approaches in the Treatment of Cancer. Nova Science Publishers, New York, USA.
- Ramadan AEMM (2012) Macrocyclic nickel (II) complexes: Synthesis, characterization, superoxide scavenging activity and DNA-binding. Journal of Molecular Structure 1015: 56-66.
- Pothiraj K, Baskaran T, Raman N (2012) DNA interaction studies of d9 and d10 metal complexes having Schiff base and polypyridyl ligands. J Coord Chem 65: 2110-2126.
- Rajendiran V, Karthik R, Palaniandavar M, Stoeckli-Evans H, Periasamy VS, et al. (2007) Mixed-ligand copper(II)-phenolate complexes: effect of coligand on enhanced DNA and protein binding, DNA cleavage, and anticancer activity. Inorg Chem 46: 8208-8221.
- Williams RJP, Frausto da Silva JJR (1997) The Natural Selection of the Chemical Elements, Clarendon Press, Oxford.
- Fraústo da Silva JJR, Williams RJP (2001) The Biological Chemistry of the Elements(2ndedn.), Oxford University Press, Oxford.
- Siegel A, Siegel H, Siegel RKO (2007) Nickel and its Surprising Impact in Nature. Wiley, New York, USA.
- Kaim W, Rall J (1996) Copper-A “Modern†Bioelement. Angew Chem Int Ed 35: 43-60.
- Jaouen G (2006) Bioorganometallics: Biomolecules, Labeling, Medicine. Wiley-VCH, Weinheim
- Lippard SJ, Berg JM (1994) Principles of Bioinorganic Chemistry, University Science Books, Mill Valley (California).
- Holder AA (2012) Inorganic pharmaceuticals. Annu Rep Prog Chem, Sect A: Inorg Chem 108: 350-368.
- Ronconi L, Sadler PJ (2007)Using coordination chemistry to design new medicines. Coord Chem Rev 251: 1633-1648.
- Schwietert CW,McCue JP (1999)Coordination compounds in medicinal chemistry. Coord Chem Rev 184: 67-89.
- Kraatz HB, Metzler-Nolte N (2006) Concepts and Models in Bioinorganic Chemistry. Wiley-VCH: Weinheim, Germany.
- Jones CJ, Thornback JR (2007) Medicinal Applications of Coordination Chemistry. Royal Society of Chemistry: Cambridge, UK.
- Alessio E (2011) Bioinorganic Medicinal Chemistry(1stedn.), Wiley-VCH: Weinheim, Germany.
- Dabrowiak JC (2009) Metals in Medicine. John Wiley & Sons Ltd., Chichester, UK.
- Farrell NP (1999) Uses of Inorganic Chemistry in Medicine(1st edn.), Royal Society of Chemistry, Cambridge, UK.
- Gielen M, Tiekink ERT (2005) Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine (1stedn.), John Wiley & Sons Ltd.: Chichester, UK.
- Gaynor D, Griffith DM (2012) The prevalence of metal-based drugs as therapeutic or diagnostic agents: beyond platinum. Dalton Trans 41: 13239-13257.
- Casini A (2012) Exploring the mechanisms of metal-based pharmacological agents via an integrated approach. J Inorg Biochem 109: 97-106.
- Guo Z, Sadler PJ (1999) Metals in Medicine. Angew Chem Int Ed 38: 1512-1531.
- Sun RWY, Che CM (2009) The anti-cancer properties of gold (III) compounds with dianionic porphyrin and tetradentate ligands. Coord Chem Rev 253: 1682.
- Komeda S, Casini A (2012) Next-generation anticancer metallodrugs. Curr Top Med Chem 12: 219-235.
- P Farrell N (2011) Platinum formulations as anticancer drugs clinical and pre-clinical studies. Curr Top Med Chem 11: 2623-2631.
- Ott I (2009) On the medicinal chemistry of gold complexes as anticancer drugs. Coord Chem Rev 253: 1670-1681.
- da Silva MDR, Gonçalves JM, Silva AL, Oliveira PC, Schröder B, et al. (2004) Molecular thermochemical study of Ni (II), Cu (II) and Zn (II) complexes with N, N′-bis (salicylaldehydo) ethylenediamine, J Mol Catal A Chem 224: 207.
- Spinu C, Kriza A (2000) Co (II), Ni (II) and Cu (II) complexes of bidentate Schiff bases. Acta Chem Slov 47: 179-186.
- Jayamani A, Thamilarasan V, Sengottuvelan N, Manisankar P, Kang SK, et al. (2014) Synthesis of mononuclear copper(II) complexes of acyclic Schiff's base ligands: spectral, structural, electrochemical, antibacterial, DNA binding and cleavage activity. Spectrochim Acta A Mol Biomol Spectrosc 122: 365-374.
- Tan XJ, Liu HZ, Ye CZ, Lou JF, Liu Y, et al. (2014) Synthesis, characterization and in vitro cytotoxic properties of new silver (I) complexes of two novel Schiff bases derived from thiazole and pyrazine. Polyhedron 71: 119-132.
- Zianna A, Psomas G, Hatzidimitriou A, Coutouli-Argyropoulou E, Lalia-Kantouri M (2013) Zinc complexes of salicylaldehydes: synthesis, characterization and DNA-binding properties. J Inorg Biochem 127: 116-126.
- Parveen S, Arjmand F, Ahmad I (2014) Enantiomeric in vitro DNA binding, pBR322 DNA cleavage and molecular docking studies of chiral L- and D-ternary copper(II) complexes of histidine and picolinic acid. J Photochem Photobiol B 130: 170-178.
- Gwaram NS, Ali HM, Khaledi H, Abdulla MA, Hadi AH, et al. (2012) Antibacterial evaluation of some Schiff bases derived from 2-acetylpyridine and their metal complexes. Molecules 17: 5952-5971.
- Tisato F, Refosco F, Bandoli G (1994) Structural survey of technetium complexes. Coord Chem Rev 135: 325-397.
- Bell SC, Conklin GL, Childress SJ (1963) The separation of ketimine isomers. J Am Chem Soc 85: 2868-2869
- Sadler PJ, Guo Z (1998) Metal complexes in medicine: design and mechanism of action. Pure Appl Chem 70: 863-871.
- Tabassum S, Zaki M, Arjmand F, Ahmad I (2012) Synthesis of heterobimetallic complexes: In vitro DNA binding, cleavage and antimicrobial studies. J Photochem Photobiol B 114: 108-118.
- Arora K, Gupta A, Agarwal DD (2002)Semi-empirical AM1 and PM3 calculations for electronic structure of Schiff base. Asian J Chem 14: 1611-1615.
- Refat MS, El-Korashy SA, Kumar DN, Ahmed AS (2007) Syntheses and characterization of Ru (III) with chelating containing ONNO donor quadridentate Schiff bases. Spectrochim. Acta Part A 70: 898-906.
- Pandey A, Dewangan D, Verma S, Mishra A, Dubey RD (2011) Synthesis of schiff bases of 2-amino-5-aryl-1,3,4-thiadiazole and its analgesic, anti-inflammatory, antibacterial and antitubercular activity. Int J Chem Tech Res 3: 178-184.
- Zuo J, Bi C, Fan Y, Buac D, Nardon C, et al. (2013) Cellular and computational studies of proteasome inhibition and apoptosis induction in human cancer cells by amino acid Schiff base-copper complexes. J Inorg Biochem 118: 83-93.
- Khoo TJ, bin Break MK, Crouse KA, Tahir MIM, Ali AM, et al. (2014) Synthesis, characterization and biological activity of two Schiff base ligands and their nickel (II), copper (II), zinc (II) and cadmium (II) complexes derived from S-4-picolyldithiocarbazate and X-ray crystal structure of cadmium (II) complex derived from pyridine-2-carboxaldehyde. Inorg Chim Acta 413: 68-76.
- Nirmal R, Prakash C, Meenakshi K, Shanmugapandiyan P (2010) Synthesis and Pharmacological Evaluation of Novel Schiff Base Analogues of 3-(4-amino) Phenylimino) 5-fluoroindolin-2-one. J Young Pharm 2: 162-168.
- Sun RW, Ma DL, Wong EL, Che CM (2007) Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Trans, pp: 4884-4892.
- Mathew B, Vakketh SS, Kumar SS (2010) Synthesis, molecular properties and anthelmintic activity of some schiff bases of 1,3,4 thiadiazole derivatives. Der Pharma Chemica 2: 337-343.
- Raman N, Joseph J, Sakthivel A, Jeyamurugan R (2009) Synthesis, structural characterization and antimicrobial studies of novel Schiff base copper (II) complexes. J Chil Chem Soc 54: 354-357.
- Sadeek SA, Refat MS (2006) Preparation and Characterization of Tin (II) Complexes with Isomeric Series of Schiff Bases as Ligands. J Korea Chem Soc 50: 107.
- Tabassum S, Amir S, Arjmand F, Pettinari C, Marchetti F, et al. (2013) Mixed-ligand Cu(II)-vanillin Schiff base complexes; effect of coligands on their DNA binding, DNA cleavage, SOD mimetic and anticancer activity. Eur J Med Chem 60: 216-232.
- El-Ayaan U, Abdel-Aziz AA, Al-Shihry S (2007) Solvatochromism, DNA binding, antitumor activity and molecular modeling study of mixed-ligand copper(II) complexes containing the bulky ligand: bis[N-(p-tolyl)imino]acenaphthene. Eur J Med Chem 42: 1325-1333.
- Raman N, Jeyamurugan R, Senthilkumar R, Rajkapoor B, Franzblau SG (2010) In vivo and in vitro evaluation of highly specific thiolate carrier group copper(II) and zinc(II) complexes on Ehrlich ascites carcinoma tumor model. Eur J Med Chem 45: 5438-5451.
- Karamkar R, Choudhary CR, Mitra S, Dahlenburg L (2005) One novel Cu (II)–amino acid Schiff base complex derived from salicylaldehyde and L-serine: identification of unusual monodentate 4,4′-bipyridine. Struct Chem 16: 611-616.
- Hudasi KB, Patil MS, Vadavi RS, Shenoy RV, Patil SA (2006)X-ray crystal structure of the N-(2-hydroxy-1-naphthalidene) phenylglycine Schiff base. Synthesis and characterization of its transition metal complexes. Transit Met Chem 31: 580-585.
- Vittal JJ, Tiekink ERT (2006) Frontiers in Crystal Engineering. Wiley, England, p: 297
- Sreenivasulu B, Vetrichelvan M, Zhao F, Gas S, Vittal JJ (2005) Copper (II) complexes of Schiff?base and reduced Schiff?base ligands: Influence of weakly coordinating sulfonate groups on the structure and oxidation of 3, 5?DTBC. Eur J Inorg Chem 2005: 4635-4645.
- Wang XB, Ranford JD, Vittal JJ (2006) One-dimensional coordination polymers: Cu (II) and Zn (II) complexes of N-(2-pyridylmethyl)-glycine and N-(2-pyridylmethyl)-l-alanine. J Mol Struct 796: 28-35
- Ganguly R, Sreenivasulu B, Vittal JJ (2008) Amino acid-containing reduced Schiff bases as the building blocks for metallasupramolecular structures. Coord Chem Rev 252: 1027-1050.
- Barret GC, Elmore DT (1998) Amino Acids and Peptides. Cambridge University Press, Cambridge, p: 224.
- Gilman AG, Hardman JG, Limbird LE (1996) The Pharmacological Basis of Therapeutics (9th edn.). McGraw-Hill, New York, p: 1905.
- Reddy PR, Shilpa A (2011) Oxidative and hydrolytic DNA cleavage by Cu (II) complexes of salicylidene tyrosine schiff base and 1, 10 phenanthroline/bipyridine. Polyhedron 30: 565-572.
- Nagesh GY, Mruthyunjayaswamy BHM (2015) Synthesis, characterization and biological relevance of some metal (II) complexes with oxygen, nitrogen and oxygen (ONO) donor Schiff base ligand derived from thiazole and 2-hydroxy-1-naphthaldehyde. J Mol Struct 1085: 198-206.
- Reddy PR, Shilpa A, Raju N, Raghavaiah P (2011) Synthesis, structure, DNA binding and cleavage properties of ternary amino acid Schiff base-phen/bipy Cu(II) complexes. J Inorg Biochem 105: 1603-1612.
- Yang CT, Moubaraki B, Murray KS, Ranford JD, Vittal JJ (2001) Interconversion of a monomer and two coordination polymers of a copper(II)-reduced Schiff base ligand-1,10-phenanthroline complex based on hydrogen- and coordinative-bonding. Inorg Chem 40: 5934-5941.
- Yang CT, Moubaraki B, Murray KS, Vittal JJ (2003) Synthesis, characterization and properties of ternary copper (II) complexes containing reduced Schiff base N-(2-hydroxybenzyl)-α-amino acids and 1, 10-phenanthroline. J Chem Soc Dalton Trans 880-889.
- Reddy PAN, Nethaji M, Chakravarthy AR (2004) Hydrolytic cleavage of DNA by ternary amino acid Schiff base copper (II) complexes having planar heterocyclic ligands. Eur J Inorg Chem 7: 1440-1446.
- Pradhan R, Thomas AM, Mukherjee A, Dhar S, Nethaji M, et al. (2005) Synthesis, crystal structure and DNA hydrolysis activity of ternary (N-salicylidene-L-methioninato) copper (II) complexes of heterocyclic bases. Indian J Chem 44: 18-26.
- Keypour H, Shooshtari A, Rezaeivala M, Kup FO, Rudbari HA (2015) Synthesis of two new N2O4 macroacyclic Schiff base ligands and their mononuclear complexes: Spectral, X-ray crystal structural, antibacterial and DNA cleavage activity. Polyhedron 97: 75-82.
- Wang MZ, Meng ZX, Liu BL, Cai GL, Zhang CL, et al. (2005) Novel tumor chemotherapeutic agents and tumor radio-imaging agents: potential tumor pharmaceuticals of ternary copper (II) complexes. Inorg Chem Commun 8: 368-371.
- Sasmal PK, Patra AK, Nethaji M, Chakravarty AR (2007) DNA cleavage by new oxovanadium(IV) complexes of N-salicylidene alpha-amino acids and phenanthroline bases in the photodynamic therapy window. Inorg Chem 46: 11112-11121.
- Patra AK, Bhowmick T, Roy S, Ramakumar S, Chakravarty AR (2009) Copper(II) complexes of L-arginine as netropsin mimics showing DNA cleavage activity in red light. Inorg Chem 48: 2932-2943.
- Sasmal PK, Majumdar R, Dighe RR, Chakravarty AR (2010) Photocytotoxicity and DNA cleavage activity of L-arg and L-lys Schiff base oxovanadium(IV) complexes having phenanthroline bases. Dalton Trans 39: 7104-7113.
- Singh BK, Rajour HK, Prakash A (2012) Synthesis, characterization and biological activity of transition metal complexes with Schiff bases derived from 2-nitrobenzaldehyde with glycine and methionine. Spectrochim Acta A Mol Biomol Spectrosc 94: 143-151.
- Sathiyaraj S, Sampath K, Raja G, Butcher RJ, Gupta SK, et al. (2013) DNA binding/cleavage, antioxidant and cytotoxic activities of water soluble cobalt (II) and copper (II) antipyrine complexes. Inorganica Chimica Acta 406: 44-52.
- Sondhi SM, Sharma VK, Verma RP, Singhal N, Shukla R, et al. (1999) Synthesis, anti-inflammatory and analgesic activity evaluation of some mercapto pyrimidine and pyrimidobenzimidazole derivatives. Synthesis 1999: 878-884.
- Mishra AP (1999) Physicochemical and antimicrobial studies on nickel (II) and copper (II) Schiff base complexes derived from 2-furfuraldehyde. J Indian Chem Soc 76: 35-37.
- Cechinel Filho V, Corrêa R, Vaz Z, Calixto JB, Nunes RJ, et al. (1998) Further studies on analgesic activity of cyclic imides. Farmaco 53: 55-57.
- Bose R, Murty DSR, Chakrapani G (2005) Extraction of thorium (IV) as perchlorate and chloroacetate complexes with 1-phenyl-2,3-dimethyl-5-pyrazolone (antipyrine). J Radioanal Nucl Chem 265: 115-122.
- Ito T, Goto C, Noguchi K (2001)Lanthanoid ion-selective solvent polymeric membrane electrode based on 1-phenyl-3-methyl-4-octadecanoyl-5-pyrazolone. Anal Chim Acta 443: 41-51.
- Sondhi SM, Singhal N, Verma RP, Arora SK, Dastidar SG (2001)Synthesis of hemin and prophyrin derivatives and their evaluation for anticancer activity. Indian J Chem B 40: 113-119.
- Bao F, Lu X, Kang B, Wu Q (2006) Vinyl polymerization of norbornene catalyzed by a series of bis (β-ketoiminato) nickel (II) complexes in the presence of methylaluminoxane. Eur Polym J 42: 928-934.
- Sathiyaraj S, Sampath K, Butcher RJ, Pallepogu R, Jayabalakrishnan C (2013) Designing, structural elucidation, comparison of DNA binding, cleavage, radical scavenging activity and anticancer activity of copper(I) complex with 5-dimethyl-2-phenyl-4-[(pyridin-2-ylmethylene)-amino]-1,2-dihydro-pyrazol-3-one Schiff base ligand. Eur J Med Chem 64: 81-89.
- Swavey S, Brewer KJ (2004) Comprehensive Coordination Chemistry II. Pergamon Press, Oxford 1: 135.
- Steel PJ (1990) Aromatic nitrogen heterocycles as bridging ligands; a survey. Coord Chem Rev 106: 227
- Balzani V, Juris A, Venturi M, Campagna S, Serroni S (1996) Luminescent and Redox-Active Polynuclear Transition Metal Complexes. Chem Rev 96: 759-834.
- Kaes C, Katz A, Hosseini MW (2000) Bipyridine: the most widely used ligand. A review of molecules comprising at least two 2,2'-bipyridine units. Chem Rev 100: 3553-3590.
- Dumur F, Dumas E, Mayer CR (2007) Targets Heterocycl. In Syst 11: 70-103.
- Vos JG, Kelly JM (2006) Ruthenium polypyridyl chemistry; from basic research to applications and back again. Dalton Trans, pp: 4869-4883.
- Steel PJ (2005) Ligand design in multimetallic architectures: six lessons learned. Acc Chem Res 38: 243-250.
- Glasson CR, Lindoy LF, Meehan GV (2008) Recent developments in the d-block metallo-supramolecular chemistry of polypyridyls. Coord Chem Rev 252: 940-963.
- Leong WL, Vittal JJ (2011) One-dimensional coordination polymers: complexity and diversity in structures, properties, and applications. Chem Rev 111: 688-764.
- Liu TF, Lü J, Cao R (2010) Coordination polymers based on flexible ditopic carboxylate or nitrogen-donor ligands. Cryst Eng Comm 12: 660-670.
- Aakeröy CB, Champness NR, Janiak C (2010) Recent advances in crystal engineering. Cryst Eng Comm 12: 22-43.
- Zhang JP, Huang XC, Chen XM (2009) Supramolecular isomerism in coordination polymers. Chem Soc Rev 38: 2385-2396.
- Robson R (2008) Design and its limitations in the construction of bi-and poly-nuclear coordination complexes and coordination polymers (aka MOFs): a personal view. Dalton Transactions 38: 5113-5131.
- Robin AY, Fromm KM (2006) Coordination polymer networks with O-and N-donors: What they are, why and how they are made. Coord Chem Rev 250: 2127-2157.
- Kitagawa S, Noro S, Nakamura T (2006) Pore surface engineering of microporous coordination polymers. Chem Commun (Camb), pp: 701-707.
- Kitagawa S, Kitaura R, Noro S (2004) Functional porous coordination polymers. Angew Chem Int Ed Engl 43: 2334-2375.
- Sammes PG, Yahioglu G (1994) 1,10-Phenanthroline: a versatile ligand. Chem Soc Rev 23: 327-334.
- Luman CR, Castellano FN (2004)ComprehensiveCoordination Chemistry. In: McCleverty JA, Meyer TJ, Lever ABP (eds.), Elsevier, Oxford, UK, p: 25.
- Bencini A, Lippolis V (2010) 1,10-Phenanthroline: a versatile building block for the construction of ligands for various purposes. Coord Chem Rev 254: 2096-2180.
- Leiter J, Hartwell JL, Kahler JS, Kline I, Shear MJ (1953) Damage induced in sarcoma 37 with chemical agents. VI. Biphenyl, fluorene, phenanthrene, and tropolone derivatives. J Natl Cancer Inst 14: 365-374.
- Krishnamurty C, Byryan LA, Petering DH (1980) Effects of ethylenediaminetetraacetic acid and 1, 10-phenanthroline on cell proliferation and DNA synthesis of Ehrlich ascites cells. Cancer Res 40: 4092-4099.
- Cleare MJ, Hydes PC (1980) Metal Ions in Biological Systems, Sigel H(eds.), Dekker, New York, p: 11.
- Takamiya K (1960) Anti-tumour activities of copper chelates. Nature 185: 190-191.
- Ahmad M, Afzal M, Tabassum S, Kali?ska B, Mrozinski J, et al. (2014) Synthesis and structure elucidation of a cobalt(II) complex as topoisomerase I inhibitor: in vitro DNA binding, nuclease and RBC hemolysis. Eur J Med Chem 74: 683-693.
- Raman N, Mahalakshmi R, Mitu L (2014) Bio-sensitive activities of coordination compounds containing 1,10-phenanthroline as co-ligand: synthesis, structural elucidation and dna binding properties of metal(II) complexes. Spectrochim Acta A Mol Biomol Spectrosc 131: 355-364.
- Pravin N, Devaraji V, Raman N (2015) Targeting protein kinase and DNA molecules by diimine-phthalate complexes in antiproliferative activity. Int J Biol Macromol 79: 837-855.
- Sumby CJ (2011) Bridging ligands comprising two or more di-2-pyridylmethyl or amine arms: Alternatives to 2,2′-bipyridyl-containing bridging ligands. Coord Chem Rev 255: 1937-1967.
- Köpf -Maier P (1994) Complexes of metals other than platinum as antitumour agents. Eur J Clin Pharmacol 47: 1-16.
- Sathyaraj G, Weyhermüller T, Nair BU (2010) Synthesis, characterization and DNA binding studies of new ruthenium(II)bisterpyridine complexes. Eur J Med Chem 45: 284-291.
- Ali AQ, Teoh SG, Salhin A, Eltayeb NE, Ahamed MBK, et al. (2014) Synthesis of platinum (II) complexes of isatin thiosemicarbazones derivatives: In vitro anti-cancer and deoxyribose nucleic acid binding activities. Inorganica Chimica Acta 416: 235-244.
- Singh R, Afzal M, Zaki M, Ahmad M, Tabassum S, et al. (2014) Synthesis, structure elucidation and DFT studies of a new coumarin-derived Zn (ii) complex: in vitro DNA/HSA binding profile and pBR322 cleavage pathway. RSC Adv 4: 43504-43515.
- Patra AK, Bhowmick T, Ramakumar S, Nethaji M, Chakravarty AR (2008) DNA cleavage in red light promoted by copper(II) complexes of alpha-amino acids and photoactive phenanthroline bases. Dalton Trans, pp: 6966-6976.
- Zianna A, Psomas G, Hatzidimitriou A, Lalia-Kantouri M (2015) Copper (II) complexes of salicylaldehydes and 2-hydroxyphenones: synthesis, structure, thermal decomposition study and interaction with calf-thymus DNA and albumins. RSC Advances 5: 37495-37511.
- Duan RR, Ou ZB, Wang W, Chen S, Zhou XH (2015) Synthesis, crystal structures, photoluminescence properties and DNA binding of triazine-nickel(II) complexes for DNA detection. Spectrochim Acta A Mol Biomol Spectrosc 151: 64-71.
- Zheng K, Zhu L, Li YT, Wu ZY, Yan CW (2015) Synthesis and crystal structure of new dicopper(II) complexes having asymmetric N,N'-bis(substituted)oxamides with DNA/protein binding ability: In vitro anticancer activity and molecular docking studies. J Photochem Photobiol B 149: 129-142.
- Song WJ, Lin QY, Jiang WJ, Du FY, Qi QY, et al. (2015) Synthesis, interaction with DNA and antiproliferative activities of two novel Cu(II) complexes with norcantharidin and benzimidazole derivatives. Spectrochim Acta A Mol Biomol Spectrosc 137: 122-128.
- Duskova K, Sierra S, Fernandez MJ, Gude L, Lorente A (2012) Synthesis and DNA interaction of ethylenediamine platinum(II) complexes linked to DNA intercalants. Bioorg Med Chem 20: 7112-7118.
- Pravin N, Raman N (2014) Investigation of in vitro anticancer and DNA strap interactions in live cells using carboplatin type Cu (II) and Zn (II) metalloinsertors. Eur J Med Chem 85: 675-687.
- da Silveira VC, Benezra H, Luz JS, Georg RC, Oliveira CC, et al. (2011) Binding of oxindole-Schiff base copper (II) complexes to DNA and its modulation by the ligand. Journal of Inorganic Biochemistry 105: 1692-1703.
- Kozlyuk N, Lopez T, Roth P, Acquaye JH (2015) Synthesis and the characterization of Schiff-base copper complexes: reactivity with DNA, 4-NPP and BNPP. Inorg Chim Acta 428: 176-184.
- Yanga XB, Wanga Q, Huangb Y, Fua PH, Zhangc JS, et al. (2012) Synthesis, DNA interaction and antimicrobial activities of copper (II) complexes with Schiff base ligands derived from kaempferol and polyamines. Inorg Chem Commun 25: 55-59.
- Songa WJ, Cheng JP, Jiang DH, Guo L, Cai MF, et al. (2014) Synthesis, interaction with DNA and antiproliferative activities of two novel Cu (II) complexes with Schiff base of benzimidazoles. Spectrochimi Acta Part A 121: 70-76.
- Gökçe C, Gup R (2013) Synthesis, characterization and DNA interaction of new copper(II) complexes of Schiff base-aroylhydrazones bearing naphthalene ring. J Photochem Photobiol B 122: 15-23.
- Li A, Liu YH, Yuan LZ, Ma ZY, Zhao CL, et al. (2015) Association of structural modifications with bioactivity in three new copper(II) complexes of Schiff base ligands derived from 5-chlorosalicylaldehyde and amino acids. J Inorg Biochem 146: 52-60.
- Hambley TW (2007) Developing new metal-based therapeutics: challenges and opportunities. Dalton Trans, pp: 4929-4937.
- Chew ST, Lo KM, Lee SK, Heng MP, Teoh WY, et al. (2014) Copper complexes with phosphonium containing hydrazone ligand: topoisomerase inhibition and cytotoxicity study. Eur J Med Chem 76: 397-407.
- Thompson KH, Orvig C (2006) Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans 35: 761-764.
- Vijesh AM, Isloor AM, Shetty P, Sundershan S, Fun HK (2013) New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem 62: 410-415.
- Nishida J, Kawabata J (2006) DPPH radical scavenging reaction of hydroxy- and methoxychalcones. Biosci Biotechnol Biochem 70: 193-202.
- Miller AL (1996) Antioxidant flavonoids: structure, function and clinical usage. Alt Med Rev 1: 103-111
- Rezk BM, Haenen GR, van der Vijgh WJ, Bast A (2002) The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem Biophys Res Commun 295: 9-13.
- Aust SD, Morehouse LA, Thomas CE (1985) Role of metals in oxygen radical reactions. J Free Radic Biol Med 1: 3-25.
- Cardoso SM, Pereira C, Oliveira CR (1998) The protective effect of vitamin E, idebenone and reduced glutathione on free radical mediated injury in rat brain synaptosomes. Biochem Biophys Res Commun 246: 703-710.
- Mates JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153: 83-104.
- Miller DM, Buettner GR, Aust SD (1990) Transition metals as catalysts of "autoxidation" reactions. Free Radic Biol Med 8: 95-108.
- Turel I (2002) The interactions of metal ions with quinolone antibacterial agents. Coord Chem Rev 232: 27-47.
- Ruiz M, Perello L, Ortiz R, Castineiras A, Maichle-Mössmer C, et al. (1995) Synthesis, characterization, and crystal structure of [Cu(cinoxacinate)2].2H2O complex: a square-planar CuO4 chromophore. Antibacterial studies. J Inorg Biochem 59: 801-810.
- Chohan ZH, Supuran CT, Scozzafava A (2005) Metal binding and antibacterial activity of ciprofloxacin complexes. J Enz Med Chem 20: 303-307.
- Â Irgi EP, Geromichalos GD, Balala S, Kljun J, Kalogiannis S, et al. (2015) Cobalt (ii) complexes with the quinolone antimicrobial drug oxolinic acid: structure and biological perspectives. RSC Advances 5: 36353-36367.
- Kumar MP, Tejaswi S, Rambabu A, Kalalbandi VKA (2015) Synthesis, crystal structure, DNA binding and cleavage studies of copper (II) complexes with isoxazole Schiff bases. Polyhedron 102: 111-120.
- Devi J, Batra N (2015) Synthesis, characterization and antimicrobial activities of mixed ligand transition metal complexes with isatin monohydrazone Schiff base ligands and heterocyclic nitrogen base. Spectrochim Acta A Mol Biomol Spectrosc 135: 710-719.
- Dhanaraj CJ, Johnson J (2015) Quinoxaline based bio-active mixed ligand transition metal complexes: Synthesis, characterization, electrochemical, antimicrobial, DNA binding, cleavage, antioxidant and molecular docking studies. Journal of Photochemistry and Photobiology B: Biology 151: 100-109.
- Chandra S, Vandana, Kumar S (2015) Synthesis, spectroscopic, anticancer, antibacterial and antifungal studies of Ni(II) and Cu(II) complexes with hydrazine carboxamide, 2-[3-methyl-2-thienyl methylene]. Spectrochim Acta A Mol Biomol Spectrosc 135: 356-363.
- O'Connor M, Kellett A, McCann M, Rosair G, McNamara M, et al. (2012) Copper(II) complexes of salicylic acid combining superoxide dismutase mimetic properties with DNA binding and cleaving capabilities display promising chemotherapeutic potential with fast acting in vitro cytotoxicity against cisplatin sensitive and resistant cancer cell lines. J Med Chem 55: 1957-1968.
- Silva PP, Guerra W, Silveira JN, Ferreira AM, Bortolotto T, et al. (2011) Two new ternary complexes of copper(II) with tetracycline or doxycycline and 1,10-phenanthroline and their potential as antitumoral: cytotoxicity and DNA cleavage. Inorg Chem 50: 6414-6424.
- Abdi K, Hadadzadeh H, Weil M, Salimi M (2012) Mononuclear copper (II) complex with terpyridine and an extended phenanthroline base, [Cu(tpy)(dppz)] 2+: Synthesis, crystal structure, DNA binding and cytotoxicity activity. Polyhedron 31: 638-648.
- Pivetta T, Cannas MD, Demartin F, Castellano C, Vascellari S, et al. (2011) Synthesis, structural characterization, formation constants and in vitro cytotoxicity of phenanthroline and imidazolidine-2-thione copper(II) complexes. J Inorg Biochem 105: 329-338.
- Maheswari PU, Roy S, den Dulk H, Barends S, van Wezel G, et al. (2006) The square-planar cytotoxic [Cu(II)(pyrimol)Cl] complex acts as an efficient DNA cleaver without reductant. J Am Chem Soc 128: 710-711.
- Ranford JD (1993) Sadler PJ and Tocher DA. J Chem Soc Dalton Trans 1993: 3393.
- Ramakrishnan S, Rajendiran V, Palaniandavar M, Periasamy VS, Srinag BS, et al. (2009) Induction of cell death by ternary copper(II) complexes of L-tyrosine and diimines: role of coligands on DNA binding and cleavage and anticancer activity. Inorg Chem 48: 1309-1322.
- Ramakrishnan S, Shakthipriya D, Suresh E, Periasamy VS, Akbarsha MA, et al. (2011) Ternary dinuclear copper(II) complexes of a hydroxybenzamide ligand with diimine coligands: the 5,6-dmp ligand enhances DNA binding and cleavage and induces apoptosis. Inorg Chem 50: 6458-6471.
- Loganathan R, Ramakrishnan S, Suresh E, Riyasdeen A, Akbarsha MA, et al. (2012) Mixed ligand copper(II) complexes of N,N-bis(benzimidazol-2-ylmethyl)amine (BBA) with diimine co-ligands: efficient chemical nuclease and protease activities and cytotoxicity. Inorg Chem 51: 5512-5532.
- Bhat SS, Kumbhar AA, Heptullah H, Khan AA, Gobre VV, et al. (2011) Synthesis, electronic structure, DNA and protein binding, DNA cleavage, and anticancer activity of fluorophore-labeled copper(II) complexes. Inorg Chem 50: 545-558.
- Foye WO (1995) Cancer Chemotherapeutic Agents. American Chemical Society, Washington DC, USA.
- Sigman DS, Bruice TW, Mazumder A, Sutton CL (1993) Targeted chemical nucleases. Acc Chem Res 26: 98-104
- Hegg EL, Burstyn JN (1998) Toward the development of metal-based synthetic nucleases and peptidases: a rationale and progress report in applying the principles of coordination chemistry. Coord Chem Rev 173: 133-165.
- Pogozelski WK, Tullius TD (1998) Oxidative Strand Scission of Nucleic Acids: Routes Initiated by Hydrogen Abstraction from the Sugar Moiety. Chem Rev 98: 1089-1108.
- Mancin F, Scrimin P, Tecilla P, Tonellato U (2005) Artificial metallonucleases. Chem Commun (Camb), pp: 2540-2548.
- Mar??n-Hernández A, Gracia-Mora I, Ruiz-RamÃÂrez L, Moreno-Sánchez R (2003) Toxic effects of copper-based antineoplastic drugs (Casiopeinas) on mitochondrial functions. Biochem Pharmacol 65: 1979-1989.
- Dey SK, Mondal N, El Fallah MS, Vicente R, Escuer A, et al. (2004) Crystal structure and magnetic interactions in nickel(II) dibridged complexes formed by two azide groups or by both phenolate oxygen-azide, -thiocyanate, -carboxylate, or -cyanate groups. Inorg Chem 43: 2427-2434.
- Choudhury CR, Dey SK, Karmakar R, Wu CD, Lu CZ, et al. (2003) First report of singly phenoxo-bridged copper (II) dimeric complexes: synthesis, crystal structure and low-temperature magnetic behaviour study. New J Chem 27: 1360-1366.
- Sen S, Talukder P, Dey SK, Mitra S, Rosair G, et al. (2006) Ligating properties of a potentially tetradentate Schiff base [(CH3)2NCH2CH2N=CHC6H3(OH)(OMe)] with zinc(II), cadmium(II), cobalt(II), cobalt(III) and manganese(III) ions: synthesis and structural studies. Dalton Trans, pp: 1758-1767.
- Das K, Datta A, Liu PH, Huang JH, Hsu CL, et al. (2014) Structural characterization of cobalt (II) complexes of an N, O donor Schiff base and their activity on carcinoma cells. Polyhedron 71: 85-90.
- Stanojkovic TP, Kovala-Demertzi D, Primikyri A, Garcia-Santos I, Castineiras A, et al. (2010) Zinc(II) complexes of 2-acetyl pyridine 1-(4-fluorophenyl)-piperazinyl thiosemicarbazone: Synthesis, spectroscopic study and crystal structures - potential anticancer drugs. J Inorg Biochem 104: 467-476.
- Gabr IM, El-Asmy HA, Emmam MS, Mostafa SI (2009) Synthesis, characterization and anticancer activity of 3-aminopyrazine-2-carboxylic acid transition metal complexes. Transition Metal Chemistry 34: 409-418.
- Mostafa SI (2007) Mixed ligand complexes with 2-piperidine-carboxylic acid as primary ligand and ethylene diamine, 2,2′-bipyridyl,1,10-phenanthroline and 2 (2′-pyridyl) quinoxaline as secondary ligands: preparation, characterization and biological activity. Transition Metal Chemistry 32: 769-775.
- Mostafa SI (2008) Synthesis, characterization and antineoplastic activity of 5-chloro-2, 3-dihydroxypyridine transition metal complexes. J Coord Chem 61: 1553-1567.
- Mostafa SI, Badria FA (2008) Synthesis, Spectroscopic, and Anticancerous Properties of Mixed Ligand Palladium(II) and Silver(I) Complexes with 4,6-Diamino-5-hydroxy-2-mercaptopyrimidine and 2,2'-Bipyridyl. Met Based Drugs 2008: 723634.
- El-Deen AAA, El-Askalany AEME, Halaoui R, Jean-Claude BJ, Butler IS, et al. (2013) New zinc (II), palladium (II) and platinum (II) complexes of dl-piperidine-2-carboxylic acid; X-ray crystal structure of trans-[Zn 2 (μ-Ca) 2 (Hpa) 2 Cl 6] and anticancer activity of some complexes. J Mol Struct 1036: 161-167.
- Elsayed SA, Jean-Claude BJ, Butler IS, Mostafa SI (2012) Synthesis, structural characterization and anticancer activity of some new complexes of 6-amino-4-hydroxy-2-thiopyrimidine. J Mol Struct 1028: 208-214.
- Elsayed SA, El-Hendawy AM, Mostafa SI, Jean-Claude BJ, Todorova M, et al. (2010) Antineoplastic activity of new transition metal complexes of 6-methylpyridine-2-carbaldehyde-n (4)-ethylthiosemicarbazone: X-Ray crystal structures of [VO2 (mpETSC)] and [Pt (mpETSC) Cl]. Bioinorg Chem Appl 2010: 149149.
- Pascu SI, Waghorn PA, Conry TD, Lin B, Betts HM, et al. (2008) Cellular confocal fluorescence studies and cytotoxic activity of new Zn (II) bis (thiosemicarbazonato) complexes. Dalton Trans 16: 2107-2110.
- Harding MM, Mokdsi G (2000) Antitumour metallocenes: structure-activity studies and interactions with biomolecules. Curr Med Chem 7: 1289-1303.
- Havelek R, Siman P, Cmielova J, Stoklasova A, Vavrova J, et al. (2012) Differences in vanadocene dichloride and cisplatin effect on MOLT-4 leukemia and human peripheral blood mononuclear cells. Med Chem 8: 615-621.
- Vinklárek J, Honz???ek J, Holubová J (2004) Interaction of the antitumor agent vanadocene dichloride with phosphate buffered saline.Inorg Chim Acta 357: 3765-3769.
- Palá?ková H, Vinklárek J, Holubová J, CÃsa?ová I, Erben M (2007) The interaction of antitumor active vanadocene dichloride with sulfur-containing amino acids. J Organomet Chem 692: 3758-3764.
- Claffey J, Deally A, Gleeson B, Hogan M, Méndez LM, et al. (2009) Pseudo-halide derivatives of titanocene: synthesis and cytotoxicity studies. Metallomics 1: 511-517.
- Manikandan R, Viswanathamurthi P, Velmurugan K, Nandhakumar R, Hashimoto T, et al. (2014) Synthesis, characterization and crystal structure of cobalt(III) complexes containing 2-acetylpyridine thiosemicarbazones: DNA/protein interaction, radical scavenging and cytotoxic activities. J Photochem Photobiol B 130: 205-216.
- Odisitse S, Jackson GE, Govender T, Kruger HG, Singh A (2007) Chemical speciation of copper(II) diaminediamide derivative of pentacycloundecane-a potential anti-inflammatory agent. Dalton Trans, pp: 1140-1149.
- Odisitse S, Jackson GE (2009) In vitro and in vivo studies of the dermally absorbed Cu (II) complexes of N5O2 donor ligands-Potential anti-inflammatory drugs. Inorg Chimi Acta 362: 125-135.
- Sondhi SM, Singhal N, Johar M, Reddy BS, Lown JW (2002) Heterocyclic compounds as inflammationinhibitors. Curr Med Chem 9: 1045-1074.
- Mohareb RM, Zaki MY, Abbas NS (2015) Synthesis, anti-inflammatory and anti-ulcer evaluations of thiazole, thiophene, pyridine and pyran derivatives derived from androstenedione. Steroids 98: 80-91.
- Booth NH, Mcdonald LE (1988) Veterinary pharmacology and therapeutics(5thedn.), Iowa State University Press, Ames.
- Ciofalo VB, Latranyi MB, Patel JB, Taber RI (1977) Flunixin meglumine: a non-narcotic analgesic. J Pharmacol Exp Ther 200: 501-507.
- Brun P, Dean A, Di Marco V, Surajit P, Castagliuolo I, et al. (2013) Peroxisome proliferator-activated receptor-γ mediates the anti-inflammatory effect of 3-hydroxy-4-pyridinecarboxylic acid derivatives: synthesis and biological evaluation. Eur J Med Chem 62: 486-497.
- Gudasi KB, Vadavi RS, Shenoy RV, Patil MS, Patil SA, et al. (2005) Five-coordinate cobalt (II), nickel (II) and zinc (II) complexes derived from 2-pyridine-2-yl-3-(pyridine-2-carboxylideneamino)-1,2-dihydroquinazolin-4 (3H)-one. The crystal structure of the cobalt (II) complex. Transit Met Chem 30: 661-668.
- Gudasi KB, Vadavi RS, Shenoy RV, Patil SA, Nethaji M (2006). Crystal Structure of 2-thiophene-2-yl-3 (thiophene-2-carboxylideneamino)-1,2-dihydroquinazolin-4(3H)-one and the Synthesis, Spectral and Thermal Studies of its Transition Metal (II) Complexes. Transit Met Chem 31: 374-381.
- Gudasi KB, Patil SA, Vadavi RS, Shenoy RV, Nethaji M (2006) Crystal structure of 2-[2-hydroxy-3-methoxyphenyl]-3-[2-hydroxy-3-methoxybenzylamino]-1,2-dihydroquinazolin-4(3H)-one and the synthesis, spectral and thermal investigation of its transition metal complexes. Transit Met Chem 31: 586-592.
- Gudasi KB, Shcnoy RV, Vadavi RS, Palil MS, Palil SA (2005) New lanthanide (llf) complexes of biologically active 2,3-disubstituted quinazoline-4-(3H)-one: Synthesis, characterization and biological studies. Indian J Chem 44A: 2247-2254.
- Gudasi KB, Havanur VC, Patil SA, Patil BR (2007) Antimicrobial Study of Newly Synthesized Lanthanide(III) Complexes of 2-[2-hydroxy-3-methoxyphenyl]-3-[2-hydroxy-3-methoxybenzylamino]-1,2-dihydroquinazolin-4(3H)-one. Met Based Drugs 2007: 37348.
- Hoonur RS, Patil BR, Badiger DS, Vadavi RS, Gudasi KB, et al. (2010) Transition metal complexes of 3-aryl-2-substituted 1, 2-dihydroquinazolin-4 (3H)-one derivatives: New class of analgesic and anti-inflammatory agents. Eur J Med Chem 45: 2277-2282.
- Masarwa AS, Herron N, Fendrick CM, Buseh DH (1994) Synthesis, Characterization, Anti-Microbial and Anti-Inflammatory activity, Studies of Novel Schiff base 3,3’-{1,2-PhenyleneBis[Nitrilo(E) Methylydine]} Diquinolin-2-ol and its Metal(II) Complexes.Inorg Chem 42: 1086.
- Bossard GE, George TA (1981)Reactions of coordinated dinitrogen. 12. Identification of intermediates in the conversion of molybdenum-bound dinitrogen into ammonia and hydrazine. Factors affecting the ammonia-forming reactionInorg Chim Acta 54: 1249.
- Hogaboam CM, Donigi-Gale D, Scott Shoupe T, Bissonnette EY, Befus AD, et al. (1992) Platelet-activating factor synthesis by peritoneal mast cells and its inhibition by two quinoline-based compounds.Br J Pharmacol 105: 87-92.
- Narayanachar, Dhumwad SD, Mutalik S, Hugar MH, Naik PN (2013) Synthesis, spectral characterization of Co (II), Ni (II), Cu (II) and Zn (II) complexes of Schiff bases derived from 3-formyl quinoline and 2,6-diaminopyridine and their biological studies. Main Group Chem 12: 87-104.
- Arayne S, Sultana N, Haroon U, Mesaik MA (2009) Synthesis, characterization, antibacterial and anti-inflammatory activities of enoxacin metal complexes. Bioinorg Chem Appl.
- Dimiza F, Papadopoulos AN, Tangoulis V, Psycharis V, Raptopoulou CP, et al. (2010) Biological evaluation of non-steroidal anti-inflammatory drugs-cobalt(II) complexes. Dalton Trans 39: 4517-4528.
Citation: Selvaganapathy M, Raman N (2016) Pharmacological Activity of a Few Transition Metal Complexes: A Short Review. J Chem Biol Ther 2: 108. DOI: 10.4172/2572-0406.1000108
Copyright: © 2016 Selvaganapathy M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 65156
- [From(publication date): 8-2016 - Sep 01, 2025]
- Breakdown by view type
- HTML page views: 61898
- PDF downloads: 3258