Real World Efficacy and Tolerability of Acotiamide, in Relieving Mealrelated Symptoms of Functional Dyspepsia
Received: 09-Feb-2018 / Accepted Date: 21-Feb-2018 / Published Date: 27-Feb-2018 DOI: 10.4172/2161-069X.1000553
Abstract
Background: Functional Dyspepsia (FD) is a highly prevalent clinical condition that imposes negative economic burden on health-care system as well as greatly impairs quality of life. Treatment of non-specific and bothersome meal-related FD symptoms like post-prandial fullness, upper abdominal bloating and early satiety, is a therapeutic challenge for the clinicians as poorly-defined and ill-understood pathogenesis has hampered efforts to develop effective treatments. Acotiamide is first-in-class drug that exerts its gastro-kinetic effect by enhancing acetylcholine release. Though evidence of its efficacy and tolerance are available through randomized clinical trials, real world data from its regular in-clinic use is lacking.
Methodology: In this study, 314 FD patients with meal-related-symptoms, visiting 63 gastroenterology clinics across India, received Acotiamide 100 mg thrice daily for 4 weeks. These patients were retrospectively evaluated with a questionnaire to record patient’s perception on improvement in the presenting symptoms, as well as tolerance to treatment.
Results: It was observed that, complete relief or significant improvement from post prandial fullness, upper abdominal bloating and early satiety was achieved by 79.2%, 74.4%, and 77.1% patients respectively. (P<0.001 for all vs. no/slight improvement). Significantly more number of patients achieved complete relief when treated for >28 days or 14-28 days than when treated for less than 2 weeks (P<0.05). Adverse events were reported by 6% patients; mainly headache, nausea, vomiting, vertigo, burning sensation, palpitation, and epigastric pain, and were all mild and transient in nature.
Conclusion: This real world study suggests that use of Acotiamide was associated with improvement of mealrelated FD symptoms with good safety profile.
Keywords: Rome III criteria; Functional dyspepsia; Acotiamide; Prokinetic; Post prandial fullness; Abdominal bloating; Early satiety; Impaired gut motility
Introduction
Rome III consensus defines functional dyspepsia as presence of symptoms thought to originate in the gastroduodenal region (postprandial fullness, early satiation, epigastric pain or burning), in the absence of any organic, systemic or metabolic disease that is likely to explain the symptoms. These symptoms are mostly chronic, occurring at least weekly and over a period of at least 6 months. According to this definition, approximately 5-11% of global population suffers from FD [1]. FD being a heterogeneous disorder significantly impairs health related quality of life, work productivity and incur direct and indirect economic burden on patients and health care system [2,3]. Overall it is difficult to estimate the magnitude of problem of FD in India as most of the prevalence studies report the estimates of uninvestigated dyspepsia (UD). Several studies have reported the frequency of UD and FD between 8%-30% and 8%-23%, respectively in Asia [4]. There are very few population-based prevalence studies in India and in one such population study from Mumbai, almost one third of the population suffered from dyspepsia (30.4%) and 12% of study patients reported significant dyspeptic symptoms [5].
Majority of FD patients complain of meal-related symptoms. However, the pathophysiology of FD remains poorly elucidated. Many of these patients have no exclusive cause of dyspepsia by standard diagnostic tests. Being a heterogeneous disorder, FD involves multiple pathogenic factors, such as excessive gastric acid secretion, gastric motility disorders, Helicobacter pylori infection, psychological factors and visceral hypersensitivity. These factors interactively contribute to the manifestation of FD symptoms. Currently available pharmacologic treatments for the management of FD have only been shown to be of limited efficacy. Therefore, it is logical to direct the therapeutic approaches towards underlying pathophysiologic factors to enhance treatment efficacy [6,7].
The mainstay of FD treatment has been targeted to (a) reduce gastric-acid secretion and (b) enhance the reduced gut motility. The role of acid inhibitory drugs, such as proton pump inhibitors in the treatment of FD is well established. Consequently, prokinetics were developed based on the concept that improving impaired gut motility could diminish the symptoms of FD. Apart from acid inhibitory therapy, gastroprokinetic drugs are the mainstay of the treatment of FD. Acotiamide is a first-in-class gastroprokinetic agent extensively studied in clinical trials. It improves upper gastrointestinal motility to relieve abdominal symptoms arising due to impaired GI motility in FD patients. Acotiamide received its first global regulatory approval in Japan in 2013 and was approved in India in 2016 for the treatment of bloating after meals, epigastric bloating and early satiety in FD patients. In USA and Europe, Acotiamide is undergoing phase III trials [8,9]. Acotiamide has been listed in Rome IV as a treatment option for FD [10]. Gastroprokinetic action of Acotiamide results from enhanced action of acetylcholine (ACh) by (a) increasing release by antagonizing the M1 and M2 muscarinic receptors in the enteric nervous system and (b) prolonging the action of the ACh by inhibiting acetylcholinesterase activity. These actions increase the availability of ACh at postsynaptic receptors in neuromuscular junctions in enteric nervous system.
Apart from these prokinetic actions, few studies have also reported that Acotiamide may also modulate gut-brain interactions via its effects on the afferent vagus nerve, modifying sensory input from the GI tract to the CNS. Unlike metoclopramide or domperidone, the gastroprokinetic activity of Acotiamide does not appear to be associated with prolongation of the QTc interval, based on preclinical research. After oral administration, maximum plasma levels of Acotiamide are achieved in 1-1.5 hours, with a plasma half-life of 7-10 hours. Approximately, 45% of Acotiamide is excreted in the feces with no marked CYP inhibition [11].
In number of clinical trials across various countries, Acotiamide has been reported to improve meal-related symptoms of FD and quality of life in patients with FD. Although clinical trials are always considered to be an acceptable standard for establishing efficacy, in general, clinical trials utilize a standardized therapy in a select patients group. As clinical trials are performed in controlled conditions such as strict inclusion and exclusion criteria, drug provided free of cost, compliance monitored, etc., they fail to assess multifaceted interactions within a study arm and fail to ascertain continuous relationship between treatment and study results. Due to these limitations, there is a need for real-world (RW) study in which data regarding actual care received by patients in clinics is recorded. Instead of having strict inclusion and exclusion criteria as in randomized clinical trials, all the patients including those with co-morbidities have to be treated. Such RW studies generate long term efficacy and safety data along with economic assessment under pragmatic conditions [12,13].
This RW study was performed to evaluate the knowledge gap between Acotiamide clinical trials and actual clinical usage, with an objective to understand how gastroprokinetic treatment with Acotiamide will work when applied in clinical practice environment. The expectation is that systematically analyzed RW data can deliver key insights which, after due validation, could help to deploy Acotiamide in a manner, which in turn, could result in a safer and more appropriate choice of FD-patients for treatment with Acotiamide, making it a better therapeutic experience for the physician and patient alike.
Methodology
Total 314 adult patients, who presented clinically with any of the symptoms of post prandial fullness, upper abdominal bloating, and/or early satiety in the Gastroenterologist’s out-patient department and were prescribed 100 mg Acotiamide thrice daily, were evaluated with a questionnaire to record patient’s perception of improvement in the presenting symptoms, as well as tolerance to treatment.
The data was captured from 63 Gastroenterologists across India (20 each in north and east zone, 14 in west zone and 9 in south zone). ROME III diagnostic criteria were used to classify patients as functional dyspepsia with predominantly post prandial distress syndrome (PDS-type). To facilitate objective and unambiguous assessment by the patients, a 4 point rating scale, comprising of (a) ‘No improvement’, (b) ‘Slightly improved’, (c) ‘Significantly improved’, and (d) ‘Complete relief ’, was used. For each patient, the duration of treatment was also recorded. Adverse events, if any, were recorded, assessed and managed. All patient-data was captured in accordance with ethical principles and with patient consent.
Results
Total 314 patients were prescribed Acotiamide for their presenting symptoms. Male: Female ratio was 3:2, and 60% patients were ≥ 40 years while 13% were ≥ 60 years. Smoking and alcohol intake was seen in 14% and 11% patients respectively and both these factors were present in 6% patients.
Results outcomes were 74%, 76% and 53% patients presented with symptoms of post prandial fullness, upper abdominal bloating and early satiety respectively. Other symptoms included epigastric pain (38%), epigastric burning (35%), constipation (26%) and diarrhea (3%) (Table 1).
| Gender distribution | 60% male; 40% female |
|---|---|
| Age distribution | 60% patients ≥ 40 years; 13% patients ≥ 60 years |
| Symptom distribution | Postprandial fullness-74% |
| Upper abdominal bloating-76% | |
| Early satiety-53% | |
| Epigastric pain-38% | |
| Epigastric burning-35% | |
| Constipation-26% | |
| Diarrhea-3% |
Table 1: Baseline demographic data.
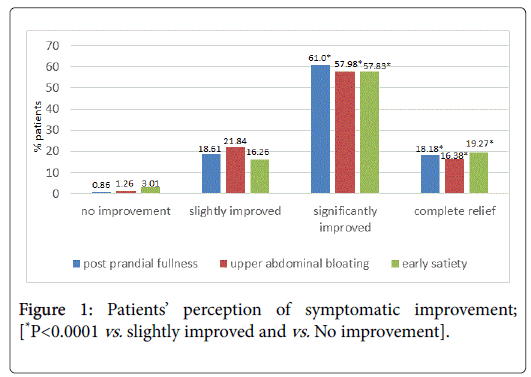
Complete relief or significant improvement from post prandial fullness, upper abdominal bloating and early satiety was achieved by 79.2%, 74.4%, and 77.1% patients respectively; (P<0.001 for all mentioned values vs. no/slight improvement Figure 1). No significant difference in complete relief or significant improvement rates was seen between men and women for the symptoms of post prandial fullness and upper abdominal bloating. However for early satiety, more women achieved a significant improvement as compared to men (63.26% vs. 47.05%; P=0.03)
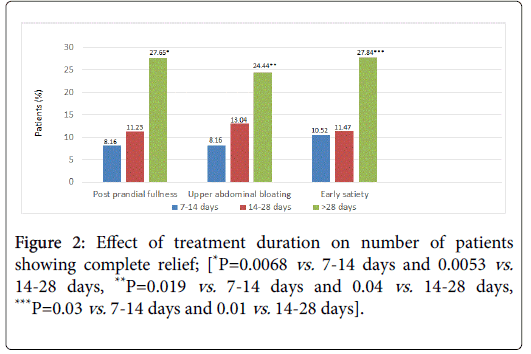
No significant difference was seen between age groups <40 and ≥ 40 years or <60 and ≥ 60 years in complete relief or significant improvement rates. Similarly, the pre-therapy duration of symptoms (≤ 6 months or >6 months) did not significantly affect the scorings of complete relief or significant symptomatic improvement. Duration of treatment did not have an effect on symptomatic relief obtained (Figure 2). Significantly more patients achieved complete relief when treated for >28 days or 14-28 days than when treated for <2 weeks; (P<0.05 for all 3 symptoms; 28 days vs. 14-18 and 7-14 days).
Adverse events were reported by 6% patients. The adverse events that were reported were headache, nausea, vomiting, vertigo, burning sensation, palpitation, and epigastric pain. All events were mild and transient in nature. Treatment discontinuation occurred in 2 patients (1.36%); (1 patient each who had palpitation, nausea, epigastric pain and 1 due to lack of efficacy).
Discussion
Despite evidence of efficacy and safety of use in clinical trials, few real world studies in clinical settings on Acotiamide have been reported. In this study, we report that Acotiamide significantly improved the symptoms of post-prandial fullness, upper abdominal bloating and early satiety in Indian patients with FD. Findings of this study will definitely assist several clinicians seeing several dyspeptic patients in general and specialized practice. To our knowledge this is the first Indian study conducted in real world settings suggesting the positive outcomes of Acotiamide in FD patients. Impaired gastricemptying and accommodation are two of the known pathophysiological mechanisms related with FD symptoms of postprandial fullness, upper abdominal bloating and early satiety. Acotiamide improves gastric emptying rate and, thereby, relieves these FD symptoms. Findings of the present study are in line with previous reports of efficacy from randomized controlled trials on Acotiamide.
Matsueda et al. performed a 4 week, phase III, randomized, placebo controlled trial with 100 mg Acotiamide in 892 FD patients in Japan to study elimination rate of all three meal-related symptoms (postprandial fullness, upper abdominal bloating and early satiation) [14]. During global assessment of treatment efficacy, researchers classified 52.2% patients receiving Acotiamide and 34.8% patients receiving placebo as respondents (P<0.001). Interestingly, at the end of 4 weeks, significantly more number of patients from Acotiamide group (P=0.004) showed improvement in all three meal related FD symptoms [14].
In a long-term 48-week study carried out by Matsueda et al. to investigate the efficacy, safety and administration pattern in 405 FD patients, researchers observed favorable outcomes with global overall treatment efficacy (OTE) [15]. The OTE improvement rate was 26.1% at week 1 and increased with time reaching 60.6% (week 8), 66.7% (week 48) and 73.2% (during the last period of treatment). Many patients who met the cessation criterion achieved remission of FD symptoms after experiencing dose interruption and re-administration. This study concluded that FD symptoms were controlled even by intermittent administration of Acotiamide in patients with relapsing FD [15].
Another study employed gastric ultrasound to measure the crosssectional area of the proximal stomach after a liquid meal to assess gastric accommodation in FD patients before and after treatment with Acotiamide 100 mg t.i.d. The study also evaluated gastric emptying rate, motility index and duodeno-gastric reflux index for assessment of gastroduodenal motility. A significant difference was found in the change of gastric accommodation between the Acotiamide group and the placebo group (21.7 vs. 4.4%) [16]. Furthermore, Acotiamide was found to significantly accelerate the gastric emptying rate, which was not seen in the placebo group. The subjective improvement rates also tended to be better in the Acotiamide group (31.6 vs. 16.7%) [17]. None of these studies reported serious adverse effect and change in ECG during or after Acotiamide treatment.
Recently, Behera et al. compared the efficacy and safety of Acotiamide with Levosulpiride in Indian FD patients [18]. 60 patients were divided in two groups to receive either Acotiamide 100 mg t.i.d. or Levosulpiride 25 mg t.i.d. for 8 weeks. Approximately 93% patients reported excellent to good improvement of FD symptoms after 4 week administration of Acotiamide compared to 80% improvement with Levosulpiride. The study concluded that, Acotiamide was superior to Levosulpiride in, both, efficacy and tolerability in FD patients [18].
In a randomized, double-blind placebo-controlled study with 46 Japanese FD patients by Nakamura et al. Acotiamide significantly increased gastric accommodation compared to placebo (P=0.04 vs. P=0.08), significantly accelerated gastric emptying (50% half-emptying time P=0.02 vs. P=0.59) and significantly improved the total GSRS scores and HADS anxiety score compared to placebo (P=0.0007 vs. P=0.14 and P=0.04 vs. P=0.20 respectively) [19].
In a retrospective study by Shinozaki et al. in 79 patients with functional dyspepsia whose symptoms improved with Acotiamide therapy and who were followed up, at one year, dyspepsia symptoms recurred in 25% of the patients [20]. Patients with severe dyspepsia before starting Acotiamide had significantly more recurrences than those with mild symptoms (P=0.004). Patients who continued Acotiamide therapy throughout the follow-up period had significantly fewer recurrences than those who stopped therapy (P<0.001).
In the European phase III open-label study [21] to evaluate the long-term safety and efficacy of Acotiamide on PDS symptoms, adult FD-PDS patients (defined by ROME III criteria) with active PDS symptoms and without predominant overlapping symptoms of epigastric pain syndrome and related disorders were enrolled to receive 100 mg Acotiamide three times daily for 1 year. 81.6% patients maintained exposure to Acotiamide for >50 weeks, with a mean duration of 320.3 days. No specific clinically significant safety concerns have been shown, with no deaths or treatment-related severe/serious adverse events, or any clinically significant laboratory test results. Acotiamide showed a change in severity larger than the minimum clinically important difference at weeks 1 and 2 for the meal related symptoms-postprandial fullness and early satiation with improvement of quality of life and work productivity from week 12 up to 1 year.
Conclusion
The efficacy assessed by patients’ assessment of their symptomatic improvement along with the safety and tolerance of Acotiamide observed in the present real world study, corroborates with the published clinical trials in literature. Since clinical trials do not address all relevant clinical requirements and real-world clinical situations. More real world studies are required which have a larger population size, longer follow up periods, capturing many more clinically relevant parameters to further supplement the earlier clinical studies.
References
- Talley NJ, Ford AC (2015) Functional dyspepsia. N Engl J Med 373: 1853-1863.
- Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ (2013) Functional dyspepsia: The economic impact to patients. Aliment Pharmacol Ther 38: 170-177.
- Aro P, Talley NJ, Agreus L, Johansson SE, Bolling-Sternevald E, et al. (2011) Functional dyspepsia impairs quality of life in the adult Population. Aliment Pharmacol Ther 33: 1215-1224.
- Ghoshal UC (2011) Epidemiology of uninvestigated and functional dyspepsia in Asia: Facts and fiction. J Neurogastroenterol Motil 17: 235-244.
- Shah SS, Bhatia SJ, Mistry FP (2001) Epidemiology of dyspepsia in the general population in Mumbai. Indian J Gastroenterol 20: 103-106.
- Futagami S (2011) Pathophysiology of functional dyspepsia. J Nippon Med Sch 78: 280-285.
- Tack J (2004) Pathophysiology and treatment of functional dyspepsia. Gastroenterol 127: 1239-1255.
- Yang YJ, Bang CS, Baik GH, Park TY, Shin SP, et al. (2017) Prokinetics for the treatment of functional dyspepsia: Bayesian network meta-analysis. BMC Gastroenterol 17: 83.
- Ueda M, Iwasaki E, Suzuki H (2016) Profile of Acotiamide in the treatment of functional dyspepsia. Clin Exp Gastroenterol 9: 83-88.
- Suzuki H (2017) The application of the Rome IV criteria to functional esophagogastroduodenal disorders in Asia. J Neurogastroenterol Motil 23: 325-333.
- Tack J, Janssen P (2011) Acotiamide (Z-338, YM443), a new drug for the treatment of functional dyspepsia. Expert Opin Investig Drugs 20: 701-712.
- Mahajan R (2015) Real world data: Additional source for making clinical decisions. Int J Appl Basic Med Res 5: 82.
- Suvarna V (2010) Phase IV of drug development. Perspect Clin Res 1: 57-60.
- Kei M, Michio H, Jan T, Youichi S, Hiroki K (2012) A placebo-controlled trial of Acotiamide for meal-related symptoms of functional dyspepsia. Gut 61: 821-828.
- Kei M, Michio H, Ushijima S, Akiho H (2011) A long-term study of acotiamide in patients with functional dyspepsia: Results from an open-label phase iii trial in Japan on efficacy, safety and pattern of administration. Digestion 84: 261-268.
- Kusunoki H, Haruma K, Manabe N (2012) Therapeutic efficacy of Acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil 24: 540-545.
- Tack J, Masclee A, Heading R (2009) A dose-ranging, placebo-controlled, pilot trial of Acotiamide in patients with functional dyspepsia. Neurogastroenterol Motil 21: 272-280.
- Behera R, Sethi S (2017) Efficacy and safety assessment of Acotiamide and Levosulpiride in functional dyspepsia. J Dent Med Sci 16: 53-57.
- Nakamura K (2017) A double-blind placebo controlled study of Acotiamide hydrochloride for efficacy on gastrointestinal motility of patients with functional dyspepsia. J Gastroenterol 52: 602-610.
- Shinozaki S (2017) Adherence to an Acotiamide therapeutic regimen improves long-term outcomes in patients with functional dyspepsia. J Gastrointestin Liver Dis 26: 345-350.
- Tack J (2018) Long-term safety and efficacy of Acotiamide in functional dyspepsia (postprandial distress syndrome)-results from the European phase 3 open-label safety trial. Neurogastroenterol Motil.
Citation: Narayanan V, Bhargava A, Pallewar S (2018) Real World Efficacy and Tolerability of Acotiamide, in Relieving Meal-related Symptoms of Functional Dyspepsia. J Gastrointest Dig Syst 8: 553. DOI: 10.4172/2161-069X.1000553
Copyright: © 2018 Narayanan V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8255
- [From(publication date): 0-2018 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 7214
- PDF downloads: 1041