Plasma Cortisol, Testosterone, Estradiol and Progesterone Levels in Children with Acute Alcohol Intoxication
Received: 24-Mar-2011 / Accepted Date: 12-May-2011 / Published Date: 15-May-2011 DOI: 10.4172/2155-6105.1000111
Abstract
Aim: To study the relationships among plasma testosterone, estradiol, progesterone and cortisol levels with plasma glucose, lactate, sodium, and potassium concentrations in children hospitalised with acute alcohol intoxication (AI).
Methods: Data was analysed from 264 children aged 8-17 years who were hospitalised at Estonia's two children's hospital over a three-year period. In each case, the on-call paediatrician completed a special form about the clinical symptoms of AI. A blood sample was taken and serum was analysed for testosterone, estradiol, progesterone, cortisol, glucose, lactate, sodium, and potassium levels.
Results: The most common finding was an increased level of cortisol in 77.7 percent of patients (N=205). Cortisol levels correlated positively with glucose levels (r=0.29; p<0.05) and progesterone levels (r=0.62; p<0.05) in girls. Three children had serum lactate level above critical 5 mmol/L.
Keywords: Alcohol intoxication; Cortisol; Progesterone; Testosterone; Glucose; Sodium
Introduction
Alcohol consumption among children and adolescents is a growing problem in most industrialised countries [1-3]. As the frequency and amount of alcohol consumption has gradually increased, medical personnel at hospitals have had to become prepared for the corresponding increased workload. Biochemical tests, such as those for glucose, lactate and electrolytes levels have been used to study the clinical condition of children with AI. The studies performed in healthy adult volunteers [4-5] showed that hyperlactinaemia, hypokalaemia [6], hypernatraemia and hypoglycaemia [7], or hyperglycaemia do occur in AI. Hypoglycaemia has been found to be a severe problem in young children with acute AI [8]. Although it is not very common [9], it still requires careful examination. Hyperglycaemia can also occur in these children [9] as a result of the increased cortisol levels due to stimulation of the adrenocorticotropic hormone. The level of cortisol, one of the main stress hormones, is increased by various stress factors, including pain, inflammation, burns and scares. Some researchers have found an association between blood alcohol concentration and increased serum cortisol concentration [10-12], although others have not [13].
Many studies have investigated changing serum sex hormone levels in adults with AI, finding that a significant relationship exists between alcohol intake and serum sex hormones levels. Testosterone concentration has decreased [14,15] and estradiol concentration has not been affected by ethanol [16]. Increased progesterone levels have been found in alcohol-intoxicated women in the lutheal phase [16], but other studies have found decreased progesterone concentrations by ethanol [17]. We aimed to study the relationships among plasma testosterone, estradiol, progesterone and cortisol levels with plasma glucose, lactate, sodium, and potassium concentrations in children hospitalised with acute alcohol intoxication.
Methods and Subjects
The study included all 8-17-year-old children hospitalised with acute AI at Estonia's two main children's hospitals - Tartu University Children's Clinic and Tallinn Children's Hospital - over a three-year period (Dec 2005-Dec 2008). No lower age limit was set; that is, all self-intoxicated by alcohol children were included. The upper age limit (17.9 years) was set by the children's hospitals. Alcohol intoxication was diagnosed by Diagnostic and Statistical Manual of Mental Disorders (DSM IV), and criteria were used for building up a medical assessment form. Upon hospitalisation, an anonymous encoded medical assessment form was completed by an on-call paediatrician. The medical assessment form included data about the child's mental status (consciousness, balance, speech, nystagmus), physical status (blood pressure, pulse rate, muscle tone), menstrual cycle in girls, consumption of alcohol (time, amount, repeatedly or first time) and the time of medical assessment. The on-call paediatrician was also asked to estimate the level of drunkenness through a clinical examination. However, this was sometimes complicated and the level of drunkenness could only be estimated by serum alcohol concentration. The children with AI were divided into three groups, based on their serum alcohol concentration: 0.21-1.49, 1.50-2.49 and =2.50 g/L. Blood samples were drawn for the measurements of serum alcohol, plasma cortisol and sex hormones level, glucose, lactate, sodium, and potassium concentrations. Urine samples were also collected in order to exclude the use of narcotic substances.
Biochemical tests were completed immediately after the collection of samples. Lithium heparin plasma was separated and kept at -20°C until hormone measurements were made within a three-month interval in the laboratory of Tartu University Hospital. Serum ethanol concentration was determined using the TDxFLx (Abbott Diagnostics) in both hospitals. At Tartu University Hospital, plasma glucose and lactate were determined by Cobas Integra 400plus (Roche), and potassium and sodium AVL 988-4 (Roche). At Tallinn Children's Hospital, glucose, lactate, potassium and sodium were determined by ABL 700 Radiometer (Radiometer Analytical). Both laboratories participate in the Lab quality control programme. Plasma cortisol, testosterone, estradiol and progesterone levels were measured by Immulite 2000 (Siemens Healthcare Diagnostics). Hormones laboratory participate in the RIQAS quality control programme.
Statistical analysis was performed using the Statistica 9.0 statistical programme and descriptive statistics were used to analyse children's general data. Spearman Rank Order Correlations was used to assess bivariate relationships. A p value <0.05 was considered statistically significant.
The study was approved by the Ethics Review Committee on Human Research of the University of Tartu, and procedures were followed in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Results
From December 2005 to December 2008, 417 children and adolescents were hospitalised at the two hospitals with suspected AI. Twenty children were excluded because their serum alcohol concentration was below 0.20 g/L; according to the International Statistical Classification of Diseases and Related Health Problems X, they were considered to have not used alcohol or all of the blood alcohol was already eliminated by the time of alcohol measurement. Narcotic intoxication was confirmed with a rapid urine test in three children, who were also excluded. One child, a newborn baby with an alcohol concentration of 2.05 g/L, was excluded because she had not consumed alcohol by herself. One hundred and twenty nine children dropped out from the study group because they refused testing, there were mistakes in blood drawing, biochemical tests had not been conducted or the medical form had not been completed.
The children's symptoms occurred mildly in the 0.21-1.49 g/L alcohol concentration group: balance was normal or slightly unbalanced; there was mild disorientation and somnolence. The children's behavior was normal or, in some cases, there was slow speech. The intensity of symptoms grew with the alcohol concentration in serum. Disorientation, somnolence and aggressiveness were more notable in the 1.50-2.49 g/L alcohol concentration group. Children in this group were unbalanced, but in some cases balance was normal. The main observed symptoms in children in the 1.50-2.49 g/L alcohol concentration group were apathy, aggressiveness (or in some cases euphoria), slow and confused speech. Children with alcohol concentration>2.50 g/L in serum were often in a bad condition; they were confused, imbalanced or in coma. Balance and behaviour was not observed in most of these children due to their poor condition. Coma was measured from five to 13 points using the Glasgow Coma Scale.
Subjects' characteristics
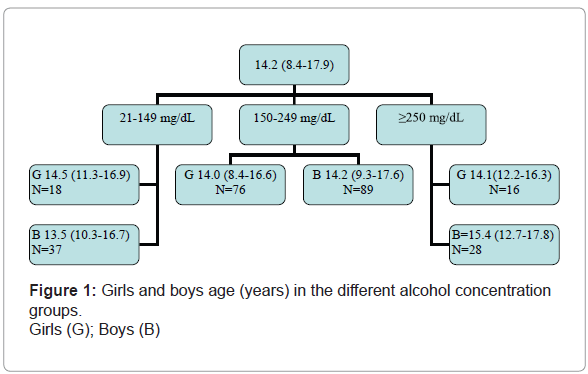
Data from the remaining 264 children was used in the analysis. The sample included 81 children from Tartu University Children's Clinic and 183 from Tallinn Children's Hospital. The mean age of children was 14.2 years (range 8.4-17.9 years). There were 154 boys, forming a male-to-female ratio of 1.4:1.
The age range was similar for girls across the different alcohol concentration groups, but the boys' age increased from one group to the next (Figure 1).
Biochemical test results
Table 1 shows the mean ± SD and range in brackets of serum ethanol and plasma glucose, lactate, potassium, sodium, and hormones concentrations of all subjects (n=264).
Glucose levels above the reference value of 6.1 mmol/L were evident in 46.6 percent of all children: 78 boys and 45 girls (sex ratio 1.7:1). None of the subjects were hypoglycaemic (i.e., had plasma glucose <3.3 mmol/L).
| Analyte | Mean ± SD (range) |
AI Gr 1 | AI Gr 2 | AI Gr 3 | Reference value |
|---|---|---|---|---|---|
| Ethanol (mg/dL) |
199 ± 58 (47-408) |
124 ± 24 (47-149) |
199 ± 27 (150-248) |
289 ± 31 (252-408) |
<20 |
| Glucose (mmol/L) | 6.1 ±1.2 (3.4-13.1) |
5.9±1.3 (3.5-9.2) |
6.3±1.2 (3.4-13.1) |
6.0±0.9 (4.8-8.3) |
3.3-6.1 |
| Lactate (mmol/L) | 2.7 ± 0.9 (0.5-6.4) |
2.8±1.0 (0.5-5.6) |
2.8±0.8 (1.1-6.4) |
2.4±0.6 (1.0-4.4) |
<2.4 |
| Sodium (mmol/L) | 142.5 ± 3.2 (132.0-150.1) |
141.8±3.1 (133-148) |
142.3±3.1 (132-150.3) |
143.0±3.0 (139-150) |
132-145 |
| Potassium (mmol/L) |
3.55 ± 0.51 (2.1-6.9) |
3.6±0.7 (2.1-6.9) |
3.5±0.4 (2.5-5.3) |
3.6±0.4 (2.9-5.0) |
3.5-5.1 |
| Estradiol (pmol/L) |
213.1±193.3 (<73-910) |
172.7±143.6 (<73-525) |
216.1±195.0 (<73-910) |
244.4±234.1 (<73-903) |
101-1468 |
| Progesterone (nmol/L) |
4.82±5.2 (<0.64-32.4) |
3.2±1.9 (0.6-7.3) |
5.4±6.0 (0.6-32.4) |
3.8±2.2 (0.6-9.7) |
1.5-67 |
| Testosterone (nmol/L) |
8.32±6.1 (<0.7-25.7) |
5.5±5.3 (<0.7-19.4) |
8.6±6.1 (<0.7-22.4) |
11.3±5.1 (1.6-25.7) |
9.9-52.4 |
| Cortisol (nmol/L) |
627.3±241 (87.5-1294) |
612.9±236 (89.4-1013) |
658±232 (139-1294) |
530±257.5 (87.5-1159) |
140-600 (mornings) ½ from morning level (evenings) |
Table 1: Ethanol, glucose, lactate, sodium and cortisol concentrations, estradiol and progesterone levels in girls, testosterone level in boys in alcohol-intoxicated children.
AI Gr 1 – alcohol intoxication group with serum ethanol concentration 21-149 mg/ dL; AI Gr 2 – serum alcohol concentration 150-249 mg/dL; AI Gr 3 – serum alcohol concentration =250 mg/dL.
Hyperlactinaemia (=2.4 mmol/L) occurred in 66.6 percent of all children: 100 boys and 76 girls (sex 1.3: 1).
Hypokalaemia (<3.5 mmol/L) was present in 46.2 percent of the children, there were 66 boys and 56 girls (sex ratio 1.2:1). Critically low levels of potassium were found in six boys and three girls, so 3.4 percent (N=9) of the children had critically low levels (<2.8 mmol/L) of potassium.
Plasma sodium concentration was higher than the reference value (>145 mmol/L) in 19.3 percent of children, and it was at a relatively high level (>150 mmol/L) in two children (0.8 percent). There were 34 boys and 17 girls (sex ratio 2:1).
Increasing cortisol levels (>460 nmol/L) occurred in 77.7 percent of the patients (N=205).
Serum alcohol concentration correlation with biochemical test results
For the entire group, potassium and sodium were positively correlated with age in all children (r=0.3; p<0.05) and age correlated negatively with glucose and lactate (r=0.2; p<0.05).
For the group of children with serum alcohol concentration between 0.21-1.49 g/L, age was positively correlated with plasma sodium (r=0.31. p<0.05) and potassium (r=0.28; p<0.05) concentration, but negatively with lactate concentration (r=-0.19; p<0.05). The mean blood glucose concentration was higher in boys than in girls (6.0 ± 1.2:5.5 ± 1.2 mmol/L) (p=0.001). There was a significant positive correlation between lactate and glucose (r=0.2; p<0.05).
When the serum alcohol concentration was 1.50-2.49 g/L, the age correlation with sodium and potassium was the same as in the 0.21- 1.49 g/L alcohol concentration group (r=0.3; p<0.05). Glucose had a positive correlation with lactate (r=0.2; p<0.05).
In the group with serum alcohol concentration more than 2.50 g/L, increased age correlated positively with sodium and potassium (r=0.4; p<0.05).
Serum alcohol concentration correlation with hormones
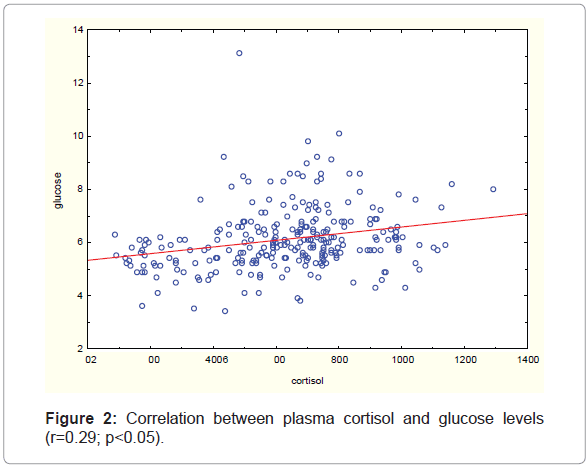
Whole group. In girls, plasma estradiol and progesterone concentration did not significantly correlate to age but for boys there was a positive correlation between plasma testosterone concentration and age (r=0.63; p0.0001). Plasma cortisol concentration in the whole group correlated positively with plasma glucose levels (r=0.29; p 0.05) (Figure 2).
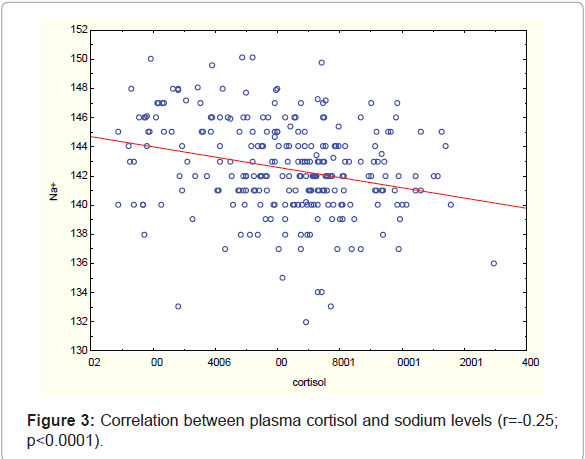
Cortisol concentration correlated positively with plasma lactate concentration (r=0.32; p<0.0001) and negatively with sodium level (r=-0.25; p<0.0001) (Figure 3).
Plasma progesterone concentration was positively correlated with cortisol (r=0.62; p<0.05), glucose (r=0.30; p<0.05) and lactate (r=0.22; p<0.05), and negatively with sodium levels (r=-0.22; p<0.05). Plasma estradiol level did not correlate to any of the measured biochemical markers.
Plasma testosterone concentration was positively correlated with plasma potassium (r=0.29; p<0.05) and sodium (r=0.23; p<0.05), and negatively with lactate levels (r=-0.17; p<0.05).
For the group of children with serum alcohol concentration 0.21- 1.49 g/L, testosterone concentration for boys correlated positively with age (r=0.80; p<0.0001) and with sodium concentration (r=0.34; p<0.05). In girls, progesterone concentration correlated positively with glucose (r=0.62; p<0.05) and cortisol (r=0.67; p<0.01) levels.
Among those with a serum alcohol concentration of 1.50-2.49 g/L, testosterone concentration for boys correlated positively with age (<0.0001; r=0.53) and with potassium level (p<0.002; r=0.32). In girls, the progesterone level correlated positively with age (p<0.05; r=0.23) and cortisol (p<0.0001; r=0.65) concentration and negatively with sodium (p<0.05; r=-0.24) level.
Among the group of children with serum alcohol concentration more than 2.50 g/L, testosterone concentration in boys correlated positively with age (p<0.03; r=0.43) and negatively with glucose (p<0.01; r=-0.48) level. Lactate level only correlated positively with estradiol (p<0.02; r=0.62) level in girls with a serum alcohol concentration of more than 2.50 g/L.
Discussion
Alcohol consumption among children is a growing problem in Estonia. The present study involved a relatively large sample of drunken children hospitalised at two regional hospitals in Estonia over a two-year period. Although 32.8% of them dropped out from the study, mostly due to incompletely filled medical forms, the study population is still representative to describe the situation of acute alcohol intoxication in children admitted to hospital in Estonia.
There have been very few studies about clinical symptoms of acute alcohol intoxication in children; most of these have been in children with relatively high level of alcohol concentrations [7,8]. Clinical symptoms of acute alcohol intoxication in children have been found similar to those seen in adults, but in children combining clinical signs and with blood alcohol measurements will significantly help to asses can help child's general status.
The ratio of girls to boys (1:1.4) was akin to similar studies [18,19], which generally included more boys. The mean age (14.2 years) of drunken children in the study was similar to that in other studies [3].
Children (N=165) in the study were most commonly hospitalised with an alcohol concentration of 1.50-2.49 g/L (mean 1.99 ± 0.58 g/L). Other studies have found similar results [3,20].
Alcohol consumption causes changes in plasma glucose, lactate, sodium, and potassium concentrations and is a complex process.
In our study we've seen a tendency towards to higher glucose level. Alcohol may increase glucose levels through inhibiting the basal insulin secretion, from increasing cortisol secretion and enhancing gluconeogenesis from lactate [21]. Increased cortisol level is one of the most imported factors in the mechanism of increasing glucose level.
Hyperlactinaemia was a common finding in our children, which was a feature similar to the other studies [22,23]. One cause for the lactic acidosis is tissue hypoxia due to mild hypothermia or ethanol induced central nervous system depression and altered blood vessel tone [24]. The other possible reason for lactic acidosis is enhanced anaerobic glucose metabolism, which leads to an increased lactate production.
According to the plasma potassium reference values (3.5-5.1 mmol/L), there was a tendency towards hypokalaemia. Critically low potassium values, i.e. <2.8 mmol/L, were present in 3.4% of children (n=9) and required correction with a potassium infusion. This might result from ethanol-induced lactic acidosis and development of high glucose level, since these two factors may decrease the cellular level of potassium and its increased loss with urine and vomiting.
There was also a tendency towards mild hypernatraemia. The most likely cause for hypernatraemia is the water deficit from alcohol intoxication and hyperaldosteronism, which commonly occur at the absorption step of alcohol [25].
Potassium and sodium were positively correlated with age in all groups of drunken children and correlated negatively with glucose and lactate [9]. The possible reason for the correlations differences is that younger children were more affected by alcohol than older children.
The serum lactate concentration above 5 mmol/L has been considered to be dangerous, because such lactate levels have been associated with increased 3- and 30-day mortality levels in intensive care patients with acute alcohol intoxication [36]. In our study, there were 3 children with lactate levels above 5 mmol/L. Thus, although hyperlactaemia according to our study was not very common finding in children, it can be severe and therefore serum lactate level should be measured in every child with acute AI.
Acute alcohol intoxication has been shown to stimulate the hypothalamic pituitary adrenal axis and to increase plasma cortisol concentration [26-28]. For most of the children hospitalised at evening or night (from 5 p.m. to 2.30 a.m.; N=191), when cortisol secretion is normally very low, about half of the amount secreted in the morning. The majority of patients had plasma cortisol levels above 110-460 nmol/l, the maximum reference value for that time of day (4-7 p.m. 80-460 nmol/L; midnight 30-110 nmol/L) [29]. Pierucci-Lagha et al. [17] found no change in cortisol levels in adult volunteers with alcohol concentration of about 1.00 g/L. Other studies have found increasing cortisol level in humans acutely intoxicated with ethanol [15,30]. In our study, cortisol concentration was increased in all alcohol concentration groups. The most likely cause for rising cortisol levels is the stress situation of an organism from the depression of central nerve system by alcohol intoxication.
The study showed a positive correlation between progesterone and cortisol. Most progesterone in males is created during testicular production of testosterone, and most in females by the ovaries; it is also produced in the brain and by the adrenal gland, however, where progesterone is an indirect precursor to cortisol [31]. Like cortisol, progesterone is released in response to the adrenocorticotropin hormone (ACTH) [30,32].
Estradiol level was not correlated with alcohol concentration in our study, which was similar to the other studies [33].
The metabolic effect of cortisol elevates the level of plasma glucose [34]. Some studies have shown that the elevation of glucose is more pronounced in the evening than in the morning [35]. The higher glucose results of our study were notable in the evenings than in the mornings.
Limitations. A statistical analysis of our study was limited from the high level of drop out (32.8%). In our study a lot of girls were in pre-menarche age or without regular menstrual cycle. Girls older than 13-14 years often were in medically serious health conditions and we didn't obtain information about their menstrual cycle.
In our further study we are planning to study the clinical signs and symptoms of acute AI in e different age and alcohol concentration groups in those children.
In conclusion, increased cortisol, sodium and glucose levels above reference values are common findings and complex processes in children with acute AI. Although increased serum lactate levels were not a common finding, it can be severe and therefore serum lactate should be measured in every child with acute AI.
Acknowledgements
The study was funded by Estonian Science Foundation Grant No. 8256 and by a Basic funding grant from Tartu University (PARPA). Acknowledgements are due to the Tartu University Children's Clinic and Tallinn Children's Hospital for the cooperation in collecting data.
References
- Woolfenden S, Dossetor D, Williams K (2002) Children and adolescents with acute alcohol intoxication/self-poisoning presenting to the emergency department. Arch Pediatr Adolesc Med 156: 345-348.
- Meyer S, Steiner M, Mueller H, Nunold H, Gottschling S, et al. (2008) Recent trends in the burden of alcohol intoxication on pediatric in-patient services in Germany. Klin Padiatr 220: 6-9.
- Schöberl S, Nickel P, Schmutzer G, Siekmeyer W, Kiess W (2008) Acute ethanol intoxication among children and adolescents. A retrospective analysis of 173 patients admitted to a university children hospital. Klin Padiatr 220: 253-258.
- Albers HM, van der Lely N (2004) Alcohol intoxication in four adolescents. Ned Tijdschr Geneeskd 148: 1504-1506.
- Lionte C, Sorodoc L, Laba V (2004) Toxic-induced hypoglycemia in clinical practice. Rom J Intern Med 42: 447-455.
- Lamminpää A, Vilska J, Korri UM, Riihimäki V (1993) Alcohol intoxication in hospitalized young teenagers. Acta Paediatr 82: 783-788.
- Madsen LP (1990) Acute alcohol intoxication in children. Diagnosis, treatment and complications. Ugeskr Laeger 152: 2362-2364.
- Lamminpää A (1995) Alcohol intoxication in childhood and adolescence. Alcohol Alcohol 30: 5-12.
- Tõnisson M, Tillmann V, Kuudeberg A, Väli M (2010) Plasma glucose, lactate, sodium, and potassium levels in children hospitalized with acute alcohol intoxication. Alcohol 44: 565-571.
- Mennella JA, Pepino MY, Teff KL (2004) Acute alcohol consumption disrupts the hormonal milieu of lactating women. J Clin Endocrinol Metab 90:1979- 1985.
- Badrick E, Bobak M, Britton A, Kirschbaum C, Marmot M, et al. (2008) The Relationship between Alcohol Consumption and Cortisol Secretion in an Aging Cohort. J Clin Endocrinol Metab 93: 750-757.
- King A, Munisamy G, de Wit H, Lin S (2006) Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol 59: 203-209.
- Davies BT, Bowen CK (1999) Total body water and peak alcohol concentration: a comparative study of young, middle-age, and older females. Alcohol Clin Exp Res 23: 969-975.
- Välimäki MJ, Härkonen M, Ylikahri RH (1983) Acute effects of alcohol on female sex hormones. Alcohol Clin Exp Res 7: 289-293.
- Välimäki MJ, Härkönen M, Eriksson CJ, Ylikahri RH (1984) Sex hormones and adrenocortical steroids in men acutely intoxicated with ethanol. Alcohol 1: 89-93.
- Holdstock L, Penland SN, Morrow AL, de Wit H (2006) Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3α-hydroxy- 5α-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology 186: 442-450.
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, et al. (2005) Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology (Berl) 186: 451-461.
- Marchi AG, Lamminpää A, Azeredo P, Williamson L, Cabecadas M, et al. (2003) Acute alcohol intoxication in adolescents. A multicentre study. Ital J Pediatr 29: 211-216.
- McIntosh J, MacDonald F, McKeganey N (2004) Pre-teenage children's experiences of drug use. Int JDrug Pol 16: 37-45.
- Weinberg L, Wyatt JP (2006) Children presenting to hospital with acute alcohol intoxication. Emerg Med J 23: 774-776.
- Shin JS, Lee JJ, Yang JW, Kim CW (2002) Ethanol decreases basal insulin secretion from HIT-T15 cells. Life Sci 70: 1989-1997.
- McDonald L, Kruse JA, Levy DB, Marulendra S, Sweeny PJ (1994) Lactic acidosis and acute ethanol intoxication. Am J Emerg Med 12: 32-35.
- Umpierrez GE, DiGirolamo M, Tuvlin JA, Isaacs SD, Bhoola SM, et al. (2000) Differences in Metabolic and Hormonal Milieu in Diabetic- and Alcohol- Induced Ketoacidosis. J Crit Care 15: 52-59.
- Lien D, Mader TJ (1999) Survival from Profound Alcohol-Related Lactic Acidosis. J Emerg Med 17: 841-846.
- Nieminen MM, Fyhrquist F, Linkola J, Tikkanen I, Tontti K (1981) Renin-aldosterone axis in ethanol intoxication. Pharm Biochem Behav 15: 879-882.
- Owens MJ, Nemeroff CB (1992) Physiology and pharmacology of corticotropin-releasing factor. Pharm Rev 43: 425-473.
- Patchev VK, Hayashi S, Orikasa C, Almeida OF (1995) Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J 9: 419-423.
- Frias J, Torres JM, Miranda MT, Ruiz E, Ortega E (2002) Effects of acute alcohol intoxication on pituitary-gonadal axis hormones, pituitary-adrenal axis hormones, β-endorphin and prolactin in human adults of both sexes. Alcohol Alcohol 37: 169-173.
- Marks V, Cantor T, Mesko D, Pullmann R, Nosalova G (2002) Differential Diagnosis by Laboratory Medicine. A Quick Reference for Physicians. Springer, Germany.
- Wirth MM, Meier EA, Fredrickson BL, Schultheiss OC (2007) Relationship between salivary cortisol and progesterone levels in humans. Biol Psychol 74: 104-107.
- Baulieu EE, Robel P, Schumacher M (2001) Neurosteroids: beginning of the story. Int Rev Neurobiol 46: 1-32.
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, et al. (1998) Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab 83: 2099-2103.
- Sarkola T, Mäkisalo H, Fukunaga T, Eriksson CJ (1999) Acute effect of alcohol on estradiol, estrone, progesterone, prolactin, cortisol, and luteinizing hormone in premenopausal women. Alcohol Clin Exp Res 23: 976-982.
- Garrel DR, Moussali R, De Oliveira A, Lesiéqe D, Lariviére F (1995) RU 486 prevents the acute effects of cortisol on glucose and leucine metabolism. J Clin Endocrinol Metab 80: 379-385.
- Plat L, Leproult R, L´HermiteBaleriaux M, Fery F, Mockel J, et al. (1999) Metabolic Effects of Short-Term Elevations of Plasma Cortisol Are More Pronounced in the Evening Than in the Morning. J Clin Endocrinol Metab 84: 3082-3092.
- Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, et al (1994) Natural history and course of acquired lactic acidosis in adults. Am J Med 97: 47-54.
Citation: Tõnisson M, Tillmann V, Kuudeberg A, Väli M (2011) Effect of CBT on Depressive Symptoms in Methadone Maintenance Patients Undergoing Treatment for Hepatitis C. J Addict Res Ther 2:111. DOI: 10.4172/2155-6105.1000111
Copyright: © 2011 Tõnisson M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16881
- [From(publication date): 4-2011 - Aug 31, 2025]
- Breakdown by view type
- HTML page views: 12142
- PDF downloads: 4739