Preliminary Study on the Significance of BRCA1 and PARP1 Immunohistochemical Expression in Ovarian Cancer
Received: 06-Mar-2018 / Accepted Date: 26-Mar-2018 / Published Date: 30-Mar-2018 DOI: 10.4172/2161-0681.1000342
Abstract
Role of BRCA1 and PARP1 has been studied by immunohistochemistry in a cohort of ovarian cancers. Their expression has been related to overall survival and disease free disease.
Objective: To investigate the clinical outcome of an heterogeneous population of ovarian cancers as respect to immunohistochemical expression of BRCA1 and PARP1 in order to identify a histological threshold for positivity and negativity and to predict possible candidates to anti-PARP therapy
Material and methods: 97 cases of ovarian cancers were collected in a multicentre study and immunohistochemically tested for BRCA1 and PARP1. The immunohistochemical expression of BRCA1 and PARP1 was studied on formalin-fixed and paraffin-embedded samples. Overall survival and disease free survival were evaluated. Statistical analysis was performed including cancers of all types and a subgroup of high grade serous carcinoma that represents the predominant histotype.
Results: A possible threshold to discriminate positive and negative cases has been proposed. BRCA1 and PARP1 immunohistochemical expression did not significantly correlate with overall survival. The evaluation of survival for the combinations of BRCA1+/-, PARP1+/- showed a longer patient survival when the positivity and negativity were in contrast compared to when they were in agreement.
Conclusions: Although BRCA1 and PARP1 expression do not appear to be correlated with overall survival, further investigation and follow-up together with a larger number of cases could clarify the function; the standardised immunohistochemical method could better select the patient group sensitive to PARP1 inhibitors.
Keywords: Ovarian cancer; BRCA1; PARP1; Immunohistochemistry; Overall survival; Disease free survival; Anti- PARP therapy
Introduction
One of the new frontiers in the medical treatment of tumours is that of the biomarker as it enables the neoplasia’s personal profile to be defined which then can be linked with a corresponding suitable targeted therapy, preferably having a high likelihood of success.
At the same time, discoveries in human genetics have allowed the identification of those germinal mutations that are family risk factors for some neoplasia. Among these, BRCA1/2 mutations are risk factors not only for the breast but also for the ovary, the colon and several other tumours. Somatic mutations of BRCA1/2 can also occur in tumour tissues, that is mutations during cancerogenesis.
Recently an interaction has been observed between BRCA1/2 mutation and the expression of PARP1, a molecule which is important in the maintenance of DNA integrity [1]. Consequently, PARP inhibitors have begun to be utilised in ovarian tumour therapy, initially as part of a combination treatment and then as mono-therapy, with success in patients with mutated BRCA.
In 2005, two important studies stimulated interest in PARP targeted strategies showing that the inactivation of PARP1 is synthetically lethal with a BRCA1/2 deficiency, both in vitro and in vivo [2,3].
The first real evidence of the clinical benefit of PARP1 inhibition as the only active agency was given by Fong et al., who conducted a study with olaparib (a powerful PARP inhibitor in vitro ) in patients with an advanced ovarian, breast or prostate cancer, with antitumor activity exclusively in the presence of BRCA1/2 germinal mutation [4,5]. These results were subsequently confirmed in a larger group of BRCA mutated ovarian cancer patients [6] and in phase II clinical studies [7,8]. Ulterior studies with olaparib and other PARP1 inhibitors in monotherapy have been carried out with results that are largely (though not uniformly) promising, especially in ovarian cancer [9,10].
In 2004, Ashworth introduced the concept of BRCAness and included some sporadic tumours with clinical and biological characteristics similar to those of germinal mutated BRCA tumours [11]. The fact that the depletion of homologous recombination (HR) repair components different from BRCA1/2 (such as the complex MRN, RAD51, ATR, ATM, e FANCC) creates a synthetic lethality with PARP1 inhibition is evidence, independently of the genetic lesion, for the expansion of the group of patients with a right to treatment by PARP1 inhibitors [12,13].
Recently, interest has mainly been concentrated on the loss of PTEN as a determining factor for the characteristics of BRCA-like tumours which show susceptibility to PARP1 inhibitors and agents harmful to DNA [14,15]. However, these observations remain controversial [16-18].
These developments have however taken place with little understanding of the pharmacological mechanisms involved and above all with no assessment of the mechanisms of pre-selection of those women candidates for anti-Parp treatment.
The belief that high-grade ovarian cancer are to be considered as homologous to triple negative breast cancer [1] has no basis in reality in that approximately 2/3 of ovarian tumours have oestrogen receptors [19] and anti-PARP treatment in breast cancer was less effective than in ovarian cancer.
Since there is very little information on the possibility of immunohistochemically documenting the expression of BRCA and PARP, we decided to undertake a multicentre study to test the possibility of defining the expression of the two proteins, assess the characteristics of the patients involved and eventually draw operating conclusions for a treatment plan.
Methods
Patients recruitment
A cohort of 111 patients with ovarian cancer was recruited, diagnosed at the Department of Pathology at University of Bari (I) (77 patients) and at Pathology Division, Catholic University of Rome (I) (34 patients) between the years of 2010 to 2016. The study was approved by the Independent Ethics Committee of the Policlinico- Hospital, Bari, Italy (accession number 556, April 15-2016). Patients were included on the basis of histological diagnosis of ovarian cancer, availability of adequate tissue blocks and clinical follow-up. All patients underwent a hysterectomy with or without lymphadenectomy and peritoneal biopsy.
Immunohistochemical analysis
Paraffin sections were cut at 3-μm thick, then deparaffinised and placed in a citrate buffer at 70°C. Mouse monoclonal BRCA1 antibody (1:150 dilution, clone MS110, Abcam) and polyclonal PARP1 antibody (1:500 dilution, Thermoscientific) were used in an automated immunostainer (DAKO® AUTOSTAINER LINK 48). The epitope unmasking was carried out using a citrate buffer solution at pH 6.1 for 15 minutes at 97°C, followed by 10 minutes of buffer washing at 70°C in a solution of 0.05 mol/L Tris/HCl, 0.15 mol/L NaCl, 0.05% Tween 20, pH 7.6. Staining was done automatically with the EnVision FLEX kit.
Peroxidase Blocking Reagent from DAKO® is utilised for 5 minutes, the slides are then immersed in a buffer wash and incubation is carried out with the primary antibody for 20 minutes. Amplification of BRCA1 only is carried out with the reagent FLEX+ Mouse (DAKO®).
Both antibodies are again buffer washed and the secondary antibody (HRP) is released for 20 minutes. This unique immunochemistry based on dextran polymer technology permits binding of a large number of enzyme molecules, horseradish peroxidase, to a secondary antibody via the dextran backbone. The benefits are many, including increased sensitivity, minimized nonspecific background staining and a reduction in the total number of assay steps as compared to conventional techniques. The technique allows the binding of a maximum of 100 molecules of HRP with a maximum of 20 molecules of primary antibody for each chain.
After further buffer washing, the chromogen (a solution of diaminobenzidine) is added for 10 minutes, revealing the Ag/Ab reaction through an intense brown staining.
After another buffer wash, counterstaining with Haematoxylin for 10 minutes reveals the clear blue staining of the nucleus.
Positive control was a slide of a high grade serous ovarian cancer positive in patients with a known BRCA mutation, while the negative control was the analysis without the primary antibody.
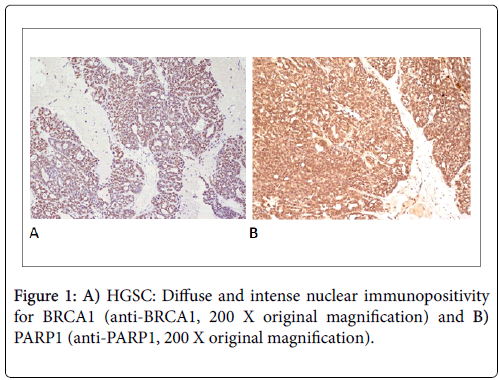
Only a nuclear immunopositivity was considered. A cytoplasmic positivity for both proteins, occasionally observed, was not considered. All specimens were evaluated by two blinded observers and any disagreements were later consensually resolved in re-examination. According to Domagala et al. [20], a semiquantitative analysis was performed based on the combination of immunohistochemical staining intensity and the percentage of positive cells observed in 10 fields at 200 magnifications.
Staining intensity was measured on a 0-3 scale as follows: Score 0: no staining; Score 1: weak staining; Score 2: moderate staining; Score 3: strong staining.
The percentage of positive cells was transformed into a 0-4 scale. Score 0: no staining; Score 1: 1-10% of positive cells; Score 2: 11-50% of positive cells; Score 3: 51-80% of positive cells; Score 4: 81-100% of positive cells (Figures 1A and 1B).
For each case, the two score were multiplied, and the median used as a threshold value: 10.4 for BRCA1 and 8.4 for PARP1 respectively.
This threshold value was chosen as there is no previous study in the literature on the immunohistochemical expression of the two proteins in ovarian cancers and therefore there is no known sensitivity threshold to separate positive and negative cases. To be able to separate them we preferred to consider as positive those cases with tumours with an expression result higher than the median value, and as negative those equal to or less than this value.
Statistical analysis
Statistical analysis was performed with the MedCalc version 16.4.3 (Acacialaan 22 8400 Ostend, Belgium).
For the correlations of BRCA1 and PARP1 with the other clinicopathological features, the Pearson’s χ2 test was utilised. Survival curve were constructed using the Kaplan-Meier methods. The difference in survival estimator was assessed by the Log-rank test. Analysis of variance (ANOVA) was carried out using Cox regression test. P<0.05 was considered statistically significant.
Results
In Table 1 are detailed the clinic-pathologic data of patients enrolled for the study. Tables 2 and 3 show the relationship between expression of BRCA1/PARP1 and clinic-pathological features.
| Count (%) | |
|---|---|
| Age, mean (range) | 55, 8 [31-83] |
| ≤ 55, 8 | 60 (54) |
| >55, 8 | 51 (46) |
| Histology | |
| High grade seous (HGSC) | 69 (62) |
| Low grade serous (LGSC) | 6 (5) |
| Mucinous (MC) | 5 (5) |
| Clear cell (CCC) | 18 (16) |
| Endometrioid (EC) | 13 (12) |
| FIGO staging | |
| I | 34 (31) |
| II | 17 (15) |
| III | 59 (53) |
| IV | 1 (1) |
| Follow-up | |
| survivors | 69 (62) |
| died | 28 (25) |
| Lost to follow-up | 14 (13) |
| Survival (months) | 40 [0-145] |
| ≤ 40 | 50 (45) |
| >40 | 47 (42) |
| Lost to follow-up | 14 (13) |
| Sum | 111 |
Table 1: Clinico-pathological data of the patients involved in the study.
| BRCA1 negative | (n=58) | BRCA1 positive | (n=53) | p | |
|---|---|---|---|---|---|
| N° patients | % | N° patients | % | ||
| Age | 0.0358 | ||||
| ≤ 55, 8 | 37 | 0.64 | 23 | 0.43 | |
| >55, 8 | 21 | 0.36 | 30 | 0.57 | |
| Histology | <0.0001 | ||||
| HGSC | 18 | 0.31 | 51 | 0.96 | |
| LGSC | 4 | 0.07 | 2 | 0.04 | |
| MC | 5 | 0.09 | 0 | 0 | |
| CCC | 18 | 0.31 | 0 | 0 | |
| EC | 13 | 0.22 | 0 | 0 | |
| PARP1 | <0.0001 | ||||
| negative | 47 | 0.81 | 9 | 0.17 | |
| positive | 11 | 0.19 | 44 | 0.83 |
Table 2: Relationship between BRCA1 expression and clinicopathologic data.
| PARP1 negative | (n=56) | PARP1 positive | (n=55) | p | |
|---|---|---|---|---|---|
| N° patients | % | N° patients | % | ||
| Age | 0.0744 | ||||
| ≤ 55,8 | 35 | 0.63 | 25 | 0.45 | |
| >55,8 | 21 | 0.38 | 30 | 0.55 | |
| Histology | <0.0001 | ||||
| HGSC | 20 | 0.36 | 49 | 0.89 | |
| LGSC | 5 | 0.09 | 1 | 0.02 | |
| MC | 3 | 0.05 | 2 | 0.04 | |
| CCC | 16 | 0.29 | 2 | 0.04 | |
| EC | 12 | 0.21 | 1 | 0.02 | |
| BRCA1 | <0.0001 | ||||
| negative | 47 | 0.84 | 11 | 0.2 | |
| positive | 9 | 0.16 | 44 | 0.8 |
Table 3: Relationship between PARP1 expression and clinicopathologic data.
BRCA1 was statistically significant (p=0.036) in older patients (age>55 years) and in high grade serous ovarian cancer (p=0.0001). Also PARP1 was a statistically significant in high grade serous ovarian cancer (p=0.0001).
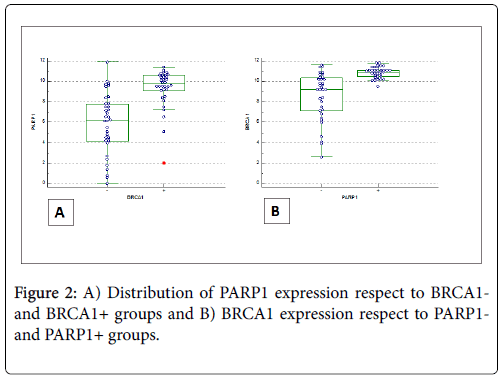
Figure 2 shows the distribution of BRCA1 (BRCA1-positivity and BRCA1-negativity) and PARP1 (positive/negative) immunoexpression according to ANOVA test.
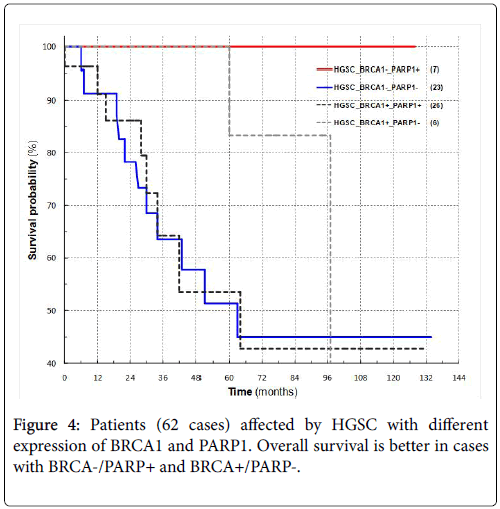
Patients survival was available for 97 of 111 cases and in particular for 62 high grade serous carcinomas; survival progressively deteriorated from BRCA1-postitive (51 cases) and PARP1-positive (55 cases) for all cases (p=0.29 and p=0.61 respectively) and for high grade serous cancers (p=0.98 and p=0.84) (Figures 3A-3D).
Patients with either BRCA1- and PARP1+ or BRCA1+ and PARP1- have a longer survival rate than those patients where both markers are either positive or negative (Figure 4).
Disease free survival was detected in 49 cancers; it was significantly longer in patients with a low immunohistochemical expression of either BRCA1 (23 cases) or PARP1(21 cases) (p=0.048 and p=0.012) (Figures 5A and 5B). Disease free survival was available for 34 high grade serous carcinomas; differences of disease free survival between positive and negative cases for both BRCA1 and PARP1 were not significant (p=0.81 and p=0.3 respectively) (Figures 5C and 5D and Table 3).
Discussion
However, one looks at the entire population of women suffering from ovarian cancer, the percentage of patients with BAP1-germinal mutation is markedly low, not exceeding 20% of those affected by high grade serous cancer [21]. Again, occur add to these those patients with BRCA1/2 somatic mutations and again other patients that do not have genetically demonstrable mutations but present a neoplasia with BRCAness that is mutation of the genes involved in DNA (HR) repair mechanisms.
It is widely accepted that there is a correlation between BRCA1/2 mutation and the predisposition for the onset of breast and ovarian cancer. During the lifetime, this predisposition has a 44-60% of chance of develops an ovarian cancer.
The success of PARP1 inhibitors in ovarian tumours, better than in breast tumours, would imply that there is a strong link in the activity in the cell cycle of the contemporary mutation of BRCA and the hyperexpression of PARP1 (synthetic lethality) [2,3].
However, there are no studies as yet capable of assessing the candidature of patients to anti-PARP treatment through an analysis of BRCA1 germinal mutation and PARP1 hyper-expression. Immunohistochemical analysis, similar to that done in breast cancer, can help to identify patients in whom anti-PARP medication could be more effective. It is known that immunohistochemical analysis of the protein PARP1 is able to show the overexpression of the mRNA for PARP1, its gene polymorphisms and post-transcriptional modifications [22] as well as its increased nuclear activity [23].
We can hypothesise the same type of analysis for BRCA1 whose immunohistochemical expression could correlate with the somatic or germinal mutations or with the activity of proteins similar to BRCA1.
The immunohistochemical expression could allow its application in many laboratories once standardised the method. Actually, Wysham et al. [24] and Gan et al. [25] reported their BAP and PARP immunohistochemical experiences, with which they showed the significant correlation between the two proteins and survival; a longer survival was observed in women with low PARP and BRCA expression.
Interestingly, Marques et al. [26] shows a reduction of BRCA1 expression after chemotherapy. The definition of an immunohistochemical cut-off point to define a case positive, does not established and, in the literature, the different antibodies and different laboratory methods are reported. For this reason, in our work we have used the median values of the BRCA and PARP obtained by a semiquantitative evaluation and also, this has been an inspired choice seeing as our positive cases were almost exclusively high grade serous cancers (the histotype most frequently BRCA1 mutated).
Wysham et al. [24] and Gan et al. [25] showed a significant and more frequent cancer’s relapse in subjects with high expression of PARP1, p53 e FANCD2. This is also present in our cases while survival is not statistically significant.
Not only is a correlation between PARP1 and BRCA1 expression fully confirmed but also confirmed is the direct relation between the expressions of the two proteins [27].
Previous studies of ovarian tumours had identified an inverse correlation between them showing that the loss of efficiency in the HR pathway, mediated by BRCA1, pushed the repair of DNA damage towards the mechanisms of the BER pathway, guided by PARP1 [24-28]. It has been suggested that BRCA1 inactivation may be involved in the induction of NAD+ synthesis, and therefore responsible for the regulation of PARP1 expression and for a raised NAD+ - PARP1 activity.
Understanding the mechanism that regulates cross-talk between the two proteins is still being studied [29,30]. PARP1 is able to PARylate BRCA1 at the level of its DNA binding-domain, regulating its activity with regards to the same DNA molecule, during the carrying out of repair function through the HR repair pathway.
PARylation is not a fundamental process for the carrying out of repair function by BRCA1, but it is a critical phase in its regulation and can have genome instability as a final consequence. Albeit, BRCA1 and PARP1 are part of the PARP1-RAP80-BRCA1 complex, therefore the interaction between these two proteins would logically seem to be influenced by the activity and state of other protein factors.
BRCA1 and PARP1 expression correlates with some clinicopathological parameters. First of all, age in that evidently older women have a greater likelihood of gene transformation. The correlation with histological type is obviously with high grade serous cancer.
None of the patients in our study was treated with PARP inhibitors and so the predictive value of PARP1 immunohistochemical expression was not studied.
The evaluation of survival for the combinations of BRCA1+/-, PARP1+/- showed a clearly longer patient survival when the positivity and negativity were in contrast compared to when they were in agreement.
In the case of breast cancer, the women candidates for anti-PARP are those with BRCA- and PARP+; our preliminary data would agree with those reported in breast cancer since patients with high grade serous cancers show the best overall survival.
These data are not easy to interpret, and it can be supposed that if BRCA1 is not mutated, PARP1 activation anyway would go towards the activation of alternative pathways able to repair DNA. The anti- PARP therapy could be the alternative pathway which would launch tumoral necrosis.
Conclusion
Our study, based on cases from two different hospitals where many women are treated for ovarian cancer, has shown that: it is possible to evaluate with immunohistochemistry the expression of proteins linked to the gene BRCA1, though it is still extremely complicated to establish a real cut-off between positive and negative cases. In the same way, it is possible to evaluate the expression of the protein PARP1; although BRCA1 and PARP1 expression do not appear to be correlated with overall survival, further investigation and follow-up together with a larger number of cases could clarify the function. The standardised immunohistochemical method could better select the patient group sensitive to PARP1 inhibitors to better regulate the administration of this therapy currently used in second-line.
Our data are still in an initial and experimental phase and many other studies of many cases are needed to reach any reliable conclusions.
Conflicts of Interest
Leonardo Resta, Maria Arcangela Cascarano, Gennaro Cormio, Gianfranco Zannoni, Damiano Arciuolo, Gabriella Serio and Andrea Marzullo have no conflict of interest.
References
- Mazzotta A, Partipilo G, De Summa S, Giotta F, Simone G, et al. (2016) Nuclear PARP1 expression and its prognostic significance in breast cancer patients. Tumour Biol 37: 6143-6153.
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, et al. (2005) Specific killing of BRCA2-deficient tumors with inhibitors of poly(ADP-ribose) polymerase. Nature 434: 913-917.
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, et al. (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434: 917-921.
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, et al. (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361: 123-134.
- Steffen JD, Brody JR, Armen RS, Pascal JM (2013) Structural implications for Selective Targeting of PARPS. Front Oncol 3: 301.
- Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, et al. (2010) Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 28: 2512-2519.
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, et al. (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer a proof-of-concept trial. Lancet 376: 245-251.
- Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, et al. (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA] or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376: 235-244.
- Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, et al. (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12: 852-861.
- Sandhu SK, Schelman WR, Wilding C, Moreno V, Baird RD, et al. (2013) The poly (ADP-ribose) polymerase inhibitor nÃraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer a phase 1 dose-escalation trial. Lancet Oncol 14: 882-892.
- Turner N, Tutt A, Ashworth A (2004) Hallmarks of 'BRCA-ness' in sporadic cancers. Nat Rev Cancer 4: 814-819.
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, et al. (2006) Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polyrnerase inhibition. Cancer Res 66: 8109-8115.
- Dedes KJ, Wilkerson PM, Wetterskog D, Weigelt B, 'Ashworth A, et al. (2011) Synthetic lethality of PARP inhibition in cancers lacking BRCA1 and BRCA2 mutations. Cell Cycle 10: 1192-1199
- McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, et al. (2010) PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res 70: 5457- 5464
- Forster MD, Dedes KJ, Sandhu S, Frentzas S, Kristeleit R, et al. (2011) Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol 8: 302-306.
- Gupta A, Yang Q, Pandita RK, Hunt CR, Xiang T, et al. (2009) Cell cycle checkpoint defects contribute to genornic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle 8: 2198-2210
- Fraser M, Zhao H, Luoto KR, Lundin C, Coackley C, et al. (2012) PTEN deletion in prostate cancer cells does not associate with loss of RAD51 function: implications for radiotherapy and chemotherapy. Clin Cancer Res 18: 1015-1027.
- Hunt CR, Gupta A, Horikoshi N, Pandita TK (2012) Does PTEN loss impair DNA double-strand break repair by homologous recombination? Clin Cancer Res 18: 920-922.
- Prat J (2012) New insights into ovarian cancer pathology. Ann Oncol 23: 111-117.
- Domagala P, Huzarski T, Lubinski J, Gugala K, Domagala W (2011) PARP-1 expression in breast cancer including BRCA1-associated, triple negative and basal-like tumors: possible implications for PARP-1 inhibitor therapy. Breast Cancer Res Treat 127: 86l-869.
- Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, et al. (2008) ‘‘BRCAness’’ syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol 26: 5530-5536.
- Lockett KL, Hall MC, Xu J, Zheng SL, Berwick M, et al. (2004) The ADPRT V762A genetic Variant contributes to prostate cancer susceptibility and deficient enzyme function. Cancer Res 64: 634-638
- Zaremba T, Ketzer P, Cole M, Coulthard S, Plummer ER, et al. (2009) Poly (ADP-ribose) polyrnerase-1 polymorphisms, expression and activity in selected human tumour cell lines. Br J Cancer 101: 256-262.
- Wysham WZ, Mhawech-Fauceglia P, Li H, Hays L, Syriac S, et al. (2012) BRCAness profile of sporadic ovarian cancer predicts disease recurrence. PLOS One 7: e30042
- Gan A, Green AR, Nolan CC, Martin S, Deen S (2013) Poly(adenosine diphosphate-ribose) polymerase expression in BRCA-proficient ovarian high-grade serous carcinoma; association with patient survival. Hum Pathol 44: 1638-1647.
- Marques M, Beauchamp MC, Fleury H, Laskov I, Qiang S, et al. (2015) Chemotherapy reduces PARP1 in cancers of the ovary: implications for future clinical trials involving PARP inhibitors. BMC Med 13: 217.
- Green AR, Caracappa D, Benhasouna AA, Alshareeda A, Nolan CC, et al. (2015) Biological and clinical significance of PARP1 protein expression in breast cancer. Breast Cancer Res Treat 149: 353-362
- Ossovskaya V, Koo IC, Kaldjian EP, Alvares C, Sherman BM (2010) Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 1: 812-821
- Hu Y, Petit SA, Ficarro SB, Toomire KJ, Xie A (2014) PARP1-driver: poly-ADP- ribosylation regulates BRCA1 function in homologous recombination-mediated DNA repair. Cancer Discov 4: 1430-1447.
- Li D, Bi FF, Chen NN, Cao JM, Sun WP, et al. (2014) A novel crosstalk between BRCA1 and poly (ADP-ribose) polymerase 1 in breast cancer. Cell Cycle 13: 3442-3449.
Citation: Resta L, Cascarano MA, Cormio G, Zannoni F, Arciuolo D, et al. (2018) Preliminary Study on the Significance of BRCA1 and PARP1 Immunohistochemical Expression in Ovarian Cancer. J Clin Exp Pathol 8:342. DOI: 10.4172/2161-0681.1000342
Copyright: © 2018 Resta L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8042
- [From(publication date): 0-2018 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 7011
- PDF downloads: 1031