Research Article Open Access
Asymmetric Mono- and Bis-Squarylium Dyes as Pre-Column and On-Column Labels for Protein Analysis by Capillary Electrophoresis with Laser-Induced Fluorescence Detection
Xiuli Lin1, Stephanie Rockett1, Tara L. Massie1,2, Geoffrey B. Turner1,3, Takeshi Maeda4, Hiroyuki Nakazumi4, and Christa L. Colyer1*1Department of Chemistry, Wake Forest University, Winston-Salem, NC 27109, United States
2NC BioNetwork Pharmaceutical Center, 391 Technology Way, Suite 162, Winston-Salem, NC 27101, United States
3National Renewable Energy Lab, Colorado, USA
4Graduate School of Engineering, Osaka Prefecture University, 1-1 Gakuen-cho, Naka-ku, Sakai, Osaka
- *Corresponding Author:
- C. L. Colyer
Department of Chemistry
Wake Forest University
1834 Wake Forest Road
Winston-Salem, NC 27109, United States
Fax: 336-758-4656;
E-mail: colyercl@wfu.edu
Received date: April 07, 2012; Accepted May 26, 2012; Published date: June 06, 2012
Citation: Lin X, Rockett S, Massie TL, Turner GB, Maeda T, et al. (2012) Asymmetric Mono- and Bis-Squarylium Dyes as Pre-Column and On-Column Labels for Protein Analysis by Capillary Electrophoresis with Laser-Induced Fluorescence Detection. J Anal Bioanal Tech S9:001. doi: 10.4172/2155-9872.S9-001
Copyright: © 2012 Lin X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Labeling proteins with fluorescent dyes offers a powerful tool for monitoring protein interactions in vitro and in vivo. In order for this tool to be effective, the nature of the dyes - absorbance and emission properties, solution stability, pH range, and mechanisms for protein interaction - must first be considered. Two new asymmetric, squarylium dyes, bis-SQHN-4d and SQHN-3c, were shown to be only weakly fluorescent in aqueous buffers in the absence of proteins. However, their spectra showed a dramatic increase in fluorescence intensity upon the addition of Human Serum Albumin (HSA) or Bovine Serum Albumin (BSA) as model proteins. The enhanced fluorescence properties, attributed to noncovalent binding, allowed the use of the new squarylium dyes as probes for the low-level detection of proteins in a mixture (including myoglobin (pI=7.16), transferrin (pI=5.9), and HSA (pI=4.8)), separated by Capillary Electrophoresis with Laser-Induced Fluorescence detection (CE-LIF). Because of the low background fluorescence of these probes, on-column labeling was feasible and led to simple and rapid protein detection. This labeling protocol offered greater sensitivity than the more conventional pre-column labeling protocol (with a 10-fold lower limit of detection for HSA with bis-SQHN-4d). A limit of detection for HSA (by CE-LIF with on-column labeling with bis-SQHN-4d) of 3.42 x 10-8 M indicates that this dye is well suited to the development of other protein assays.
Introduction
The implication of proteins in various biological functions and as markers of various states of disease provides the impetus for developing improved protein assays. Various techniques, including two-dimensional Polyacrylamide Gel Electrophoresis (PAGE) [1], High-Performance Liquid Chromatography (HPLC) [2,3], and mass spectrometry [4], have been successfully employed for protein analysis. Alternatively, fluorescence-based methods have been shown to be some of the most sensitive methods for protein analysis [5]. By combining the sensitivity and selectivity of Laser-Induced Fluorescence (LIF) detection with the high efficiency and speed of Capillary Electrophoresis (CE) separations of proteins, many have shown the utility of this system for protein analysis [6-13]. However, since just a small number of proteins possess natural fluorescence in a suitable spectral range (beyond UV), CE-LIF requires protein analytes to be labeled with a fluorescent tag or dye. Covalent labeling (e.g. via the ε-amino group of lysine, the α-amino group of the N-terminus, or the thiol group of a cysteine residue) often necessitates increased sample preparation, and may be difficult to achieve with very dilute samples. Instead, non covalent dye–protein interactions can be exploited to facilitate protein determination by CE–LIF. Non covalent labeling can occur by a variety of physical mechanisms, including hydrophobic interactions, electrostatic interactions and hydrogen bonding. The exact nature of these interactions is often difficult to determine, but evidence of interaction is clearly provided by a change in the emission (wavelength and/or intensity) of the fluorophore–protein complex relative to that of the free, uncomplexed fluorophore, which can be easily monitored by fluorimetric studies [8].
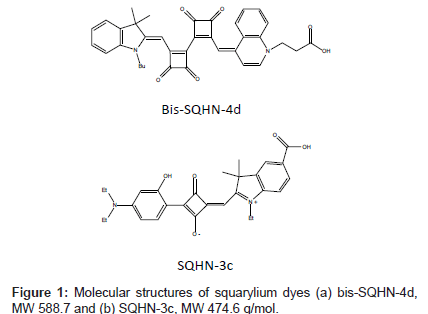
Squarylium dye molecules are polymethine dyes with a cyclobutene core in the middle of the pi-conjugation systems. These dyes and their derivatives are gaining significant interest in many fields such as solarenergy conversion [9], biochemical labeling [10], chromo/fluorogenic probes [11], and neutral molecule recognition [12]. The properties of squarylium dye molecules have been extensively studied by Liang et al. [13]. Typically, they exhibit a characteristically intense absorption band in the range of 640–850 nm [14]. Nakazumi et al. [15], showed that squarylium dyes experience enhanced fluorescence emission upon binding to BSA and HSA. By modifying the structure of the molecule, it is possible to design different kinds of squarylium dyes that will function in specific media or exhibit enhanced selectivity and sensitivity towards specific analytes. Two squarylium dyes designed with these goals in mind are shown in Figure 1.They exhibit different selectivity and sensitivity for protein labeling because of their different molecular structures.
The aim of the current work is to determine the effects of solution conditions on the nature of protein-dye interactions for bis-SQHN- 4d and SQHN-3c dyes; the emission properties of the two novel squarylium dyes in their bound and Free states; and the efficiency of the separation of dye-labeled proteins. To this end, the absorbance and emission properties of the new dyes were determined in a variety of solvents and aqueous buffers at different pH’s prior to titration of fixed quantities of the dyes with increasing concentrations of model proteins Human Serum Albumin (HSA) or Bovine Serum Albumin (BSA).An investigation of aggregation effects along with protein-dye incubation time and solution age was conducted. Finally, the utility of the new dyes bis-SQHN-4d and SQHN-3c as noncovalent protein probes was explored by designing and quantifying pre-column and on-column labeling protocols for CE-LIF.
Materials and Methods
Reagents, buffers, and sample solutions
3-((Z)-4-((2’-((E)-(1-Butyl-3,3-dimethylindolin-2-ylidene) methyl)-3,3’,4,4’-tetraoxo-[1,1’-bi(cyclobutane)]-1,1’-dien-2-yl) methylene)quinolin-1(4H)-yl)propanoic acid (bis-SQHN-4d) and (Z)-4-((5-carboxy-1-ethyl-3,3-dimethyl-3H-indol-1-ium-2-yl) methylene)-2-(4-(diethylamino)-2-hydroxyphenyl)-3-oxocyclobut- 1-enolate (SQHN-3c) dyes (Figure. 1) were synthesized by modifying previously published procedures by Nakazumi and co-workers [16,17]. The solid dyes are stable in the dark at room temperature for several months or longer. A 1.70 x 10-4 M stock solution of bis-SQHN-4d was prepared in dimethylformamide, DMF (Fisher, Fair Lawn, NJ, USA) and subsequently diluted in HPLC- grade methanol (G.J. Chemical Company, Newark, NJ, USA) to the desired concentration (1.00 or 5.00 μM) just prior to use. A 1.30 x 10-4 M stock solution of SQHN-3c was prepared in dimethyl sulfoxide, DMSO (Fisher) and subsequently diluted to a working solution of 5.00 x 10-6 M in DMSO just prior to use. Dye stock solutions were stored in the dark at 4°C when not in use. Proteins were used as-received from Sigma-Aldrich (St. Louis, MO, USA) and prepared as stock solutions in water to the specified concentrations: human serum albumin ( HSA, 96%, pI 4.8,1.00 x 10-4 M); bovine serum albumin(BSA, 98%, pI 4.9, 1.00 x 10-4 M); myoglobin (from horse heart, 90%, pI 7.16, 2.00 x 10-4 M); trypsinogen (from bovine pancreas, 98%, pI 9.3, 1.00 x 10-4 M); transferrin (human, 98%, pI 5.9, 3.83 x 10-5 M). Protein solutions were stored at 4°C in the dark when not in use. Pre-column mixtures of protein with dye were prepared by combining the proper volumes of dye and protein stock solutions, diluted with water or buffer prior to analysis.
Buffers for sample dilution and/or CE separation were prepared from citric acid (Mallinckrodt, St. Louis, MO, USA), tris(hydroxymethyl) aminomethane (Tris, Mallinckrodt); boric acid (J.T. Baker, Phillipsburg, NJ, USA); or disodium phosphate (Fisher). Buffers were prepared by dissolving the correct amount of reagent in MilliQ distilled, deionized water (Millipore, Bedford, MA, USA) and adjusting the pH by the addition of either 1 M NaOH or 1 M HCl (Fisher, Pittsburgh, PA, USA). Before use, buffers were filtered through 0.2 μm nylon syringe filters (Corning, NY, USA).
Instrumentation
An Agilent HP3DCE system (Waldbronn, Germany) was used for the electrophoretic separations of protein mixtures labeled oncolumn and pre-column with bis-SQHN-4d. The instrument was coupled with an external Picometrics Zeta LIF detector (model: LIF SA-03, Ramonville, France) equipped with a Melles Griot 230 kV, 406 nm diode laser. Uncoated, fused-silica capillaries were purchased from Polymicro Technologies (Phoenix, AZ, USA), and were 75 μm x 75 cm x 62.5 cm or 50 μm x 75 cm x 62.5 cm (ID x total length x effective length). Variations in capillary dimensions were governed by optimization considerations and system compatibilities. The new capillary was conditioned for 10 min using purified water, 20 min using 1 M sodium hydroxide, then 10 min using purified water, and finally 20 min using the running buffer. Between each run, the capillary was rinsed with the running buffer for 3 min. Injections were by pressure at 50 mbar for 5 s. The applied separation voltage was 20 kV, and the temperature of the capillary and sample was held constant at 20°C.
A BioRad Biofocus 3000 CE system (Hercules, CA, USA) with an LIF detector equipped with a 650 nm external diode laser (OZ Optics, Carp, Canada) and a 664 nm DF20 emission filter (Omega Optical, Brattleboro, VT, USA) was used for the electrophoretic separations of protein mixtures labeled on-column and pre-column with SQHN-3c. Unless otherwise noted, uncoated, fused silica capillaries (25 μm ID x 30.0 cm total length x 25.4 cm effective length) were used and were stored overnight, when not in use, filled with water; and a separation voltage of 20 kV with 4 psi*sec injection was also employed. Between each run, the capillary was rinsed with purified water for two minutes, then running buffer for 2 min.
A Perkin-Elmer LS50B Luminescence Spectrometer (Shelton, CT, USA) was used for fluorimetric studies. For bis-SQHN-4d, excitation and emission slit widths were 10 nm; scan speed was 300 nm/min; and scan range was from 425 to 725 nm. For SQHN-3c, excitation and emission slit widths were 5 nm; scan speed was 100 nm/min; and scan range was from 640 to 750 nm. Fluorescence spectra of dye and protein–dye solutions were measured using a 1-cm quartz cuvette (VWR, Suwanee, GA, USA).
Absorbance studies for both dyes were conducted over a scan range of 190-900 nm using a Hewlett-Packard HP8453 UV–Vis spectrometer (Waldbronn, Germany) and a 1-cm quartz cuvette.
Results and Discussion
Spectral properties of bis-SQHN-4d and SQHN-3c alone and with added protein
Spectral properties of the novel squarylium dyes (7.00 x 10-6 M bis- SQHN-4d or 7.50 x 10-6 M SQHN-3c) were determined under various solution conditions in order to assess their suitability as fluorescent probes for proteins. Absorbance maxima for bis-SQHN-4d were observed at 220, 414, 607, and 657 nm in methanol, and red shifts of all but the shortest wavelength absorbance band were observed for dye samples prepared in aqueous buffers. However, the absorbance maximum for SQHN-3c was slightly blue shifted relative to methanol (640 nm) in all aqueous buffers except under acidic conditions (citric acid buffer), for which a slight red shift was observed. A summary of the wavelengths of maximum absorbance and emission of the dyes, both with and without added protein, is presented in Table 1. Significant differences between absorbance and emission wavelengths for both dyes in the presence of protein (for example, 52 nm in the case of bis- SQHN-4d with BSA in Tris) indicate good analytical discrimination between excitation and emission signals, thus allowing for more sensitive detection. The wavelengths of maximum absorbance and emission for SQHN-3c exceeded those for bis-SQHN-4dThese longer wavelengths can be beneficial because they allow the optical detection to be shifted away from the region of native protein absorbance bands and possible autofluorescence of biological matrices, therefore reducing interference and improving detection limits.
| Solvent or Buffer | 7.00 x 10-6 M bis-SQHN-4d | |||
| no added protein | with 1.00 x 10-6 M BSA | |||
| λabs,max (nm) | λemis,max (nm) | λabs, max (nm) | λemis, max (nm) | |
| methanol | 414 | 550 | --- | --- |
| citric acid (25.0 mM, pH 3.1) | 421 | 470 | 421 | 470 |
| Tris-HCl (25.0 mM, pH 7.4) | 418 | 470 | 418 | 470 |
| boric acid (25.0 mM, pH 9.1) | 424 | 470 | 424 | 470 |
| Na2HPO4 (25.0 mM, pH 11.3) | 420 | 475 | 423 | 620 |
| 7.00 x 10-6 M (abs) or 7.00 x 10-7 M (emis) SQHN-3c | ||||
| no added protein | with 7.00 x 10-6 M BSA | |||
| λabs,max (nm) | λemis,max (nm) | λabs, max (nm) | λemis, max (nm) | |
| methanol | 640 | 650 | --- | --- |
| citric acid (25.0 mM, pH 3.1) | 643 | 656 | 650 | 659 |
| Tris-HCl (25.0 mM, pH 7.4) | 634 | 655 | 643 | 655 |
| boric acid (25.0 mM, pH 9.1) | 636 | 654 | 647 | 652 |
| Na2HPO4 (25.0 mM, pH 11.3) | 634 | 651 | 637 | 651 |
Table 1: Absorbance and fluorescence properties for bis-SQHN-4d and SQHN-3c dyes in various solvent systems.
As noted above, there are four absorbance bands in methanol and in aqueous buffered solutions for the bis-SQHN-4d dye. The band around 420 nm was the strongest and is reported in Table 1. In Na2HPO4 buffer (pH 11.3, 25.0 mM), the fluorescence emission of bis- SQHN-4d alone was very weak with a maximum at 475 nm (and the dye color in Na2HPO4 buffer at pH 11.3 is light purple to the eye, but it is blue in other buffers). When an equimolar quantity of BSA was added, the fluorescence emission was very strong with a maximum at 620 nm. It should be noted that there was a substantial increase (98%) in the fluorescence emission for bis-SQHN-4d with BSA in Na2HPO4 buffer (pH 11.3, 25.0 mM) relative to the emission of the dye alone with no added protein in this same buffer system. No other increases of this magnitude were observed for other buffer systems, which indicated that the interaction of bis-SQHN-4d with BSA is significantly affected by the pH of the solution. At pH 11.3, both the dye and protein are predicted to be negatively charged, and so electrostatic repulsion would suggest an unfavorable condition for interaction at this pH. However, if the dye forms H-type (parallel) aggregates at low solution concentrations, as documented recently by Saito et al. [18], for another squarylium-based protein label, then it is possible that the higher solution pH instigates disaggregation, availing the dye for protein labeling. Even though electrostatic interactions are not likely favored under these solution conditions, other interactions (i.e. hydrophobic interactions, hydrogen bonds and Van der Waals forces) between dye-proteins will exist and could subsequently affect the noncovalent interactions between dye and proteins as reflected in changes in fluorescence, causing a more significant enhancement in fluorescence to be observed.
Unlike bis-SQHN-4d, there was no fluorescence enhancement observed for SQHN-3c upon addition of BSA in the phosphate buffer (pH 11.3, 25.0 mM); however, there was a fluorescence enhancement upon the addition of BSA in all other buffers, with the enhancement in the citric acid buffer (pH 3.1, 25.0 mM) being the most significant. In the citric acid buffer the fluorescence emission of the unbound SQHN-3c was the weakest; however, upon the addition of BSA the fluorescence emission was strong with a substantial 96% increase in signal relative to the emission of SQHN-3c alone. In the borate and Tris buffers there were 89% and 58% increases in signal, respectively. While these increases are not as significant as in the citric acid buffer, this does demonstrate that the binding of SQHN-3c with protein is not as pH dependent as the binding of bis-SQHN-4d with protein.
To further explore the nature of bis-SQHN-4d−BSA interactions, a fixed concentration (7.00 x 10-7 M) of dye was titrated with increasing concentrations of BSA to achieve dye: protein ratios ranging from 1:0 to 1:30 in the Na2HPO4 buffer (pH 11.3). The enhancement of fluorescence increased with increasing concentration of added protein up to 2.10 x 10-5 M added protein (a 30-fold molar excess of protein relative to dye). A correlation coefficient of r2 = 0.991 affirmed a linear correlation between emission intensity and protein concentration over the range of 0−1.00 x 10-5 M BSA, with a regression equation as follows: [fluorescence emission, RFU] = 4.02 x 105[concentration BSA, mol/L]. However, the addition of BSA to a fixed concentration of bis- SQHN-4d beyond a molar ratio of 1:14 (dye: protein) did not result in proportional increases in fluorescence. This indicates a saturation, whereby all available dye molecules are bound by protein and so the addition of still more protein could not significantly alter the emission properties of the dye.
Stability of dye and dye-protein mixtures
Since the stability of most dyes in aqueous solution is poor [19], we investigated aging effects for both the dye solution and the dyeprotein mixture in Na2HPO buffer on fluorescence for bis-SQHN- 4d. The stability of bis-SQHN-4d (1.00 x 10-6 M) and a bis-SQHN-4d (1.00 x 10-6 M) − BSA (1.00 x 10-5 M) mixture was analyzed by CELIF. The fluorescence of the CE-LIF peak observed for bis-SQHN-4d samples alone remained the same over a period of 48 hours, indicating a stable dye solution in Na2HPO buffer. However, the fluorescence of the peak attributable to the dye−BSA complex increased during the 48 hour period, but stabilized thereafter. There was a substantial peak area increase (63%) for bis-SQHN-4d with BSA in Na2HPO4 buffer (pH 11.3, 25.0 mM) after 24 hours relative to the total fluorescence response of the dye with BSA within one hour of mixing in this same buffer system. This might imply a slow rate of association for the bis squarylium dye with protein, whereas previous research confirmed a fast reaction rate [20]. Even though our own subsequent studies employing bis-SQHN- 4d for on-column labeling reactions confirm a fast enough reaction to yield sufficient sensitivity for protein detection (see Section 3.4), it is possible that greater sensitivities could be achieved by allowing longer reaction times.
The effect of age of the dye–protein mixture on fluorescence in citrate buffer (pH 3.1, 25 mM) for SQHN-3c was also investigated. The stability of a SQHN-3c (7.00 x 10-7 M) – BSA (7.00 x 10-7 M) mixture was analyzed by fluorimetry. The fluorescence of the peak attributable to the complex in the resulting spectra rapidly degraded during the first 4 hours after mixing, after which time the signal remained very weak. There was a substantial decrease (30%) in fluorescence signal from time 0 to 10 minutes, and another substantial decrease (38%) from 10 minutes to 15 minutes. The most stable fluorescence was observed between 1-2 hours after mixing; however the strongest fluorescence signal was observed immediately after mixing. As such, SQHN-3c may be considered suitable for on-column or pre-column labeling of protein samples in cases when the subsequent separation can be completed in a very short period of time, although quantitation of protein based on CE-LIF peak height in such instances may not necessarily be attained through a simple linear calibration.
CE-LIF analysis of protein-dye mixtures
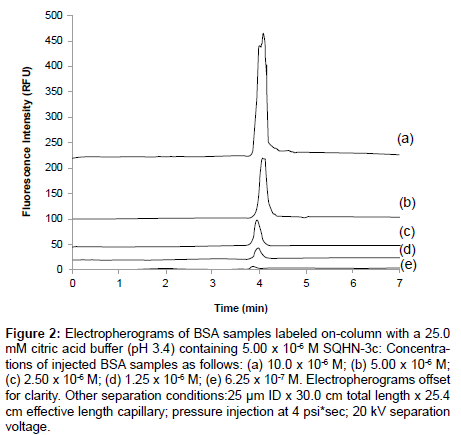
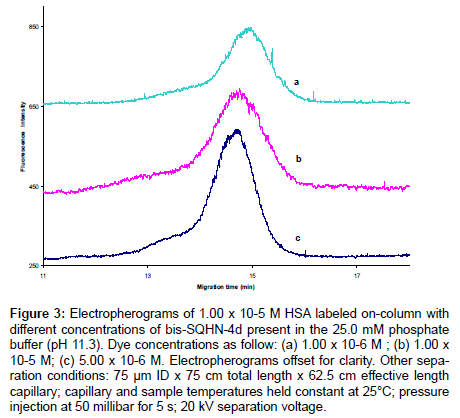
CE-LIF can be used analogously to fluorimetry to monitor changes in fluorescence emission of a dye upon titration with increasing concentrations of protein. In Figure 2, a linear increase in peak height for BSA−SQHN-3c complex (regression data reported in Table 2) is seen for increasing BSA concentration via on-column labeling . In these experiments, the citric acid running buffer contained a fixed concentration of SQHN-3c dye (5.00 x 10-6 M), while injected protein samples (prepared in water) ranged in concentration from 0.625 x 10-6 M to 10.0 x 10-6 M. Peak shape degraded as protein concentration increased in this calibration study, presumably due to competitive equilibria between protein-dye binding and protein-protein interactions. Reduced concentrations of injected protein should mitigate this problem; however, limits of detection for this particular dye/protein/buffer system need to be taken into consideration (for a complete discussion of limits of detection, refer to Section 3.4). It is possible to affect a change in CE-LIF signal for a given probe dye concentration by changing the protein analyte concentration, and indeed, this forms the basis of any calibration procedure to enable the use of this technique as a quantitative tool for protein determination. However, as seen in Figure 3, the amount of dye available for complexation can also affect the CE-LIF response, and it is therefore necessary to optimize the concentration of dye for any given analytical protocol. In Figure 3, on-column labeling of a given HSA sample (1.00 x 10-5 M) was conducted with three different concentrations of bis- SQHN-4d added to the Na2HPO4 buffer (pH 11.3, 25.0 mM).As can be seen, when the injected HSA concentration was held constant at 1.00 x 10-5 M, the peak area of the complex increased when the bis-SQHN- 4d dye concentration increased from 1.0 x 10-6 M (Figure 3a) to 5.0 x 10-6 M (Figure 3c), but the peak area of the complex did not show a further increase (and, in fact, decreased) when the dye concentration was further increased to 1.0 x 10-5 M (Figure 3b).Thus, 5.0 x 10-6 M dye in running buffer was chosen for further on-column labeling studies to maximize dye–protein signal. Similarly, a study of optimal SQHN- 3c dye concentration for on-column protein labeling revealed that 1.0 x 10-6 M SQHN-3c provided the best compromise between sensitivity towards added protein and minimal dye background signal (data not shown).
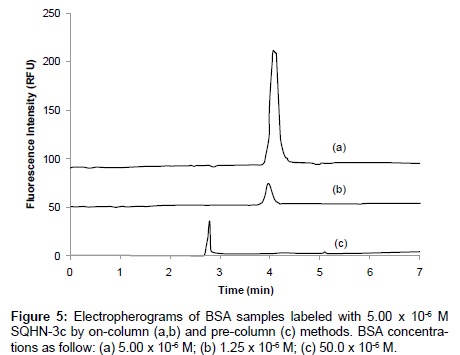
Figure 2: Electropherograms of BSA samples labeled on-column with a 25.0 mM citric acid buffer (pH 3.4) containing 5.00 x 10-6 M SQHN-3c: Concentrations of injected BSA samples as follows: (a) 10.0 x 10-6 M; (b) 5.00 x 10-6 M; (c) 2.50 x 10-6 M; (d) 1.25 x 10-6 M; (e) 6.25 x 10-7 M. Electropherograms offset for clarity. Other separation conditions:25 μm ID x 30.0 cm total length x 25.4 cm effective length capillary; pressure injection at 4 psi*sec; 20 kV separation voltage.
| Dye | Labeling Mode | Linear Regression [protein concentration (M) vs. peak height] | Correlation Coefficient | Limit of Detection (M) |
|---|---|---|---|---|
| bis-SQHN-4d | on-column | y =1.00 x 10-9x + 117 | 0.9998 | 3.42 x 10-8 |
| pre-column | y=2.93x + 0.0018 | 0.9996 | 8.70 x 10-7 | |
| SQHN-3c | on-column | y =256000x – 118000 | 0.9997 | 0.216 x 10-6 |
Table 2: LOD for pre-column and on-column labeling of HSA with bis-SQHN-4d and for on-column labeling of BSA with SQHN-3c, analyzed by CE-LIF.
On-column vs. pre-column labeling protocols
In the previous section, on-column, noncovalent protein labeling was employed to establish optimal dye concentrations for analytical method development. For noncovalent protein labeling in CE-LIF, the commonly used modes are on-column and pre-column labeling [21-23]. The mode of pre-column labeling involves mixing the protein and fluorophore together prior to introduction of a discrete plug of the mixture at the inlet of a buffer-filled capillary. On-column labeling, however, involves the injection of a discrete plug of protein alone into a capillary filled with buffer that contains the dye. In this case, the dye concentration is essentially constant in the buffer throughout the capillary, and the equilibrium between the free dye and the protein-bound dye is readily established and maintained as the protein migrates through the capillary. On-column labeling provides a simpler protocol with no sample pretreatment required, no risk of sample contamination, and no sample dilution, and it typically offers an enhancement in sensitivity as demonstrated in previous work with related squarylium dyes [24].
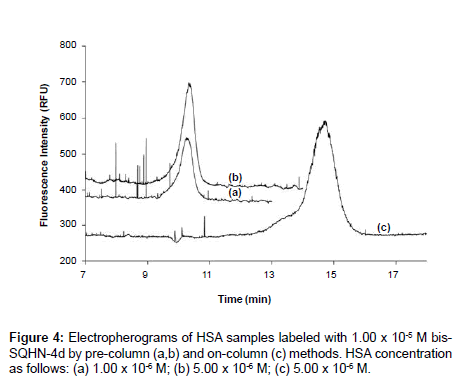
A comparative study was undertaken with bis-SQHN-4d, to determine which method is more effective for protein labeling and analysis in the present work. Figure 4 shows electropherograms resulting from pre-column labeling of two different concentrations (1.00 x 10-6 M,5.00 x 10-6 M) of HSA with 1.00 x 10-5 M bis-SQHN-4d dye, 24 hours after sample preparation (Figure 4(a) and Figure (b)), along with an electropherogram resulting from on-column labeling of a 5.00 x 10-6 M HSA samplewith 1.00 x 10-5 M dye in the Na2HPO4 separation buffer (Figure 4(c)). In (Figure 4(a) and Figure (b)), it can be seen that the protein−dye complex peak increased with increasing concentration of protein, due to the formation of additional complex. No peaks are observed for free dye in these pre-column labeling experiments, presumably because an excess of protein in the samples ensured that all dye was present in its bound form. Any small amount of dye liberated by dissociation during the separation itself would have been insufficient to be detected as an independent peak given the lower fluorescence quantum yield of the free dye relative to the bound dye. A single peak for dye-protein complex was similarly observed in the electropherogram for the on-column labeling experiment (Figure 4(c)). Any unbound dye in this instance would simply contribute to the baseline fluorescence signal rather than appear as a discrete peak since the dye is present everywhere in the buffer, but again, the quantum yield of the unbound dye is lower than that of the bound dye and so background fluorescence in the case of on-column labeling with bis- SQHN-4d is not likely to reduce the overall sensitivity of the method. It can be seen that the HSA−bis-SQHN-4d peak resulting from oncolumn labeling (Figure 4(c)) is larger (by 51% in terms of peak area) than the comparable peak from pre-column labeling (Figure 4(c)), which confirms the increased sensitivity achieved for on-column labeling in other work [24]. In addition, on-column labeling offers the advantage of reduced sample handling. The increase in migration time observed for the protein-dye complex peak in the on-column labeling experiment arises due to the presence of dye in the running buffer, which increases the ionic strength of the buffer, thus decreasing the overall electroosmotic mobility and concomitantly increasing the migration time.
Figure 3: Electropherograms of 1.00 x 10-5 M HSA labeled on-column with different concentrations of bis-SQHN-4d present in the 25.0 mM phosphate buffer (pH 11.3). Dye concentrations as follow: (a) 1.00 x 10-6 M ; (b) 1.00 x 10-5 M; (c) 5.00 x 10-6 M. Electropherograms offset for clarity. Other separation conditions: 75 μm ID x 75 cm total length x 62.5 cm effective length capillary; capillary and sample temperatures held constant at 25°C; pressure injection at 50 millibar for 5 s; 20 kV separation voltage.
A similar comparative study of noncovalent, pre-column vs. oncolumn labeling of BSA with SQHN-3c was conducted by CE-LIF, and the resulting electropherograms are shown in Figure 5. In this instance, 5.00 x 10-6 M SQHN-3c dye was used for on-column labeling of two different injected concentrations of BSA sample (Figure 5(a) 5.00 x 10-6 M and (b) 1.25 x 10-6 M), and pre-column labeling of 50.0 x 10-6 M BSA (Figure 5(c)) in a 25.0 mM citric acid buffer system (pH 3.4). Analogous to the studies with bis-SQHN-4d, on-column labeling with SQHN-3c resulted in a 54-fold increase in peak area (or an approximately 10- fold increase in peak height) relative to pre-column labeling, which confirms the increased sensitivity achieved for on-column labeling in other work [24]. In Figure 5 (c) it can be seen that a single peak for the protein-dye complex was observed. No peak was observed for free dye, presumably because an excess of protein in the sample, as described for the bis-SQHN-4d labeling experiments in . In Figure 5(a) and Figure (b), it can be seen that the (single) protein−dye complex peak increased with increasing concentration of injected protein, due to the formation of additional complex. As in the case of bis-SQHN-4d, the increase in migration time observed for the protein-dye complex peak in the on-column labeling experiment with SQHN-3c likely arises due to the presence of dye in the running buffer, which increases the ionic strength of the buffer, thus decreasing the overall electroosmotic mobility and concomitantly increasing the migration time.
A quantitative comparison of sensitivity differences between oncolumn and pre-column labeling methods were conducted. In this work, the Limit of Detection (LOD) was calculated as 3s/m, where ‘s’ is the standard deviation of the baseline signal, found from 1/5 of the peak-to-peak noise of a blank signal, and m is the slope or sensitivity of a calibration curve constructed from peak height versus injected protein concentration. Protein concentration ranges were from 0.78 − 3.5 μM HSA (pre-column labeled with bis-SQHN-4d), 0.70 - 7.0 μM HSA (oFigure 4n-column labeled with bis-SQHN-4d), and 0.625 - 10 μM BSA (on-column SQHN-3c). The LODs for pre-column and on-column labeling of HSA with bis-SQHN-4d and on-column labeling of BSA with SQHN-3c are reported in Table 2. As can be seen, the LOD for pre-column labeling with bis-SQHN-4d is more than an order of magnitude higher (worse) than that for on-column labeling. As the protein analyte migrates through a buffer-filled capillary in which the labeling dye is dissolved, the equilibrium between free and bound forms of the protein (dye + protein − dye �?? protein) is driven towards the bound form by the presence of excess dye in the buffer serving as a ‘reactant.’ Additionally, it can be seen that the LOD for HSA obtained with bis-SQHN-4d is about one order of magnitude lower than the LOD for BSA obtained with SQHN-3c, indicating that the former interacts more strongly with proteins, possibly through the kind of ‘clamshell’ interactions described in work by Patonay et al. [20].
Separations of protein mixtures
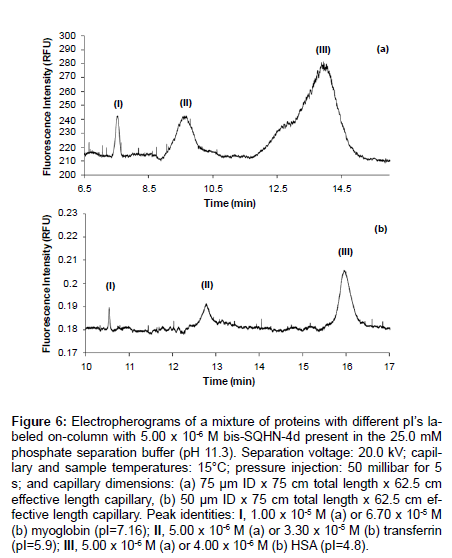
A mixture containing three model proteins with different pIs including myoglobin (pI=7.16), transferrin (pI=5.9), and HSA (pI=4.8) was labeled on-column with bis-SQHN-4d and fully resolved by CE-LIF, as shown in Figure 6. Trypsinogen was also labeled and resolved in subsequent experiments, although separation efficiency was significantly lower for this protein (data not shown). A thorough study of separation parameters was undertaken, including capillary dimensions, injection times, cartridge temperatures, separation voltage, buffer composition and ionic strength. Greater separation efficiencies were achieved for on-column labeling of a protein mixture employing a 25.0 mM phosphate separation buffer (pH 11.53) with 5.00 x 10-6 M bis-SQHN-4dusing a 50 μm ID capillary (Figure 6b) rather than a 75 μm ID capillary (Figure 6a), as had been employed for previous CE-LIF studies in this paper.
Figure 6: Electropherograms of a mixture of proteins with different pI’s labeled on-column with 5.00 x 10-6 M bis-SQHN-4d present in the 25.0 mM phosphate separation buffer (pH 11.3). Separation voltage: 20.0 kV; capillary and sample temperatures: 15°C; pressure injection: 50 millibar for 5 s; and capillary dimensions: (a) 75 μm ID x 75 cm total length x 62.5 cm effective length capillary, (b) 50 μm ID x 75 cm total length x 62.5 cm effective length capillary. Peak identities: I, 1.00 x 10-5 M (a) or 6.70 x 10-5 M (b) myoglobin (pI=7.16); II, 5.00 x 10-6 M (a) or 3.30 x 10-5 M (b) transferrin (pI=5.9); III, 5.00 x 10-6 M (a) or 4.00 x 10-6 M (b) HSA (pI=4.8).
Unfortunately, similar CE-LIF conditions did not yield successful protein separations with SQHN-3c as the labeling probe. The lower sensitivities achieved with SQHN-3c may have been a contributing factor, or perhaps SQHN-3c interacted more strongly with charged sites on the inside capillary wall and so separation and labeling efficiencies were reduced relative to the work with bis-SQHN-4d. As noted previously, at the high pH (11.3) employed for the on-column labeling and separation of the mixture of model proteins in this work, all proteins would be negatively charged, as would be the squarylium dyes (due to the deprotonation of the pendant carboxylic acid group on each dye), and so pure electrostatic interactions alone would not favor noncovalent binding. This would imply that other interactions such as hydrophobic interactions, hydrogen bonds and Van der Waals forces (i.e. Dipole-dipole bonds) are critical to dye-protein binding, as confirmed in previous work [19].
Conclusions
The spectral properties of two novel squarylium dyes, bis-SQHN-4d and SQHN-3c, were explored under a variety of solution conditions in order to determine the best conditions for noncovalent protein labeling and analysis by CE-LIF. Although these dyes are not water soluble, stable stock solutions can be prepared in organic solvents and subsequently diluted with aqueous buffers to the appropriate working concentration just prior to use. In particular, bis-SQHN-4d shows great potential as a noncovalent label for a wide range of proteins, and the enhanced detection sensitivity observed for this dye with on-column labeling protocols also bodes well for its promise as a protein probe. On-column labeling methods have the advantage of reduced sample handling (and thus lower risk of contamination or loss). Sample derivatization prior to injection typically necessitates larger sample sizes (to ensure proper mixing of reagents and a complete reaction) than does any given CELIF injection, and sample derivatization prior to injection often results in sample dilution, thus impinging on detection limits. However, oncolumn, noncovalent protein labeling as described herein obviates the need for sample derivatization prior to injection, which holds much promise for analytical method development for clinical, forensic, and environmental samples. By combining the high resolving power of CE with the sensitivity of LIF detection and the convenience of on-column labeling, applications for novel, asymmetric squarylium dyes as protein probes are expected to grow.
Acknowledgements
This work was supported, in part, by Wake Forest University and the National Science Foundation under grant no. 0809756.
References
- Klose J, Kobalz U (1995) Two-dimensional electrophoresis of proteins: An updated protocol and implications for a functional analysis of the genome. Electrophoresis 16: 1034-1059.
- Liu F, Goshe MB (2010) Combinatorial Electrostatic Collision-Induced Dissociative Chemical Cross-linking Reagents for Probing Protein Surface Topology. Anal Chem 82: 6215-6223.
- Bachhav YG, Kalia YN (2010) Development and validation of an analytical method for the quantification of cytochrome c in skin transport studies. Biomed Chromatogr 24: 732-736.
- Wang M, Feng WY, Zhao YL, Chai ZF (2010) ICP-MS-Based strategies for protein quantification. Mass Spectrom Rev 29: 326-348.
- Eggertson MJ, Craig DB (2000) Laser-induced fluorescence detector for liquid chromatography: applications to protein analysis. Biomed Chromatogr 14: 156-159.
- Lacroix M, Poinsot V, Fournier C, Couderc F (2005) Laser-induced fluorescence detection schemes for the analysis of proteins and peptides using capillary electrophoresis. Electrophoresis 26: 2608-2621.
- Sloat AL, Roper MG, Lin XL, Ferrance JP, Landers JP, et al. (2008) Protein determination by microchip capillary electrophoresis using an asymmetric squarylium dye: Noncovalent labeling and nonequilibrium measurement of association constants. Electrophoresis 29: 3446-3455.
- Welder F, Paul B, Nakazumi H, Yagi S, Colyer CL (2003) Symmetric and asymmetric squarylium dyes as noncovalent protein labels: a study by fluorimetry and capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci 793: 93-105.
- Burke A, Schmidt-Mende L, Ito S, Gratzel M (2007) A novel blue dye for near-IR ‘dye-sensitised’ solar cell applications. Chem Commun 43: 234-236.
- Jisha VS, Arun KT, Hariharan M, Ramaiah D (2006) Site-selective binding and dual mode recognition of serum albumin by a squaraine dye. J Am Chem Soc 128: 6024-6025.
- Ajayaghosh A (2005) Chemistry of squaraine-derived materials:?near-IR dyes, low band gap systems, and cation sensors. Acc Chem Res 38: 449-459.
- Thomas J, Sherman DB, Amiss TJ, Andaluz SA, Pitner JB (2007) Synthesis and biosensor performance of a near-IR thiol-reactive fluorophore based on benzothiazolium squaraine. Bioconjug Chem 18: 1841-1846.
- Liang K, Farahat MS, Perlstein J, Law KY, Whitten DG (1997) Exciton interactions in nonconjugated squaraine dimers. Mechanisms for coupling and consequences for photophysics and photochemistry. J Am Chem Soc 119: 830-831.
- Bonnett R, Montevalli M, Siu J (2004) Squaraines based on 2-arylpyrroles. Tetrahedron 60: 8913-8918.
- Nakazumi H, Ohta T, Etoh H, Uno T, Colyer CL, et al. (2005) Near-infrared luminescent bis-squaraine dyes linked by a thiophene or pyrene spacer for noncovalent protein labeling. Synthetic Met 153: 33-36.
- Yagi S, Ohta T, Akagi N, Nakazumi H (2008) The synthesis and optical properties of bis-squarylium dyes bearing arene and thiophene spacers. Dyes Pigments 77: 525-536.
- Hyodo Y, Nakazumi H, Yagi S (2002) Synthesis and light absorption/emission properties of novel squarylium dimers bearing a ferrocene spacer. Dyes Pigments 54: 163-171.
- Saito S, Massie TL, Maeda T, Nakazumi H, Colyer CL (2012) On-column labeling of gram-positive bacteria with a boronic acid functionalized squarylium cyanine dye for analysis by polymer-enhanced capillary transient isotachophoresis. Anal Chem 84: 2452-2458.
- Yan W, Colyer CL (2005) Fluorimetric studies and noncovalent labeling of protein with the near-infrared dye HITCI for analysis by CE-LIF. J Sep Sci 28: 1409-1415.
- Patonay G, Kim JS, Kodagahally R, Strekowski L (2005) Spectroscopic study of a novel bis(heptamethine cyanine) dye and its interaction with human serum albumin. Appl Spectrosc 59: 682-690.
- Yan W, Colyer CL (2006) Investigating noncovalent squarylium dye–protein interactions by capillary electrophoresis–frontal analysis. J Chromatogr A 1135: 115-121.
- McCorquodale EM, Colyer CL (2001) Indocyanine green as a noncovalent, pseudofluorogenic label for protein determination by capillary electrophoresis. Electrophoresis 22: 2403-2408.
- Chichester KD, Silcott DB, Colyer CL (2008) Analysis of Bacillus globigii spores by CE. Electrophoresis 29: 641-651.
- Yan WY, Sloat AS, Yagi SY, Nakazumi HK, Colyer CL (2006) Protein labeling with red squarylium dyes for analysis by capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 27: 1347-1354.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14864
- [From(publication date):
specialissue-2015 - May 20, 2024] - Breakdown by view type
- HTML page views : 10444
- PDF downloads : 4420