Rapid Pain Relief and Functional Improvement after Local Radiofrequency Ablation and Cementing Combined with Internal Fixation for Long Bone Metastases: A Prospective Study
Received: 17-Dec-2020 / Accepted Date: 30-Dec-2020 / Published Date: 06-Jan-2021 DOI: 10.4172/2472-016X.1000145
Abstract
Background: Metastatic patients are living longer. Bone metastases are associated to pathological fractures, severe pain, and functional impairment. There is evidence of pain relief and functional improvement after Radiofrequency Ablation (RFA) and/or cementing. These procedures present a synergistic effect. Intramedullary nailing allows immediate weight bearing and complements stabilization, pain and functional improvements. In this study, a combined palliative local treatment for multiple bone metastases aims to relief pain and improve function.
Methods: Prospectively were analysed 30 patients and a total of 32 long bone metastases treated with local curettage, RFA, cement filling, and internal fixation. It was evaluated pain (Visual Analogue Scale), function (Karnofsky Performance Score), treatment complications, and survival. Local tumour progression was evaluated on radiographs and CT scan.
Results: Mean follow-up was 17.5 months (range 1-63). All patients experienced immediate pain relief 15 days after treatment. Mean VAS improved from 8.63 (SD 0.75, range 7-10) to 3.72 (SD 1.92, range 0-7). The mean VAS reduced even more at 6 weeks to 2.5 (SD 1.77, range 0-6) and at final follow up stabilized at 2.19 (SD 1.77, range 0-6). The postoperative improvement is statistically significant for all time visits (p<0.0001). The preoperative mean functional KPS was 57.7 (SD 14.55, range 30-80) compared to 74 (SD 16.32, range 40-100) postoperatively (p<0.0001). There was no evidence of local disease progression. There were no reported complications. Mean survival was 23.0 months (SD 4.35, 95% CI range 14.5-31.6).
Conclusion: The combined treatment presented rapid pain relief and significant functional improvement impacting on quality of life. This study shows an effective local tumour control. RFA, cementing, and internal fixation should be incorporated in multidisciplinary discussion since the beginning in order to be used early in the treatment algorithm. Patients with short to medium-term survival significantly benefit from this treatment.
Keywords: Long one metastases; Radiofrequency ablation; Cementing; Internal fixation; Pain; Function; Local tumour control; Quality of life
Introduction
The prevalence of the metastatic disease is increasing over the years in cancer patients and is associated with a poor prognosis accounting for more than 90% of all cancer related deaths [1-4]. Despite recent advances and improving understanding of metastatic mechanism its treatment is still a challenge.
Bone is the third most frequent location for haematogenous dissemination of malignant tumours. In fact, around 50-84% of patients with cancer develop bone metastasis [5,6]. Bone metastases are largely derive from breast, prostate, kidney, and lung, and spine is the most frequently involved area [4-20]. Long bone metastases are mainly located in femur followed by humerus [16].
Actually, metastases is the most common malignant process affecting bones and predispose bones to fractures [21]. Furthermore, bone metastases can cause severe pain, including localized, radicular and axial pain, functional impairment and spinal cord compression with neurological deficits and paraplegia [22,23]. They are associated to high morbidity and have an important psycho-socioeconomic impact [4,24].
Distressing, drug-resistant chronic pain is the main symptom of bone metastasis [25]. For instance, the presence of bone metastases is the most common cause of cancer-related pain [26]. Uncontrolled pain disturbs function and remarkably interferes with daily activity and quality of life [27]. Currently available therapeutic modalities to treat metastatic pain include specific anticancer therapy, analgesic drugs, and invasive procedures including regional analgesia, axial narcotics, injection of neurolytic agents, ablative neurosurgical techniques, radiofrequency ablation and cementoplasty [28].
After external beam radiation therapy 20%-30% of patients do not experience pain relief and complete response rates range from 10 to 50% [29-31]. Developments on oncological treatments are extending survival of metastatic patients and the pain management is important to provide the highest quality of life possible. On the one hand, survival estimate and patients selection for treatment are extremely difficulty [32]. On the other hand, optimal metastatic palliative treatment must be individually customized [32]. presented the OPTIMAL study and launched the OPTIModel App for long bone metastases. This is a prognostic model for estimating survival in patients with cancer and symptomatic metastases of the long bones (femur and humerus) with treatment recommendations for decision-making [32]. The quality of bone stock, location of the lesion, primary tumour, expected survival, and patient general condition, are factors to support decision-make [32]. Authors summarize that the treatment of bone metastases highly depends on the fracture risk in relation to expected survival [32].
The most important factors to take into account for survival estimative are a poor/good general health status, old/younger age, multiple/solitary lesion, short/long-free interval of disease (time frame from primary), and unfavourable/favourable histotype. Indications of treatment will greatly depend on life expectancy [32].
Few options exist for patients who fail to obtain adequate pain relief following radiation [33]. Furthermore, patients who have current pain at a site previously irradiated may not be eligible for further radiotherapy secondary to limitations in normal tissue tolerance [33].
Percutaneous cementoplasty is a minimally invasive technique that has been shown to alleviate pain and reduce metastatic activity in addition to mechanical stability [34]. Cementoplasty was reported to relief pain in 80 to 97% of cases whatever the bone site treated; whether vertebrae, long bones or flat bones [32]. Cortical bone is much more resistant to heating [35]. Nevertheless, bone cement generates heat up to 80ºC, which may help to potentiate the anticancer effects of RFA: tumor cells necrosis, pain relief, minimization of pathological fracture risk, and functional improvement [36]. Pain relief is achieved within 4 weeks in 82-97% of patients treated with vertebroplasty and in 90%- 100% of patients treated with RFA [37]. Success may be increased with a combination of both procedures, RFA and cementing [38].
RFA is a percutaneous procedure, target tumour destruction, is cost effective, and has a low complication rate [27,36] Also significant decrease in opioids requirements was verified but not persistent despite continuous reduction in pain scores [33]. However, there is a technically inability to expand the RFA tips into the blastic metastases, so being suitable only for osteolytic or mixed type lesions [33].
Radiofrequency ablation combined with conventional therapies such as cementoplasty for palliative treatment of painful metastasis may induce a faster pain relief and mechanical stability, and consequently improving the quality of life [39]. The combined procedure can be performed even in patients where radiotherapy is contraindicated or without previous response. The combined technique has many theoretical advantages. The intense heat from RFA may be destroying local sensory nerves, thereby interrupting pain transmission [33]. Also, by destroying tumour cells, RFA may decrease the production of tumour-induced nerve-stimulating cytokines such as tumour necrosis factor alpha (TNF-α) and interleukins (IL-1, IL-6) or systemic inflammatory markers, and consequently it attenuates or inhibits tumour osteoclast activity and reliefs pain [33,40,41]. Furthermore, RFA may prevent local disease progression by inhibiting tumour growth into the periosteum and surrounding soft tissues, and by preventing the development of painful micro and macro fractures secondary to the mechanical stress of weakened bone [33]. On the other hand, cementing has biomechanical properties with a direct necrotic thermal effect on bone-tumour cells, adding an ablation effect on C- nociceptive fibers and a structural support through the mechanical stability effect to reduce periosteum stress, prevent or treat micro and macro pathological fractures [42]. The additional pain relief obtained by cementoplasty has the advantage of rapid onset; generally 1–3 days after the procedure, with subsequent early postoperative mobilization [43].
The RFA and cementing can even be combined with internal fixation keeping a minimally invasive and cost-effective technique, with low complications risk. Surgical treatment options for long bones pathological fractures in patients with multiple bone metastases include internal fixation (intramedullary nail or plate), or prosthetic reconstruction [32]. The selection will depend on the lesion anatomical location, bone stock, life expectancy, patients’ characteristics (primary tumour, age, functional status, obesity and other co-morbidities), associated risk of complications, rehabilitation, and surgeon experience [32]. There is no consensus in literature on what procedure is the gold standard for long bone pathologic fractures [32]. The recommended procedures are mostly based on retrospective observational studies and clinical experience [32,44]. For instance, if life expectancy is less than 3 months, in generally the orthopaedic oncologist surgeons recommend intramedullary nailing for long bones [45]. For patients with estimate survival above 6 months and up to 12 months, should be preferred a prosthetic reconstruction [32,45-49]. Patients that are expected to have a long survival above 12 months, may have more complex reconstructions, wide resection and endoprostheses [32].
Intramedullary nails offer several advantages: minimally invasive procedure, the periosteum blood supply is preserved, rigid fixation is achieved with proximal and distal interlocking screws and/or cement, and allows for immediate weight bearing [45]. Additionally, for poor (lytic) bone stock, proximal fixation can be achieved with large lag screw or spiral blade [32]. However, it is not suitable for (peri)articular lesions and with time intramedullary nails without cement are at risk of failure because they are load-sharing devices [32]. Plate fixation has disadvantages such as the open approach, the large incision needed, longer procedure, and does not allow prophylactic fixation of the entire bone [32]. Nevertheless, it allows rigid fixation for very distal fractures using locking screws, and facilitates curettage, fracture reduction and cement filling [32,50].
Prosthetic reconstruction provides immediate stability, is independent of fracture healing and minimizes the risk of local disease progression or implant failure [45,47,50-53]. The principal drawback of this procedure is the high risk of complications [45,50- 52]. Prosthetic reconstruction increases blood loss, has considerable risk of infection in these patients, and is not the optimal option for patients who will need adjuvant radiotherapy [32,49,54,55]. The high costs of endoprostheses are no longer a disadvantage.
In this context, for patients with multiple bone metastases and a short to medium-term life expectancy, the combined treatment of RFA, cementing, and internal fixation (intramedullary nail, preferable) is expected to present a beneficial, complementary and synergistic effect with impact on patients’ quality of life. Furthermore, it is a cost-effective palliative treatment, minimally invasive procedure, and has a low risk of complications.
The goal of the combined procedure is to relief localized pain and improve the overall functional performance, providing local control of disease progression and consequently improving the quality of life, and hopefully maybe to extend patients survival. The main issue relays on cost-effectiveness and minimization of surgical risks related to the palliative treatment of bone metastases in a way that patients are able to benefit from treatment outcome.
Materials and Methods
Patients admitted to the surgical intervention were prospectively followed and observational clinical and imaging data were collected. The minimum follow- up was determined as 12 months or until death, whichever come first. Radiological follow-up with digital radiographs was performed at 6 weeks, 3 months, 6 months, 12 months, and each 6 months after that in the living patients. CT scan was also performed after 3 and 12 months. It was evaluated the worst pain related to the treated lesion site (Visual Analogue Scale, VAS), function (Karnofsky Performance Score, KPS), surgical treatment complications, and patients’ survival. Local and systemic complications, intraoperative death, and postoperative death up to 1 month of surgery, were all reported. Re-operation or revision surgery was also reported. Patients were additionally questioned about reduction of pain medication and quality of life, daily activities and sports. All patients had to consent participating in this study and after adequate information they accepted to be submitted to the proposed surgical treatment.
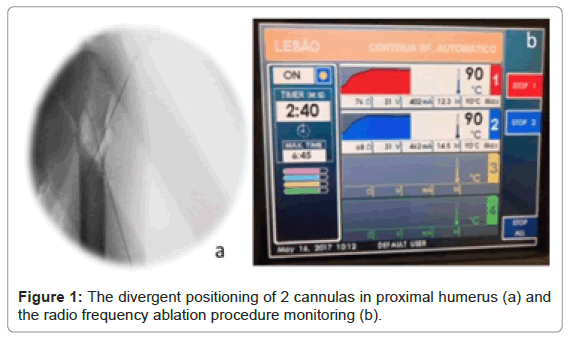
Surgical treatment is minimally invasive and consists in the combination of local curettage, radiofrequency ablation, bone cement filling and internal fixation with titanium or Carbon-Fiber reinforced Polyetheretherketone (CF- PEEK) intramedullary nailing (static locking; femoral neck screw locked to the proximal nail to prevent protrusion through the femoral head; for proximal humerus, a spiral blade was chosen for proximal fixation when used the titanium intramedullary nail). Exception for two cases stabilized with a distal femur or humerus plate due to the location of the lesion. The radiofrequency ablation (Figure 1) was performed with 2 divergent cannulas simultaneously (DirosTM, 2.5 cm active tip under 90°C for 6 minutes, with extra initial time to reach temperature first; or 2 cm active tip, OsteoCoolTM Radiofrequency Ablation System, 80°C for 15 minutes, including ramp time).
Inclusion criteria were mature skeleton (age 18 or older), advanced disease (multiple bone metastases), osteolytic or mixed type metastasis, long bone symptomatic lesion (VAS ≥ 4), risk for imminent fracture (Mirels score >8; ≥ 3 cm axial cortex destruction at femur), and pathological fracture (with or without soft tissue extension).
Patients were excluded from this procedure if presenting solitary bone metastasis, osteoblastic lesions, active infection, articular invasion, neurological compromise, abnormal coagulopathy profile (haemorrhagic diathesis) or insufficient general condition for the surgical procedure, multiple myeloma lesions, plasmacytoma, and bone lymphoma.
Statistical analysis
Survival was calculated using the Kaplan-Meier survival analysis with 95% confidence intervals (CI). The results were analysed statistically with SPSS v26.0 (IBM SPSS Statistics, Chicago, Illinois) and a p-value< 0.05 was used to denote statistical significance.
Results
A total of 32 long bone metastatic lesions (30 lytic and 2 mixed type) were treated in 30 patients. Mean age was 63 years old (range 29-88). Table 1 summarizes patient and metastatic lesion characteristics.
| Characteristic (n,%) | n = 30 | ||
|---|---|---|---|
| Lesions = 32 | |||
| Gender | |||
| Male | 19 (63) | ||
| Female | 11 (37) | ||
| Site | |||
| Femur | 18 (56) | ||
| Humerus | 12 (38) | ||
| Tibia | 2 (6) | ||
| Metastatic lesion characteristics | |||
| Impending fracture | 18 (56) | ||
| Pathological fracture | 14 (44) | ||
| Lytic lesion | 30 (94) | ||
| Mix lesion | 2 (6) | ||
| Top primary tumouR | |||
| Lung | 14 | ||
| Breast | 4 | ||
| Renal Cell | 4 | ||
| Brain metastases | 6 | ||
| Visceral metastases | 8 | ||
| Lung metastases | 16 | ||
| Spinal metastases (Thoracolumbar) | 18 | ||
| Symptomatic lesion | 32 (100) | ||
| Previous treatment | |||
| Primary tumour resection | 9 | ||
| Spinal metastases (thoracolumbar) | 2 | ||
| Systemic treatment | 9 | ||
| Radiotherapy | 7 | ||
| Spinal metastases (thoracolumbar) | 3 | ||
| Proximal humerus | 2 | ||
| Lung metastases | 2 | ||
| Treatment | |||
| FA, Cement filling and internal fixation | 32 (100) | ||
| Complications | 0 | ||
| Deaths | 22 (73) |
Table 1: Patient and metastatic lesion characteristics.
Patients presented a large variety of primary tumours: lung (14), breast (4), renal cell (4), hepatoid renal cell (1), urothelial (1), larynx epidermoid (1), Pankreatobiliar (1), gastric (1), gastrointestinal (1), colon sigmoid (1), Colorectal (1).
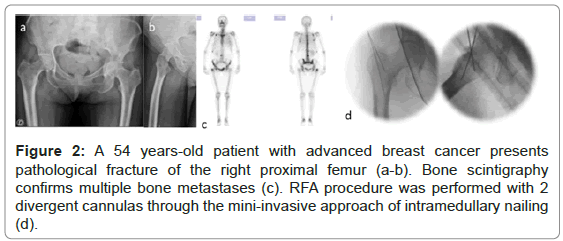
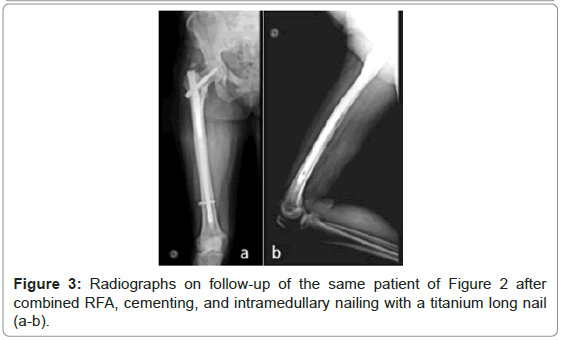
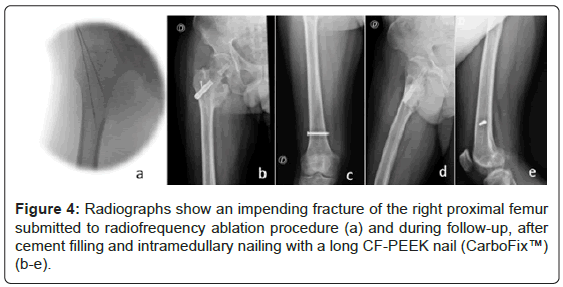
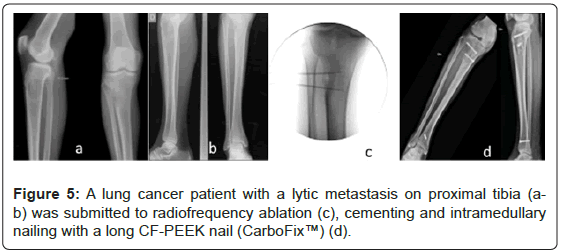
From the total of 32 lesions, 18 lesions were considered impending fractures, and the other 14 lesions presented pathological fractures (Figures 2-5).
Figure 2: A 54 years-old patient with advanced breast cancer presents pathological fracture of the right proximal femur (a-b). Bone scintigraphy confirms multiple bone metastases (c). RFA procedure was performed with 2 divergent cannulas through the mini-invasive approach of intramedullary nailing (d).
Patients treated for 2 lesions sites were asked to evaluate the worst discriminated pain related to each location separately.
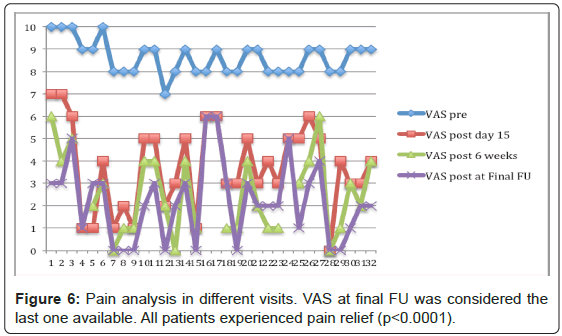
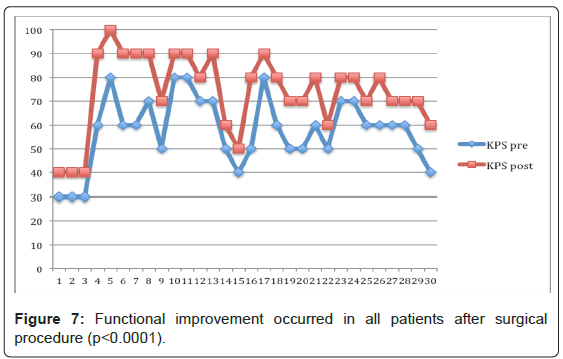
All patients experienced immediate pain relief (≥ 2 point change) 15 days after treatment. The mean VAS improved from 8.63 (SD 0.75, range 7-10) to 3.72 (SD 1.92, range 0-7). Four patients died just after 1 month and were only evaluated at day 15. For the other 26 patients (28 lesions), the mean VAS score reduced even more at 6 weeks postoperatively to 2.5 (SD 1.77, range 0-6). At final follow up was considered the last available evaluation for all patients, the mean VAS stabilized at 2.19 (SD 1.77, range 0-6) (Figure 6). The postoperative improvement is statistically significant for all time visits (p<0.0001). The 95% CI of the difference between VAS pre- and VAS postop day 15 range from 4.15 to 5.60, or 5.44 to 6.81 VAS postop at 6 weeks, or 5.76 to 7.12 VAS postop at final FU (Figure 7). The same way, all patients improved function significantly after treatment, minimum 10-point change. The preoperative mean KPS was 57.7 (SD 14.55, range 30-80) and postoperatively it reached 74 (SD 16.32, range 40-100) (p<0.0001). The 95% CI of KPS pre- and postop difference range from -24.32 to -8.34. After treatment, 28 patients were discharged home after mean 1.9 days (range 1-6) and 2 patients had no family support and were admitted to palliative care unit. Mean follow-up was 17.5 months (range 1-63).
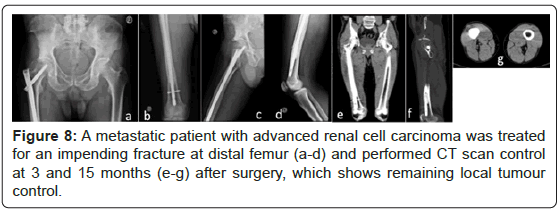
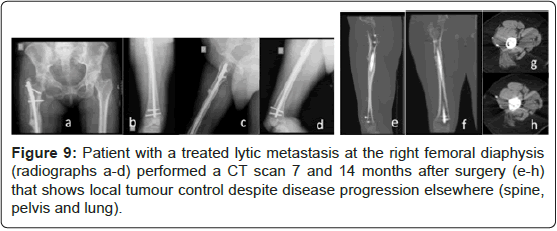
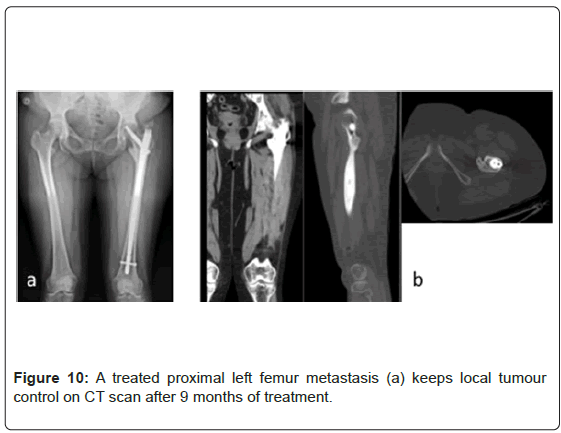
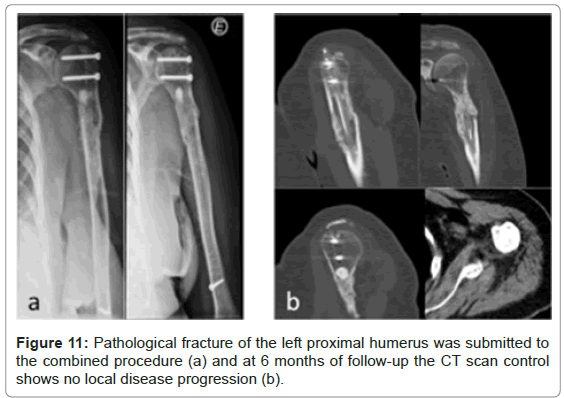
There was no evidence of local disease progression on radiographs. For different factors was not possible to realize CT scan systematically at a fixed time on follow-up. Between 3 and 15 months, local tumour control without disease progression on the operated bone was verified in CT scan of 8 patients (26.7%), despite disease progression elsewhere (spine, pelvis, visceral, and lung) after 12 months. Three of those patients repeated the CT scan control twice through that time. Figures 8 to 11 present some of those cases and show supressed progression of metastases.
After the surgical treatment, all patients confirmed initial reduction on painkillers, in agreement with local pain relief, but not sustained in time, despite the continuous reduction in pain scores, because of referred pain elsewhere.
All previous active patients had abandoned sports for some time pre-operatively and likewise none returned to sports activity after treatment. Despite the functional improvement, 26% of patients were not able to perform all daily activities they liked to. All patients especially valued the recovery of limb function.
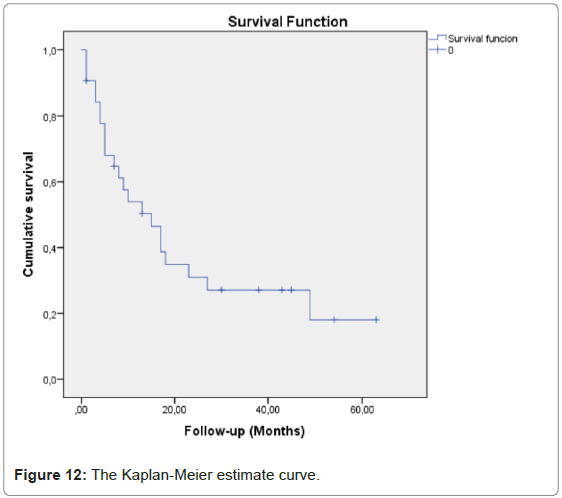
There were no reported complications. During follow-up, 22 patients died (73.3%), one case from acute respiratory infection 6 weeks after treatment, all the others from disease progression (Figure 12).
Mean survival was 23.0 months (SD 4.35, 95% CI range 14.5-31.6).
Median Survival was 15 months (SD 3.9, 95% CI range 7.3-22.7).
The 1-year estimate Survival was 50.3%.
Discussion
Although metastatic patients have limited life expectancy, systemic and local treatments for cancer patients are improving leading to longer survival even for patients with disseminated disease [32,56,57].
Evidence-based medicine is still poor and treatment is mainly based on expert opinion and experience [32]. There is no consensus on the best treatment for each case and in practice it is individually optimized. For that, early diagnosis of bone metastases, and tumour and patient prognostic factors to better estimate life expectancy are essential for decision-make. The goal is to relief pain and improve function, and for that accurately identify impending fractures, prevent pathological fracture and act before it happens, while taking into account the surgical risks and rehabilitation time in order to achieve the highest quality of active life.
Although the limited survival, the combined treatment showed effective and rapid pain relief and significant functional improvement. Local progression-free survival was verified. Further, there was no complications related to the treatment. Accordingly to Goetz et al. [39], after the surgical treatment all patients confirmed initial reduction on painkillers but not sustained in time, despite the continuous reduction in pain scores, because of referred pain elsewhere related to the systemic metastatic disease.
Despite the significant functional improvement, some patients were not able to perform all daily activities they liked to. This reflects the associated psychosocial component of living with an advanced disease status under palliative care.
These patients need to eliminate pain quickly, need to stabilize fractures, need to get ahead of delayed skeletal events, need to keep an active life, opioid consumption should be controlled, and they need to stay on course with multidisciplinary and systemic regimens.
This treatment combination is a minimally invasive technique, with extremely low complications risks, and it seems to greatly increase the efficacy of local tumour control.
A multicentre study of 43 patients reported a significant decrease in pain 12 and 24 weeks after treatment with significant reduction of opioid consumption at weeks 8 and 12 [39]. Adverse events were recorded in only 3 patients. Recently, Fares et al. [58] verified that combined radiofrequency ablation and cementoplasty was a safe and effective pain relief treatment in 30 patients with extraspinal painful bone metastases, without any major complications through 12 weeks follow-up [58]. Most treated lesions were at pelvis location and internal fixation technique was not used. Authors showed decreased pain intensity and disability from day 1 up to 12 weeks. They reported no major complications and 2 cases of minor cement leakage. This study shows the benefit of a synergistic effect of RFA plus cementoplasty for painful bone metastases.
The OPuS ONE multicentre study [59,60]. Published the results of the first 100 patients followed through 6 months after RFA treatment. Most lesions were located at the axial skeleton being 87% in thoracolumbar spine and 13% in pelvis/sacrum. In total, were performed 134 ablations and in the majority of patients (96%) balloon kyphoplasty/ kyphoplasty/ vertebroplasty/ cementoplasty was combined to the RFA. Patients in this study experienced significant improvement in worst pain, average pain, and pain interference at all time points. For instance, the worst pain pre RFA mean score was 8.2; post RFA decreased to 5.6, 4.7, 3.9, 3.7, and 3.5 at 3 days, 1 week, 1 month, 3 months, and 6 months, respectively. Furthermore, 59% experienced immediate improvement (≥ 2 points change) in worst pain 3 days after the ablation. In addition, the QOL evaluation significantly improved post treatment for all visits, improving function for daily activities, despite the advanced disease status.
After treatment, 46% of patients reported a decrease in transdermal and/or narcotic use at 3 months. Adverse events were very rare and occurred in 4 cases (2 hospitalizations: pneumonia and respiratory failure). Authors verified 30 deaths during the course of the study, 29 through 6 months visit and all deaths were attributed to disease progression. This study analyses pain and QOL, does not analyse local tumour control (necrosis, tumour growth, local recurrence) or fracture follow-up. Nevertheless, it shows clinically impactful results. The RFA and cementing association showed to be a safe and effective option for palliative care and demonstrated significant pain relief as early as 3 days and sustained through 6 months, function and QOL improvement, covering the treatment goals.
Accordingly to literature, in the current study RFA and cementing combination demonstrated to be a safe and effective procedure for local palliative treatment of long bone metastases.
Intramedullary nailing is frequently used to stabilize an impending or actual fracture of long bones because it´s a minimally invasive procedure, minimizes complications risk, reliefs localized pain, allows for immediate weight bearing in femur, and rapid rehabilitation [61,62]. The use of a long nail also provides mechanical support and prophylactic treatment against future fractures in other regions of the long bone. It should be assumed the minimal healing tendencies or non-union of pathological fractures. Nevertheless, as a result of the limited survival of these patients, the load- sharing device may not exceed the fatigue life of the implant [61,63-66]. Therefore, the palliative treatment goals may be fulfilled when including intramedullary nailing for patients with a short or medium-term life expectancy. Long survival is an important risk factor for failure [61,67-69]. Studies on the use of intramedullary nails are mostly limited because of small series, heterogeneity, and short follow-up [46,61,62,65,66,68,69]. Willeumier et al. [61] reported 5 Centers retrospective study of 245 patients treated for impending or actual fracture of the femur. Indications for intramedullary nailing were impending fractures in 49% and actual fractures in 51% for lesions of the trochanter region and large femoral shaft lesions or if multiple lesions were present at the same bone femur. Mean follow-up was 14.4 months and 67% of patients died before 1 year. Authors reported a local complications rate of 12% with 6-month cumulative incidence of 8%. Implant breakage occurred in 8% of patients and 6 month cumulative incidence was 4% [61]. Authors reported that 3% of the nails fractured, all at the junction with the neck screw [57]. Independent factors associated with increased risk of implant breakage were actual fracture and previous radiotherapy [61]. Revision occurred in 5% of patients [61]. An actual fracture was independently associated with a higher revision risk, and use of cement was independently associated with a lower risk of revision [61]. Overall, the incidence of local complications (lasting pain despite surgery and analgesics, and tumour progression needing further adjuvant treatments such as radiotherapy or surgery), implant breakage, and revision were low, also as a result of the short survival of patients [61].
Intramedullary nailing for pathological humeral fractures demonstrate good overall results, although may present complications with longer survival. Actually Willeumier et al. Reported 12.6% of failures associated to intramedullary nailing of humerus. Nevertheless, the short survival of these patients may underestimate the complications rate 70. Willeumier et al. [70] also verified that mechanical failure develops mainly 6 to 12 months postoperatively. The oncological complications predominantly arise very shortly postoperatively or after 1 year [71]. Thus, resection and reconstruction should be considered for patients with expected long survival, especially for renal carcinoma patients, and for patients with massive involvement of metaphyseal area [71,72]. After endoprosthetic reconstruction, shoulder function is generally limited although it provides a stable platform for elbow and hand function [46,53,70]. Although a small series and heterogeneous tumour diagnoses at proximal humerus, Trovarelli et al. [49] showed that in patients in whom the deltoid can be partly spared, reverse total shoulder prostheses appears to reasonably restore short-term shoulder function.
Laitinen et al. [73]. showed that patients treated with cement experienced faster pain relief. Also, Choi et al. [74]. Used cement in femur and humerus to achieve a stable fixation despite extensive osteolysis and thin cortex. In the same way, Willeumier et al. [61]. showed that cement association to intramedullary nailing of femur reduces the risk of revision, particularly important for actual fractures treatment [61]. In addition, studies on percutaneous cement augmentation while performing close intramedullary nailing of long bones demonstrate significant and rapid pain relief, and 50% suppressed progression of the metastasis on PET/CT scan or bone scintigraphy at mean 7-8 months of follow-up [75].
The patient’s longevity should be over estimated and must be assume that a pathological fracture will not heal to plan a construction accordingly or a reconstruction that survive the patient [76]. Perioperative steps and intraoperative care should reduce bleeding and embolic events as well as prevent or minimize the risk of infection [77].
In the current study, complications and revision rates for long bones metastases were null and are comparable to previous studies using intramedullary nails that range between 0% and 14% [46,61,65,68,77- 79]. This fact shows that patients selection and treatment option were probably adequate for each patient needs and survival.
Patients with short to medium-term survival take advantage of the intramedullary nail while not exceeding the probable timeline for its potential complications. It is important to have conscience that any major complications and re-operations have a high impact on these patients limited life Even in the 2 cases with indication for a plate due to anatomical location, the combined treatment still showed a successful outcome without complications, despite the inherent disadvantages of the plate comparing to intramedullary nailing such as the open approach and no prophylactic fixation of the entire bone. To take the most from the combined treatment an intramedullary nailing should be used. Lesions in distal femur or humerus without indication for nailing and short to medium-term expected survival still may benefit from the combined treatment although with a plate. Patients expected to live longer may benefit from resection and endoprosthetic reconstructions Willeumier et al. [45,80,81,82] verified that general orthopaedic surgeons preferred using an intramedullary nail for fractures of the humerus and femur and the oncological orthopaedic surgeons recommend a prosthetic reconstruction in patients with a long expected survival. They conclude that decision should be based on the expected survival of the patient, the type and extent of the tumour, and the location of the fracture, on a referral centralized care [45]. Although this is a prospective study it has no control group and limitations are inherent to small patients number. This may be related to the fact that Oncologists may not be familiar with RFA and cementing combination for pain relief and functional improvement (clinical evidence, patient selection, overall outcome), and procedure logistics and infrastructure may not properly support a referral flow without reorganization and multidisciplinary work. Also, for different reasons it was not possible to realize CT scan or even PET scan in all patients during follow-up.
It is important to share data, results, and patients’ outcome. Future prospective randomized trials, multicentre studies for larger series, are needed. It is also extremely important a multidisciplinary teamwork for metastatic patients palliative treatment. Furthermore, it is essential advances for an earlier diagnostic of metastatic disease and bone metastases, prevention of delayed skeletal events, and get the Oncologists be familiar with this combined treatment outcome. Patients benefit from the synergistic and complementary effect of the different procedures altogether. RFA, cementing, and internal fixation (intramedullary nailing) for the palliative treatment of long bone metastases, should be incorporated in multidisciplinary discussion since the beginning in order to be used early in the treatment algorithm.
Conclusion
This study shows an effective local tumour control and impactful results on patients’ quality of life through significant pain relief and functional improvements. RFA and cementing combination seems to minimize local tumour progression or local recurrence due to their synergistic effect. The association of intramedullary nailing for long bone lesions complements treatment goals. Further, this combined treatment is a minimally invasive and cost-effective procedure, and showed a null complications rate. Patients with short or medium-term survival expectancy significantly benefit from this treatment.
References
- Mitchell MJ, Wayne E, Rana K, Schaffer CB, King MR (2013) TRAIL-coated leukocytes that kill cancer cells in the circulation. PNAS 1-6.
- Siegel R, Ward E, Brawlwy O, Jemal A (2011) Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212-236.
- Nielsen OS, Munro AJ, Tannock IF (1991) Bone metastases: Pathophysiology and management policy. J Clin Oncol 9:509-524.
- Cetin K, Christiansen CF, Jacobsen JB, Nørgaard M, Sørensen HT (2014) Bone metastasis, skeletal-related events, and mortality in lung cancer patients: A Danish population-based cohort study. Lung Cancer 86:247-254.
- Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, et al. (1995) Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev 14:279-301.
- Morris VL, Schmidt EE, MacDonald IC, Groom AC, Chambers AE (1997) Sequential steps in hematogenous metastasis of cancer cells studied by in vivo videomicroscopy. Invasion Metastasis 17:281-296.
- Faltas B (2012) Cornering metastases: therapeutic targeting of circulating tumor cells and stem cells. Fron in Oncol 2:1-7.
- Bravo-Cordero JJ, Hodgson L, Condeelis J (2012) Directed cell invasion and migration during metastasis. Curr Opi Cell Biol 24:277-283.
- Berrettoni BA, Carter JR (1986) Mechanisms of cancer metastasis to bone. J Bone Joint Surg Am 68:308-312.
- Chi JH, Gokaslan ZL (2008) Vertebroplasty and kyphoplasty for spinal metastases. Curr Opin Support Palliat Care 2:9-13.
- Bailar JC III, Gornik HL (1997) Cancer undefeated. N Engl J Med 336:1569-1574.
- Thiery JP (2002) Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer. 2:442-454.
- Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420-1428.
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, et al. (2011) Twist-induced invadopodia formation promotes tumor metastasis. Canc Cell 19:372-386.
- Yilmaz M, Cristofori G (2009) EMT, The cytoskeleton, and cancer cell invasion. Canc Metast Rev. 28:15-33.
- Roussos ET, Condeelis JS, Patsialou A (2011) Chemotaxis in cancer. A comprehensive review about how chemotaxis is essential to cancer progression. Nat Rev Cancer 11:573-587.
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF (2007) Fibroblast-led collective invasion of carcinoma cells qith differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9:1392-1400.
- Chen LS, Wang AX, Dong B, Pu KF, Yuan LH, et al. (2012) A new prospect in cancer therapy: targeting cancer stem cells to eradicate cancer. Chin J Cancer 31:564-572.
- Kakhki VR, Anvari K, Sadeghi R, Mahmoudian AS, Torabian-Kakhki M (2013) Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev Cent East Eur 16:66-69.
- Jawad MU, Scully SP (2010) In brief – Mirel´s classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res 468:2825-2827.
- Georgy BA (2008) Metastatic spinal lesions: State-of-the-art treatment options and future trends. Am J Neuroradiol 29:1605-1611
- Yang H, Pan J, Sun Z, Mei X, Yang Y (2012) Percutaneous augmented instrumentation of unstable thoracolumbar burst fractures: Our experience in preventing cement leakage. Eur Spine J 21:1410-1412.
- Mercadante S (1997) Malignant bone pain: pathophysiology and treatment. Pain 69:1-18.
- Lei M, Liu Y, Yang S, Jiang W, Cao Y (2016) Percutaneous cementoplasty for painful osteolytic distal femur metastases: A case report. J Pain Res 9:859-863.
- Gronemeyer DH, Schirp S, Gevargez A (2002) Image guided radiofrequency ablation of spinal tumors. Cancer J 8:33-39.
- Chow E, Ding K, Parulekar WR, Wong RK, van der Linden YM, et al. (2016) Revisiting classification of pain from bone metastases as mild, moderate, or severe based on correlation with function and quality of life. Support Care Cancer 24:1617-23.
- Gangi A, Guth S, Wong LL (2004) Percutaneous pain relief techniques in cancer patients Medic Radio 233-46.
- Callstrom MR, Charboneau JW, Goetz MP, Joseph R, Thomas DA, et al. (2006) Image-guided ablation of painful metastatic bone tumors: A new an effective approach to a difficult problem. Skeletal Radiol 35:1-15.
- Kneisl JS, Simon MA (1992) Medical management compared with operative treatment for osteoid osteoma. JBJA 74:179-183.
- Amouzegar-Hashemi F, Behrouzi H, Kazemian A, Zarpak B, Haddad P (2008) Single versus multiple fractions of palliative radiotherapy for bone metastases: A randomized clinical trial in Iranian patients. Curr Oncol 15:36-39.
- Willeumier JJ, van der Linden YM, van de Sande MAJ, Dijkstra PDS (2016) Treatment of pathological fractures of the long bones. EFFORT Open Reviews 1:136-145.
- Bollen L, de Ruiter GC, Pondaag W, Arts MP, Fiocco M, et al. (2013) A novel approach to predicting survival in patients with symptomatic spinal bone metastases. Eur Spine J 22:1408-16.
- Bollen L, Dijkstra PDS, Bartels RHMA, de Graeff A, Poelma DLH, et al. (2018) Clinical management of spinal metastases - The Dutch national guideline. Europ J Canc 104:81-90.
- Dupuy DE, Goldberg SN (2001) Image-guided radiofrequency tumour a`blation: Challenges and opportunities. Part II. J Vasc Interv Radiol 12:1135-1148.
- Santiago FR, GarcÃa MMC, Montes JLM, GarcÃa MR, Fernández JMT (2009) Treatment of bone tumours by radiofrequency thermal ablation. Curr Rev Musculoskelet Med 2:43-50.
- Bae JW, Gwak HS, Kim S, Joo J, Shin SH, et al. (2016) Percutaneous vertebroplasty for patients with metastatic compression fractures of the thoracolumbar spine: Clinical and radiological factors affecting functional outcomes. Spine J 16:355-364.
- Botton E, Edeline J, Rolland Y, Vauleon E, Le Roux C, et al. (2012) Cementoplasty for painful bone metastases: a series of 42 cases. Med Oncol 29:1378-1383.
- Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TM et al. (2004) Percutaneous image- guided radiofrequency ablation of painful metastases involving bone: A multicentre study. J Clin Oncol 22:300-306.
- Pinto CH, Taminiau AHM, Vanderschueren GM, Hogendoorn PCW, Bloem, JL, et al. (2002) Technical considerations in CT-guided radiofrequency catheter ablation of osteoid osteoma: tricks of the trade. AJR 179:1633-1642.
- Schaefer O, Lohrmann C, Herling M, Uhrmeister P, Langer M (2002) Combined radiofrequency thermal ablation and percutaneous cementoplasty treatment of a pathologic fracture. J Vasc Interv Radiol 13:1047-1050.
- Wong G, Georgy B.A (2007) Plasma-mediated radiofrequency ablation assisted percutaneous cement injection for treating advanced malignant vertebral compression factures. AJNR 28:700-705.
- Cantwell C, Obyrne J, Eustace S (2004) Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol 14-607- 617.
- Deschamps F, De Baere T (2012) Cementoplasty of bone metastases. Diagn Interv Imaging 93:685-689.
- Willeumier, JJ, van de Sande, MAJ, van der Wal, RJP, et al. (2018) Trends in the surgical treatment of pathological fractures of the long bones: Based on a questionnaire among members of the Dutch Orthopaedic Society and the European Musculo-Skeletal Oncology Society (EMSOS). Bone Joint J 100-B:1392-1398.
- Piccioli A, Maccauro G, Rossi B, Scaramuzzo L, Frenos F, et al. (2010) Surgical treatment of pathologic fractures of humerus. Injury 41-11:1112-1116.
- Forsberg JA, Eberhardt J, Boland PJ, Wedin R, healey JH (2011) Estimating survival in patients with operable skeletal metastases: An application of a Bayesian belief network. PLoS One 6-5:e19956.
- Steensma M, Healey JH (2013) Trends in the Surgical Treatment of Pathologic Proximal Femur Fractures Among Musculoskeletal Tumor Society Members. Clin Orthop Relat Res 471: 2000–2006.
- Trovarelli G, Capelliari A, Angelini A, Pala E, Ruggieri P (2019) What Is the Survival and Function of Modular Reverse Total Shoulder Prostheses in Patients Undergoing Tumor Resections in Whom an Innervated Deltoid Muscle Can Be Preserved? Clin Orthop Relat Res 477:2495-2507.
- Dijkstra S, Stapert J, Boxma H, Wiggers T (1996) Treatment of pathological fractures of the humeral shaft due to bone metastases: A comparison of intramedullary locking nail and plate osteosynthesis with adjunctive bone cement. Eur J Surg Oncol.22-6:621-626.
- Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN (2015) Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clin Orthop Relat Res 473-3:891-899.
- Issack PS, Barker J, Barker M, Kotwal SY, Lane JM (2014) Surgical management of metastatic disease of the proximal part of the femur. J Bone Joint Surg Am 96-24:2091-2098.
- Smolle MA, Andreou D, Tunn P-U, Leithner A (2019) Advances in tumour endoprostheses: a systematic review. EFORT Open Rev 4:445-459.
- Kistler BJ, Damron TA (2014) Latest developments in surgical and minimally invasive treatment of metastatic bone disease. Current Surgery Reports 2-4.
- Harvey N, Ahmann ER, Allison DC, Wang L, Menendez LR (2012) Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin Orthop Relat Res 470:684-691.
- Sundquist M, Brudin L, tejler G (2017) Improved survival in metastatic breast cancer 1985-2016. Breast 31:46-50
- Aragon-Ching JB (2017) Promises and pitfalls of primary local treatment in metastatic prostate cancer. J Clin Oncol. 35:914.
- Fares A, Shaaban MH, Reyad RM, Ragab AS, Sami MA (2018) Combined percutaneous radiofrequency ablation and cementoplasty for the treatment of extraspinal painful bone metastases: A prospective study. Journal of the Egyptian National Cancer Institute 30:117-122.
- Bagla S, Levy J, Hopkins T, Massari F, Vogel A, et al. (2019) Rapid pain improvement in patients treated for painful bone metastases with the Medtronic Osteocool RF Ablation system: the OpuS One study. J Vasc Intervent Radiol 30:S266-S267.
- Levy J, Hopkins T, Morris J, Tran N, David E, et al. (2020) Radiofrequency ablation for the palliative treatment of bone metastases: Outcomes from a multicentre study in 100 patients. J Vasc Intervent Radiol 31:S158.
- Willeumier JJ, kaynak M, van der Zwaal P, Meylaerts SAG, Mathijssen NMC, et al. (2018) What factors are associated with implant breakage and revision after intramedullary nailing for femoral metastases? Clin Orthop Relat Res 476:1823-1833.
- Tanaka T, Imanishi J, Charoenlap C, Choong PF (2016) Intramedullary nailing has sufficient durability for metastatic femoral fractures. World J Surg Oncol 14:80.
- Gainor BJ, Buchert P (1983) Fracture healing in metastatic bone disease. Clin Orthop Relat Res &NA:297-302.
- Maes M, Deboer Y, Brabants K (2012) Failure of the titanium trochanteric gamma nail in ununited metastatic fractures. Acta Orthop Belg 78:552-557.
- Wedin R, Bauer HC (2005) Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br 87:1653-1657.
- Shemesh S, Kosashvili Y, Sidon E, Yaari L, Cohen N, et al. (2014) Intramedullary nailing without curettage and cement augmentation for the treatment of impensing and complete pathological fractures of the proximal or midshaft femur. Acta Orthop Belg 80:144-150.
- Wedin R. (2001) Surgical treatment for pathologic fracture. Acta Orthop Scand Suppl. 72:1-29.
- Miller BJ, Soni EE, Gibbs CP, Scarborough MT (2011) Intramedullary nails for long bone metastases: why do they fail? Orthop. 34-34.
- Zacherl M, Gruber G, Glehr M, Ofner-Kopeinig P, Radl R, et al. (2011) Surgery for pathological proximal femur fractures, excluding femoral head and neck fractures: Resection vs. stabilization. Int Orthop 35:1537-1543.
- Willeumier JJ (2018) Treatment of actual and impending pathologic fractures of the humerus with intramedullary nails. In: Optimizing the treatment of patients with long bone metastases. GVO Drukkers & Vormgevers BV, Ede, The Netherlands.
- Mavrogenis AF, Pala E, Romagnoli C, Romantini M, Calabro T (2012) Survival analysis of patients with femoral metastases. J Surg Oncol 105:135-41.
- Angelini A, Trovarelli G, Berizzi A, Pala E, Breda A, et al. (2018) Treatment of pathologic fractures of the proximal femur. Injury 49:S77-S83.
- Laitinen M, Nieminem J, Pakarinen TK (2011) Treatment of patgological humerus shaft fractures with intramedullary nails with or without cement fixation. Arch Orthop Trauma Surg 131:503-508.
- Choi ES, Han I, Cho HS, Park IW, Park JW, et al. (2016) Intramedullary nailing for pathological fractures of the proximal humerus. Clin Orthop Surg 8:458-464.
- Kim YI, kang HG, Kim JH, Kim SK, Lin PP, et al. (2016) Closed intramedullary nailing with percutaneous cement augmentation for long bone metastases. Bone Joint J 98-B:703-709.
- Agarwal MG, Nayak P (2015) Management of skeletal metastases: An orthopaedic surgeon's guide. Indian J Orthop 49:83-100.
- Janssen SJ, Kortlever JT, ready JE, Raskin KA, Ferrone ML, et al. (2016) Complications after surgical management of proximal femoral metastasis: a retrospective study of 417 patients. J Am Acad Orthop Surg 24:483-494.
- Takaaki T, Jungo I, Charoenlap C, Choong PFM (2016) Intramedullary nailing has sufficient durability for metastatic femoral fractures. World J Surg Oncol 14:1-6.
- Van Doorn R, Stapert JW (2000) Treatment of impending and actual pathological femoral fractures with the long Gamma nail in the Netherlands. Eur J Surg 166:247-54.
- Steensma M, Boland PJ, Morris CD, Athanasian E, Healey JH (2012) Endoprosthetic treatment is more durable for pathologic proximal femur fractures. Clin Orthop Relat Res 470:920-926.
- Willeumier, JJ, Gerco van der Wal, CWP, Schoones JW, van der Wal, et al. (2019) Stop, Think, Stage, Then Act: A Multidisciplinary Guide. In: Management of Bone Metastases pp 213-224.
- Willeumier JJ, Gerco van der Wal CWP, Schoones JW, van der Wal RJ, Dijkstra PDF (2018) Pathologic fractures of the distal femur: Current concepts and treatment options. J Surg Oncol 118:883-890.
Citation: Oliveira V, Leite XF, Ribau A, Cardoso P, Araujo A (2021) Rapid Pain Relief and Functional Improvement after Local Radiofrequency Ablation and Cementing Combined with Internal Fixation for Long Bone Metastases: A Prospective Study. J Orthop Oncol 7: 145. DOI: 10.4172/2472-016X.1000145
Copyright: © 2021 Oliveira V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3455
- [From(publication date): 0-2021 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 2559
- PDF downloads: 896