Preparation and Characterization of Biodiesel Produced from Jatropha Seed Oil Using Sulphated Zirconia as Catalyst
Received: 30-Mar-2018 / Accepted Date: 10-Apr-2018 / Published Date: 16-Apr-2018
Abstract
Biodiesel has become beguiling nowadays for it is environmental benefits and it seems an opposite alternative fuel for future. In the current study, the biodiesel was produced from Jatropha seed oil using sulphated zirconia as catalyst. The catalyst was characterized using Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), X-ray diffraction (XRD), thermogravimetric analysis (TGA/DTA) and X-ray fluorescence (XRF). The GC-MS result of biodiesel produced shows 8.46% hexadecanoic acid, methyl ester, 10.41% 9-Octadecenoic acid (Z)-, methyl ester and 10.33% 9, 12-Octadecadienoic acid (Z,Z), methyl ester as major methyl ester. The physicochemical properties of the biodiesel produced are found to be within the ASTM standard hence the biodiesel maybe use as an alternative or additive to conventional diesel.
Keywords: Jatropha seed oil; Biodiesel; Characterization; Diesel
Introduction
Fossil fuels are one of the major sources of energy in world today. It can be accounted to easy usability, availability and cost effectiveness [1]. But the limited reserves of fossil fuels are a great concern owing to fast depletion of the reserve due to worldwide increase in demand [1].
Biodiesel has become beguiling nowadays for it is environmental benefits and it seems an opposite alternative fuel for future [2], it’s made from renewable biological sources such as vegetable oils and animal fats [2]. It is biodegradable and nontoxic, low emission profiles and also environmentally beneficial [3].
Vegetable oils are among the different sources of energy fuels being considered as alternatives to fossil fuels [4]. Palm-oil, coconut oil among other oil is required in refined forms to obtained quality biodiesel, in Addition to their food needs, this makes the production of biodiesel from their sources uneconomical [5]. Non-edible oils obtained from plant species such as Jatropha curcas L . and Castor seeds may provide better alternatives with great potential for biodiesel production because it does not conflict with the food crises: since it is not main food source [6]
Each Jatropha seed produces 35% to 37% of its mass in oil and it is drought resistant [1]. It is being considered a break through because of availability of various types of oil seeds in huge quantities. Vegetable oil are renewable in nature and may generate opportunities for rural employment when use on large scare [1].
Materials and Methods
Sampling
Jatropha seed was obtained from Sokoto Energy Research Centre, Usmanu Danfodiyo University Sokoto premises, Sokoto State, Nigeria.
Sample preparation and extraction of oil
The seed was sundried to remove moisture content and grinded to powdered form. The Jatropha oil extraction was carried out by soxhlet extraction method as reported by Farag et al. [7].
Preparation of catalyst
The catalyst was prepared heterogeneously by a method called Ameliorated method below.
Preparation of S-ZrO2 powder
The fine S-ZrO2 powder was prepared by ameliorated method, which was based on the method reported by Hara and Miyayama [8]. Zirconium oxychloride hydrate (ZrOCL2.8H2O), ammonia (NH3.H2O) and sulphuric acid (H2SO4) were used as starting material, precipitating agent and sulphating agent, respectively 20% NH3 aq. was gradually dropped into ZrOCL2.8H2O solution (0.20M) and the pH value was adjusted to 10, hydrated zirconia (ZrO2.nH2O) hydrogel appeared. The obtained ZrO2.nH2O hydrogel was washed with distilled water using the centrifuge till no chloride ions were detected with silver nitrate (AgNO3). The ZrO2.nH2O hydrogel was then changed into its alcogel by washing several times with anhydrous ethanol. The fine ZrO2 powder was obtained from the ZrO2.nH2O alcogel by washing several times with anhydrous ethanol. The fine ZrO2 powder was obtained from the ZrO2.nH2O alcogel supercritical drying method at 260°C and 7MPa. The ZrO2 powder was added to 0.20M H2SO4 under vigorously stirring for 30 minutes, filtered using filter paper and heated for 3 hours at 620°C to obtain the fine S-ZrO2 powder.
ZrOCl2(H2O)4(s)+4NH3(aq) → ZrO2(NH3)4(s)+2H2O+2Cl-… (1)
ZrO(NH3)4(s)+2H2SO4(aq) → ZrO2(SO3)2+2H2O+4NH3 ……(2)
The product will be:
Sulphated Zirconia ZrO2 (SO3)2.
Characterization of catalyst
The S-ZrO2 catalyst was characterized through FTIR Method of Analysis, DSC Method of Analysis, X-ray diffraction (XRD) Analysis and X-ray fluorescence (XRF) analysis.
Transesterification of the oil into biodiesel
The pretreated Jatropha oil (20 g) was placed in two necked round bottom flask, placed on hot plate magnetic stirrer heated to 50°C and stirring at 600 rv/m. the methanol was mixed with 0.6 g (3 w/w%) catalyst load of sulphated zirconia in a beaker and heated to 40°C in water bath, then transferred into two necked round bottom flask containing oil. The reaction mixture under the same condition was allowed for 2.15 hrs. The mixture was then transferred into a separating funnel and allowed to separate overnight under gravity. Two distinct layers were observed, a dark bottom layer which is the glycerol and the light upper layer which is the biodiesel, in some cases when the layer did not separate distinctly enough, equal amount of distilled water and n-Hexane is added to aid the separation. After which the biodiesel was washed by adding 20% volume of warm distilled water and agitated gently for 5 minutes. The mixture was allowed to settle into a two layer from which the biodiesel was separated from the impurities, glycerol and water. The washing was done in order to remove impurities and any remaining residue methanol [9]. The biodiesel yield was calculated using equation 3.
Biodiesel yield=Actual Weight/Theoretical Weight × 100……… (3)
Characterization of biodiesel using GC-MS
Characterization of the highest yield 97.91% of the biodiesel produced was carried out using GC-MS model QP-2010 PLUS machine for the characterization, the biodiesel was injected into the gas chromatography at injection temperature which was programmed for 70°C to 280°C for 5 minutes and at 280°C for 5 minutes. The analysis was done based on software matching with standard mass spectra.
Physicochemical characterization of the oil
The saponification value, kinematic viscosity, specific gravity, iodine value acid value and flash point were determined through the methods as reported by Indhumathi et al. [10]. The cloud and pour point were analyzed according to method by Fuduka et al. [11]. The high heating value was calculated using formula as reported by while, cetene number according to Demirbas et al. [12,13].
Results
The results obtained from the physicochemical properties of the Jatropha seed oil, biodiesel produced, and ASTM standards are shown in the Table 1.
| Parameter | Jatropha Oil | ASTM Method | Biodiesel Limit | Biodiesel Obtained |
|---|---|---|---|---|
| Yield (%) | 38.47 ± 1.20 | - | - | 97.911 |
| 31.592 | ||||
| Specific Gravity | 0.91 ± 0.05 | D1298 | 0.87-0.89 | 0.866 |
| Cetane Number | 35 | D6756 | 48 to 65 | 62.27 |
| Pour Point (°C) | 8 | D97 | -15 to 10 | 5.5 |
| Cloud point (°C) | 6.20 ± 0.20 | D2500 | -3 to 12 | 7.9 |
| Flash Point (°C) | 130.00 ± 1.00 | D93 | 100 to 170 | 140 |
| Aniline Point (°C) | - | D611 | - | - |
| Kinematic Viscosity (Mm2/s) | 58.80 ± 3.50 | D445 | 1.9 to 6.0 | 4.7 |
| Iodine Value (gl2/100 g) | 101.38 ± 1.50 | D6751 | 130 max | 104.99 |
| Acid Value (mgKOH/g) | 5.05 ± 0.50 | D6751 | 0.5 max | 2.02 |
| Saponification Value (mgKOH/g) | 175.49 ± 2.00 | D6752 | - | 186 |
| API Gravity | 23 | - | - | 35.56 |
| Higher Heating Value (MJ/Kg) | 39.92 | - | - | 43.1 |
Where Key, 1=Row Methyl Ester Yield and 2=Methyl Ester Yield Analyzed by GC-MS Machine.
Table 1: Physicochemical Properties of Jatropha seed Oil, Biodiesel and ASTM limits of Biodiesel.
Characterizations of the catalyst prepared
Characterizations of the prepared catalyst were achieved using infrared spectroscopy (FTIR), thermo gravimetric analysis (TGA), XRay Diffractions (XRD), X-Ray Fluorescence (XRF) and diffraction scanning colometry (DSC).
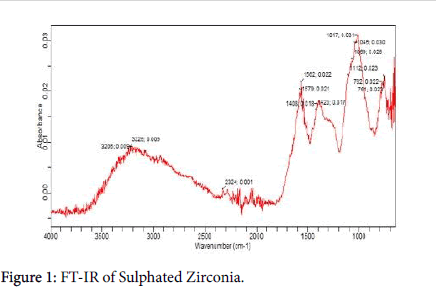
FTIR analysis: Infrared spectroscopy was reported to be used for the characterization of inorganic compound by Farag et al. [14]. Absorption peaks with FTIR spectrum of sulphated zirconia shown in Figure 1, vibrational peaks in the range of 4000-950 cm-1 represent the characteristic metal-oxygen stretching frequencies associated with the vibrations of O-S-O bonds. The absorption peak at 723.1 cm-1, represent ZrO2 the stretching vibration, the peak absorption at and the absorption peaks at 2363 and 2325 and are due to S-H stretching, the characteristic absorption of sulphated zirconia observed correspond to the absorption of SO42-/ZrO2 reported by Yunfeng et al. [15].
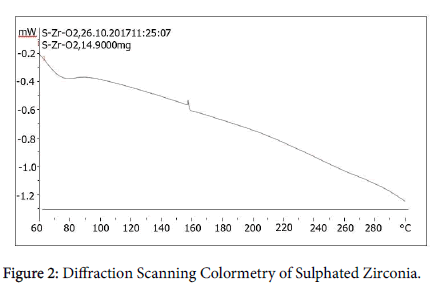
DSC analysis: The DSC (diffraction scanning colometry), analysis of S-ZrO2 as indicated in Figure 2, show glass transition temperature (the temperature at which the catalyst undergone change in heat capacity without change of state) as 74.82°C, the crystallization temperature (the temperature at which catalyst amorphous solid change to crystalline solid) is 158.31°C with exothermic heat as -46.78 × 10-3 mW/°C. As the catalyst heated from 59.996 to 299.93°C, the heat capacity change from -0.0232 mW/°C to 0.0073 mW/°C, this is due to crystallization of catalyst from amorphous to trigonal of ZrO2. The specific heat capacity of the S-ZrO2 normalized at 1.44 J/g.
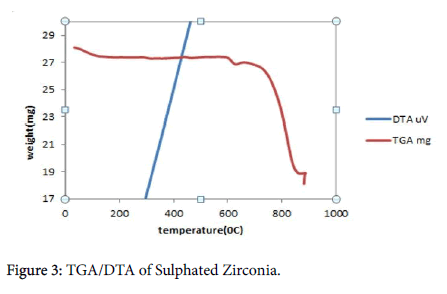
TGA/DTA analysis: The TGA and DTA curves of prepared of Sulphated Zirconia catalyst in Figure 3 show a weight lost at a temperature of 100°C, the weight of the catalyst slightly decreases from 28.1 mg to 27.3 mg when the temperature rises from 36.5°C to 120°C. The reduction maybe due to evaporation of the water at the surface of the catalyst, the weight of the catalyst slightly remains constant as the temperature rises from 120°C to 590°C. As the temperature rises above 600°C to 900°C, the catalyst weight significantly decreases from 27.3 mg to 19 mg, this may be due to decomposition of the impurities and desorption of SO2 that adsorbed at the surface of the catalyst.
XRF and XRD analysis: The results of XRF analysis as indicated show 1.728% Al2O3, 2.815% SiO2, 2.287% CaO, 1.019% SO3, 1.0137% SO3 (OS) and 74.03 ZrO2.
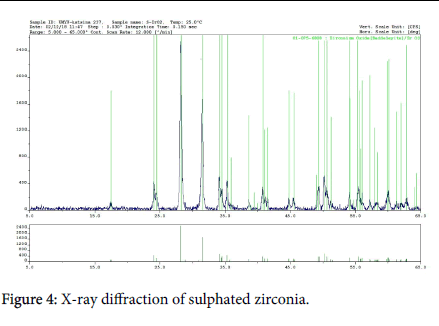
Figure 4 is the X-ray diffractogram of the S-ZrO2 and the principal minerals present. The diffractogram of the S-ZrO2 show strong absorption of various point of 2 Ø, the important peak at 28.0°C, 31.8°C, 34.0°C and 50.4°C show the characteristic absorption of mineral Baddeleyite (ZrO2). The X-ray diffraction neutron’s catering and EXAFS spectroscopy of monoclinic Zirconia analysis show crystal cell per volume of ZrO2 is 140.64 Å3.
Discussion
The results obtained might vary depending on the method and solvent used for oil extraction, nature and type of sample, environmental factors, method of transesterification etc. The results obtained from these analyses are interpreted as follow:
Percentage biodiesel yield
The yield of biodiesel produced at optimum transesterification variable were (60°C, 3 hours, 1:8 w/w%) was found to be 97.91%. This is slightly higher than the value (89.90%) for Jatropha biodiesel obtained by Teo et al. [16] using calcium oxide as catalyst, similar result (96%) was obtained by Endalew et al. [17]. The varying optimum biodiesel yield results may be due to the different methods of the catalyst preparations, catalyst loads, temperatures and oil to methanol ratios.
Physicochemical properties of biodiesel produced
Acid value: The acid value of the biodiesel determines the level of free fatty acids present in the biodiesel. The acid value of biodiesel produced was 2.02 mgKOH/g, as presented in Table 1 is higher than 0.5 maximum ASTM standards. This can be corrected by neutralizing the oil before use to suits engines in order to avoid corrosion and wear in fuel systems and storage tanks.
Iodine value: The iodine value of biodiesel produced is 104 gl2/100 g as presented in Table 1, which is within the range of 130 maximum ASTM standards. The value shows low degree of unsaturated compound in the biodiesel produced, this is upholding by GC-MS analysis of the biodiesel produced which indicate low degree of unsaturated methyl esters i.e., (8.23%) hexadecanoic acid, methyl ester, (12.14%) 9,12-octadecadienoic acid, methyl ester and (10.82%) 11- octadecanoic acid, methyl ester.
Saponification value: The saponification value of Jatropha biodiesel is 186.00 ± 2.00 mgKOH/g, as shown in Table 1, similar result (190.00 mgKOH/g) was obtained by Singh et al. [18]. The saponification value suggests that the biodiesel has higher molecular weight that may be responsible for its higher specific gravity (0.85) of the biodiesel produced. The high saponification value indicates higher methyl ester contents, as ester value is the difference between saponification value and acid value.
Flash point: The flash point of the biodiesel was 140°C as shown in Table 1, which falls within the range of 170 ASTM standards; similarly, a slightly higher result (162°C) was obtained by Singh et al. [18]. Flash point is not related to engine performance but is inversely related to fuel volatility Ramirez-Verdazeo et al. [19]. The flash point of biodiesel obtained indicates the lower volatility of the oil which in turn indicates its safety in handling.
Pour point: The pour point of biodiesel produced was 5.5°C as shown in Table 1, a lower result (-6°C) obtained by Singh et al. [18]. The value obtained is within the range (-15 to 10°C) of the ASTM standards. Considering the average temperature of our locality 22 to 40°C, the biodiesel could be handled and stored safely without solidifying, but when the fuels are to be used in cold regions, they would have to be preheated or blended with some additives that would further reduce the pour point of the biodiesel [20].
Cloud point: Cloud point of the biodiesel produced is 7.9°C as presented in Table 1, a lower result (2 ± 0.21°C) obtained by Aldo et al. [21] which are both within the range (-2 to 12) ASTM standards of biodiesel, the difference may be a result of different in weather and climatic conditions.
Specific gravity: The specific gravity of the biodiesel at optimum yield is 0.85 (Table 1). Similar result 90.89 was obtained by Ogunwole et al. [22]. The result obtained is within the range (0.95 max) ASTM standards. The higher the specific gravity of a fuel, the greater the mass of fuel injected into the engine and hence more power [23].
Kinematic viscosity: The kinematic viscosity of the biodiesel is 4.70 mm2/s, as presented in Table 1, A similar result (5.25 mm2/s) was obtained by Aldo et al. [21], and a lower result (4.51 cSt) was obtained by Previous work. The result obtained is within the range (1.6 to 6.0) of ASTM standards, which indicate the presence of short chain unsaturated methyl fatty acid esters and is likely to produce less deposits when burnt in combustion engines.
Cetane number: The result contained in Table 1 for biodiesel is 62.27, which agree with range (48-65) ASTM standard specification for biodiesel, lower result (49.50) obtained by Afaf and Salwa [4]. However, the high cetane number of the biodiesel obtained indicates its good ignition quality.
Higher heating value: The result obtains as presented in Table 1 for biodiesel is 43.10 MJ/Kg, however, the result is comparable to 47.54 MJ/Kg reported for diesel fuel [24]. Fuel having higher heating value gives higher power out and it small quality will cover long distance drive [25]. The higher heating value of biodiesel increases with increase in chain length and degree of saturation [26-44].
Conclusion
This research work demonstrated the production and characterization of biodiesel from Jatropha curcas L. seed using sulphated zirconia catalyst revealed 97.91% biodiesel yield and 31.59% methyl ester yield as analysed by GC-MS. The characterization of the catalyst shows that, the DSC analysis show glass transition and crystallization temperature of sulphated zirconia as 74.82°C and 158.31°C and specific heat capacity 1.44 J/g. The TGA/DTA show the catalyst decomposed at temperature ranged 600- 900°C. While the XRF results show 74.03% ZrO2. The physicochemical properties of biodiesel produced are within ASTM standards, this indicate that, oil might be used as alternative to conventional diesel or as an additive.
References
- Singh B, Singh K, Rejeshwar Rao G, Chikara UV, Raghuvanshi N, et al. (2013) Agro-Technology of Jatropha Curcas for Diverse Environmental Conditions in India. Biomass and Energy 48: 191-202.
- Nur ST, Sarina S (2016) Overview of Catalysts in Biodiesel Production. ARPN Journal of Engineering and Applied Sciences 11: 439-448.
- Ma F, Hanna MA (1999) Biodiesel Production: A Review. Bio-Resource Technology 70: 01-15.
- Afaf ghais A, Salwa MO (2015) Physical and Chemical Properties of Jatropha Biodiesel. International Journal of Recent Scientific Research 6: 5172-5174.
- Afaf G, Salwa M (2012) Biodiesel Production from Jatropha Oil. Faculty of Engineering. Journal of Petroleum Engineering, p: 6.
- Shuit SH, Lee KT, Kamaruddin AH, Yusup S (2010) Reactive Extraction and in Situ Esterification of Jatropha Curcas L Seed for the Production of Biodiesel. Fuel 89: 527-520.
- Hossain SA, Sallah BM, Boyce A, Chowdhury AN, Naquiddin M (2008) Biodiesel Fuel Production from Algae Renewable Energy. American Journal of Biochemistry and Biotechnology 4: 250-251.
- Hara S, Miyayama M (2004) Proton Conductivity of Super acidic Sulphated Zirconia, Solid State Ionics 269: 155-160.
- Meher LC, Vidya SS, Naik SN (2006) Optimization of Alkali Catalyzed Transesterification of Pongania Pinnata for Production of Biodiesel. Bio-resource Technology 97: 1392-1397.
- Indhumathi P, Shabudeen PS, Shoba US (2014) A Method for Production and Characterization of Biodiesel from Green Micro Algae. International Journal of Bio-Science and Bio-Technology 6: 111-122.
- Fuduka H, Kando A, Noda H (2001) Biodiesel Fuel Production by transesterification of oils. Journal of Bio-Science and Bio-Engineering 92: 405-415.
- Demirbas A (2008) Comparison of Transesterification methods for Production of Biodiesel from Vegetable Oils and Fats. Journal of Energy Conversion and Management 49: 125-130.
- Salaheldeena M, Aroua MK, Mariod AA (2015) Physico Chemical Characterization and Thermal Behaviour of Biodiesel and Biodiesel-Diesel Blends Derived from Crude Moringa Peregrinased Oil. Journal of Engineering Research and Application 3: 488-492.
- Farag HK, Hanafi ZM, Dawy M, Abdelaziz EM (2010) Characterization of ZnO Nanopowders Synthesized by the Direct Precipitation Method. Canadian Journal of Pure and Applied Sciences 4: 1303-1309.
- Yunfeng Z, Huamin Z, Jingwei H, Boalian Y (2006) Preparation and Characterization of Sulphated Zirconia (SO42-/ZrO2) /Nafion Composite Membranes for PEMFC Operation at High Temperature/ Low Humidity. Journal of Membrane Science 280: 148-155.
- Teo SH, Rashid U, Taufiq-Yap YH (2014) Biodiesel Production from Crude Jatropha Curcas Oil using Calcium Based Mixed Oxide Catalyst. Elsevier 136: 244-252.
- Endalew AK, Kiros Y, Zanzi R (2011) Heterogeneous Catalysis for Biodiesel Production from Jatropha Curcas Oil (JCO). Energy 36: 2693-700.
- Singh RK, Padhi Sarog K (2009) Characterization of Jatropha Oil for the Preparation of Biodiesel. Natural Product Radiance 8: 127-132.
- Ramirez-Verdazeo LF, Rodriguez-Rodriguez JE, Jaramillo-Jacob AR (2012) Predicting Cetane Number, Kinematic Viscosity, Density and Higher Heating. Fuel 91: 102-111.
- Hassan LG, Sani NA (2010) Preliminary Studies on Biofuel Properties of Bottle Gourd (Lagenaria Siceraria) Seeds Oil. Nigerian Journal of Renewable Energy 11: 20-25.
- Aldo O, Temu AK, Ogwok P, Ntalikwa JW (2012) Physico-Chemical Properties of Biodiesel from Jatropha and Castor Oils. International Journal of Renewable Energy Research 2: 55-68.
- Ogunwole OA (2012) Production of Biodiesel from Jatropha Oil (Curcas Oil). Research Journal of Chemical Sciences 2: 30-33.
- Rao GLN, Ramadhas AS, Nullusamy N, Sakthivel P (2010) Relationship among the Physical Properties of Biodiesel and Engine Fuel System Design Requirement. International Journal of Energy and Environment 1: 919-926.
- Oliveira LE, Silva MLCP (2013) Comparative Study of Calorific Value of Rapeseed, Soybean, Jatropha Curcas and Crambe Biodiesel. Renewable Energy and Power Quality Journal 11: 1-4
- Gulum M, Bilgin A (2015) Density, Flash Point and Heating Value Variations of Corn Oil Biodiesel- Diesel Blends. Fuel Processing Technology 134: 456-464.
- Demirbas A (1997) Calculation of Higher Heating Value of Biomass Fuels. Elsevier 78: 431-434.
- Akpan UG, Jimoh A, Mohammed AD (2006) Extraction, Characterization and Modification of Castor seed oil. Leonardo Journal of Sciences 8: 43-52.
- Alamu OJ, Dehimbo O, Sulaiman AM (2010) Producton and Testing of Coconut Oil Biodiesel Fuel and Its Blend. Leonardo Journal of sciences 95-104.
- Al-Harbawy AW, Al-Mallah K (2014) Production and Characterization of Biodiesel from Seed Oil of Castor (Ricinus Communis L.) Plants. International Journal of Science and Technology 3: 508-513.
- AOAC (1990) Official Methods of Analysis. 15th edn. Association of Official Analytical Chemists, Washington DC
- Asmare M, Gabbiye N (2014) Synthesis and Characterization of Biodiesel from Castor Bean as an Alternative Fuel for Diesel Engine. American Journal of Energy Engineering 2: 1-15.
- Asokan MA, Vijayan R (2014) The Conversoion of Kapok Seed (Ceiba Pentandra) Oil into Biodiesel and Investigation of Effects of Catalyst of Chemical Technology and Research. 6: 5709-5715.
- ASTM (2008) America Standard for testing of materials, Characteristics of Jatropha Curcas oil. Journal of American and Chemist Society 85: 2671-2675.
- Bello EI, Anjorins SA, Agge M (2008) Production of Biodiesel from Fluted Pumpkin (Telfairia Occidentalis Hook F) Seeds Oil. International Journal of Mechanical Engineering 2: 22-31.
- Dunn RO, Shocky MW, Bagby MO (1996) Improving the Low Temperature Properties of Alternative Diesel Fuels; Vegetable Oil Derived Methyl Esters. Journal of American Oil Chemist Society 73: 1719-28.
- Fakhary EM, El-Maghraby DM (2013) Fatty Acids Composition and Biodiesel Characterization of Dunaliella Salina. Journal of Water Resources and Protection 5: 894-899.
- Galadima A, Garba ZN, Ibrahim BM (2008) Homogeneous and Heterogeneous Trans-Esterification of Groundnut Oil for Synthesizing Methyl Biodiesel. International Journal of Pure and Applied Sciences 2: 138-144.
- Gerpen JV, Shanks B, Pruszko R, Clement D, Knothe G (2004) Biodiesel Production Technology. NREL, 1617 Cole Boulevard; Corado. USA.
- Hotti SR, Hebbal OD (2015) Biodiesel Production and Fuel Properties from Non-Edible Champaca Seed Oil for use in Diesel Engine. Journal of Thermal Engineering 1: 330-336.
- Krisnangkura KA (1989) Simple Method for Estimation of Cetane Index of Vegetable Oil Methyl Ester. Journal of American Oil Chemist Society 63: 552-553.
- Mukhtar M, Dabai MU (2016) Production and Fuel Properties of Biodiesel from Gingerbread Plum (Parinari Macrophylla) Seed Oil using MgO/Al2O3 Catalyst. American Journal of Environmental Protection 5: 128-133.
- Sokoto AM, Hassan LG, Dangoggo SM (2011) Influence of Fatty Acid Methyl Ester on Fuel Properties of Biodiesel Produce from Curcubita Popo. Nigeria Journal Basic Applied Science 19: 81-86.
- Ved K, Padam K (2013) Study of Physical and Chemical Properties of Biodiesel from Sorghum Oil. Research Journal of Chemical Sciences 3: 64-67.
- Verma RM (2001) Analytical Chemistry. Theory and Practice, 3rd edn. CBS Publishers and Distributors, New Delhi, India, pp: 492-498.
Citation: Dangoggo SM, Dhikrah I, Sani NA, Baki AS, Bagudo BU, et al. (2018) Preparation and Characterization of Biodiesel Produced from Jatropha Seed Oil Using Sulphated Zirconia as Catalyst. Ind Chem 4: 126. DOI:
Copyright: © 2018 Dangoggo SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 7618
- [From(publication date): 0-2018 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 6433
- PDF downloads: 1185