Role of Mitochondrial Zinc in Progression of Prostate Cancer: A Diagnostic Biomarker for Prostate Cancer
Received: 09-Nov-2020 / Accepted Date: 24-Nov-2020 / Published Date: 30-Nov-2020 DOI: 10.4172/aot.1000153
Abstract
Prostate gland is a complex organ found in men, having different components which are named as peripheral, central zone and a periurethral region. Biomarkers development for screening and detection of prostate cancer proved crucial in its easy and early management. Mitochondrial Zinc plays a crucial role in progression of prostate cancer. Peripheral zone of prostate gland has uncommon feature of zinc accumulation which result in collection and secretion of high level of citrate along with components of semen. High amount of zinc inhibit the action of m-aconitase which in turn inhibits citrate oxidation. This review describes the metabolic zinc relationships found in normal and malignant prostate. In this review paper, we will also review the current knowledge on prostate cancer biomarkers such as PSA, PCA3, TMPRSS2-ERG, α-Methylacyl–coenzyme a racemase. Validation of existing biomarkers is essential and future research should focus in validation of existing biomarkers along with discovery of new biomarkers
Keywords: Prostate cancer; Biomarkers; Metabolism; Zinc
Introduction
Prostate gland is a complex organ found in men having different components which are named as peripheral, central zone and a periurethral region. These components are ontologically, morphologically and functionally different. 70% of prostate gland is comprised by Peripheral zone which produce and secrete citrate in excessive quantity. This distinctive characteristic of production of citrate and its secretion belongs to very specialized epithelial cells of the prostate, found in peripheral zone of prostate. These cells are glandular and these are secretory in nature. Development of malignancy begins in this peripheral zone of prostate gland. Mainly 70%-80% of Prostate cancer originates from the proximal region. Pca is one of most frequent cancer found within man especially in developed countries. Which cause an economic burden in a population along with morbidity and mortality [1,2]. It is the 5th cause of cancer related deaths in males’ worldwide. More than 90% of cases are found in males having age more than 50. In India, prostate cancer cases are found mainly at the age of 65. Like age, race is also the cause for example African American population have more cases of prostate cancer in comparison to white American population. Prostate is among the top ten site for cancers in India. In India between the year of 2010 and 2015 approximate 27000 case of prostate cancer found with a survival rate of 64%. In its initial stage, this type of cancer grows slowly and remains bound to prostate gland. But sometimes it may spread to other body parts and may need surgical removal. Symptoms of Pca in severe conditions may include difficulty in urination, urine having blood, pelvis pain etc. At later stage RBC level becomes low so the patient may feel tired. However, exact cause of prostate cancer is not known. Most Primary common factors include Diet, age, Phylogenetic relationship and race. It is easy to get treated when detected at early stage. Due to increase in study of cancer metabolomics, various efforts has been made to find biomarkers to diagnose prostate cancer and efforts has been also made to analyze cellular metabolism to easy and early diagnosis and treatment of Pca. PSA (Prostate Specific Antigen) testing is one such method to detect Pca. PSA secretion is found increased in mostly men having prostate cancer so this is a widely used biomarker. [3]. PSA is secreted outside the body as a part of semen [4]. In normal conditions PSA is secreted in low amount in blood but in prostate cancer, PSA is secreted in high amount and thus increase in serum PSA represent abnormalities. Presently, PSA testing is used as early screening method to ascertain probability and gradual phenotype of Pca. PSA testing may lead to aggressive overtreatment in some patients who may have been treated sufficiently with active surveillance. Understanding the role of Zinc in prostate metabolism, it may be possible to diagnose prostate cancer easily [5]. Beside metabolic alterations, biomarkers can be proved very important in early and accurate detection of Pca. So, in this paper we will discuss about current stage of knowledge regarding role of zinc in Pca metabolism, role of biomarkers in Pca Detection and their future prospective.
Role Of Zinc In Prostate Cancer Metabolism
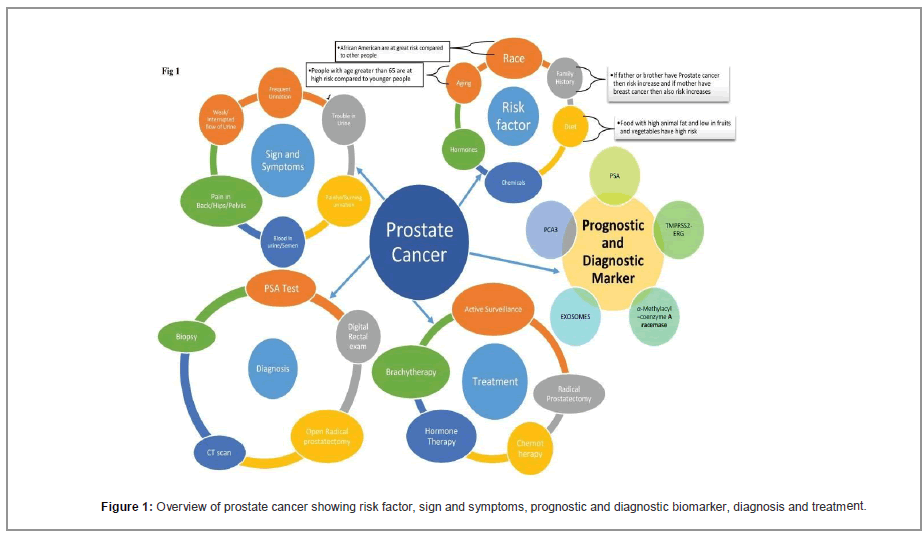
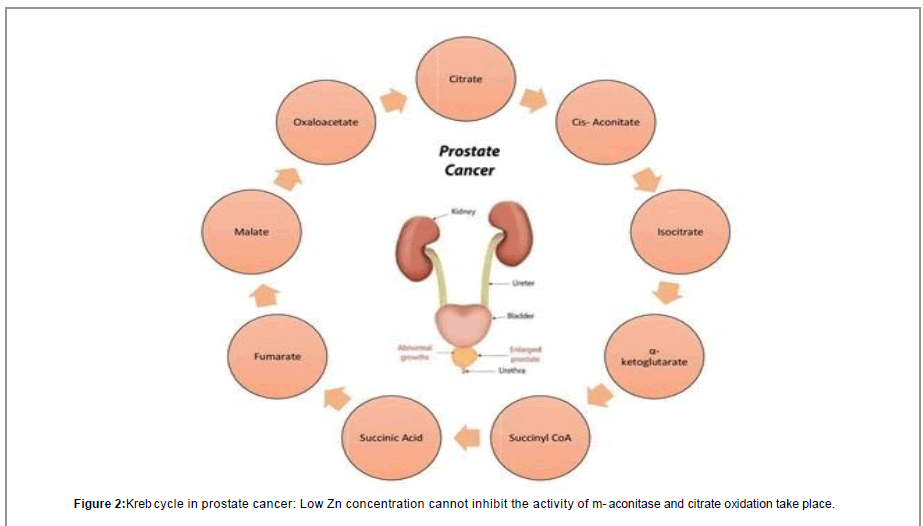
Zinc is responsible for growth, reproductive and functional activities of prostate cells and nearly all mammalian cells. Prostate cells have uncommon feature of accumulation of Zinc in very high amount [6]. Prostate zinc amount is nearly 2-5 times than amount of zinc in other organs. Also Subcellular distribution of zinc in prostate is also not equal as shown in Tables 1 and 2 [7]. It is clear from (Table 2) that mitochondrial Distribution of Zinc is high. Also the amount of Zn in mitochondria of prostate is higher than non-prostate cells mitochondria. Various reports shows that level of Zinc is remarkably low in prostate cancer in comparison to normal and BPH conditions in prostate. According to Dhar et al. [7] Zinc in normal prostate is 540 μg/g dry wt. and in prostate cancer level of zinc remains 168 μg/g dry wt. Zinc accumulation in prostate is a feature of epithelial cells of prostate which results in citrate production. Zinc accumulation in these prostate benign epithelial cells results due to increased number of zinc transporter ZIP1 in prostate. Various research’s performed on kreb cycle pathway has proved that activity of m-aconitase can be inhibited by high concentration of Zinc [8]. M-aconitase enzyme is mainly responsible for the oxidation of citrate in citrate oxidation or kreb cycle pathway. As we know, cells depends on oxidation of citrate which is a very important step in kreb cycle for the progress of aerobic respiration [9]. High amount of ATP, is produced by production and oxidation of citrate in kreb cycle which is followed by coupled phosphorylation. Various other pathways for biosynthesis of amino acid metabolism and for their degradation, are produced by the kreb cycle and recycling of their intermediates. So, Production and oxidation of citrate with the help of functional Krebs cycle is mandatory for the normal functioning of aerobic mammalian cells. But prostate epithelial cells produce citrate and they do not oxidize the citrate [10] and secrete citrate outside the body as a constituent of semen. In this epithelial cell of prostate, cancer begins [11]. Since citrate is produced and not oxidized in normal prostate cells so these cells of prostate must take on any other metabolic pathways and stop other activities which are not essential to save energy for survival and for their unique functions. Citrate producing prostate cells reportedly exhibit a high aerobic glycolysis [12-14], this results in a costly pathway of metabolism to produce energy when citrate oxidation is absent. Under normal conditions, benign prostate cells accumulate Zn and produces citrate but prostate cancer cells do not accumulate Zn and oxidation of citrate takes place by kreb cycle as shown in Figure 1.
| Zinc level | µg/g dry wt. |
|---|---|
| Whole gland | 150 |
| Lateral lobe | 211 |
| Dorsal lobe | 127 |
| Anterior lobe | 84 |
| Inner lobe | 87 |
| Prostatic fluid | 500 |
| Blood plasma | 1-2 |
| Other organs | 20-50 |
Table 1: Zinc level in prostate, blood plasma and other organs.
| Subcellular distribution of zinc | mg/g dry wt. |
|---|---|
| Total | 540 |
| Nuclear | 200 |
| Mitochondrial | 152 |
| Microsome | 92 |
| Supernatant | 64 |
Table 2: Subcellular distribution of zinc in human prostate.
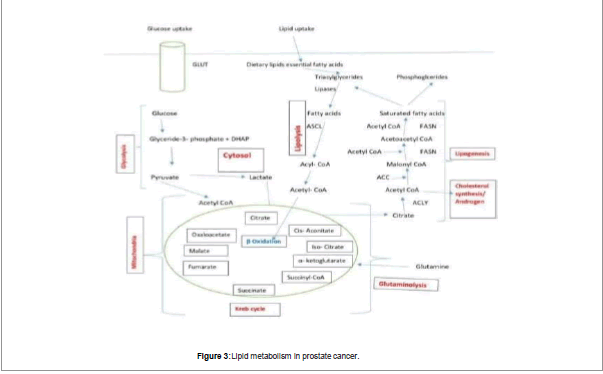
Besides this, prostate tumor cells behave differently from other types of tumors. Many types of cancer cell depend on glycolysis to fulfill their energy requirement known as Warburg phenomenon given by Otto Warburg [15]. According to Warburg increase in glucose utilization by Glycolysis is due to inherent defect in oxidative phosphorylation, which is the cause of cancer. Researchers found that cancer cells do not exhibit inherent defect in oxidative phosphorylation [16] but the observation of Warburg that cancer cells exhibit increase in glucose utilization via increase in glycolysis process proved true for most of the malignant cancers [17-19]. The increase in glucose utilization could be detected by PET which monitors glucose analogs (F18-FDG) as an indicator of malignancy in the cells [20]. Because of different phenotypes of Prostate cancer cells, these cells do not obey Warburg effect. These cells use lipids and some other molecules which are energy producing molecules for energy requirement and their proliferation and show high glucose uptake sometimes in later stages of Pca [21,22]. So, it is found that prostate cancer cells do not obey Warburg effect because increase in uptake of glucose is not found in these tumor cells [23]. So, FDG PET scans cannot detect Prostate cancers. From various studies it is found that the level of m-aconitase is same in these citrate producing cells as compared to citrate oxidizing cells [24,25]. So, it conclude that the low m-aconitase activity was not due to low level of enzyme and it was due to unique intramitochondrial conditions which inhibited enzyme activity. Liu et al. [26] provided the important information that zinc levels in mitochondria of prostate cells were uniquely in higher amount than non-prostate cells. Various studies shows that when amount of zinc becomes low in prostate then m-aconitase is not inhibited and this results in oxidation of citrate. However, it is unlikely that the decrease in citrate in prostate malignancy is solely due to its oxidation. It is not amazing that metabolism of tumor cell involves a curtailed Krebs cycle which have low amount of citrate oxidation. Actually the tumor cells in prostate are also involved in citrate production but they do not accumulate the citrate as like normal prostate cells. Instead, the citrate produced by tumor cells is exported from mitochondria to cytosol via a shuttle such as the citrate: malate shuttle where it is converted to acetyl CoA for lipogenesis. Increase in lipogenesis is very essential for proliferation of tumor cell and their growth; and essential precursor of lipogenesis is citrate. Since this process is also essential for the growth and development of malignant prostate cells, a significant proportion of synthesized citrate must be utilized for lipogenesis in addition to being oxidized. This is an essential area for further investigation regarding the involvement of citrate-related intermediary metabolism and mitochondrial function in prostate malignancy. The exact nature of bioenergetics in early Pca cells is still being worked out, and work is being done on specific pathways as depicted in Figure 2.
As we discussed that PSA testing is broadly used to diagnose Prostate Cancer. However PSA testing is not specific in nature because BPH is present more than 50% in men having more than age of 50 years [27,28]. So it is not very specific and may give false result. Beside this about 15 percent of men found with Pca having PSA at very low level (<4.0 ng/ml) and about 15% show advanced Gleason Score [29,30]. The overview of prostate cancer: risk factor, sign, symptoms, prognostic and diagnostic biomarker, diagnosis and treatment as shown in Figure 3. So in keeping view of certain limitations of PSA testing other type of biomarkers is being used to diagnose of prostate cancer so some of them are:-Extracranial locations of hemangiopericytoma were excluded.
PCA3
PCA3 formerly known as DD3 gene is highly overexpressed in prostate tumor [31,32]. PCA3 is a prominent biomarker used for testing of prostate cancer. Main importance of this is this test is not based on PSA. PCA 3 Stands for Prostate cancer antigen 3. Based on several studies, it is confirmed that malignant prostatic tissue have higher PCA3 mRNA as compared to non-malignant tissue [33-37]. Because of high sensitivity and specificity its use increased as a noninvasive biomarker. PCA 3 can be detected from cells found in urine of prostate cancer patients. Many iterations of PCA3 urine tests has been emerged [38] and presently, transcriptional amplification, a clinical grade assay based approach is used. An approach which combine reports from both PSA and PCA 3 can be used to detect prostate cancer.
TMPRSS2-ERG
Studies confirmed that high number of Pca express gene fusions in this gene fusion 5’ region of TMPRSS2 gene is fused with ERG gene. TMPRSS2 gene is regulated by androgen whereas ERG is a member of ETS family transcription factor. These are prostate cancer specific fusions and can be detected in precursor lesions. TMPRSS2-ERG RNA is investigated in prostate cancer patient urine [39,40]. Various studies shows that this is found only in about 50% prostate cancer patients; so it should be used with other biomarkers like PCA3. A study on PCA3 and TMPRSS-ERG jointly in urine is performed for diagnosis of Pca relative to PSA. Some reports say that TMPRSS2-ERG fusion can be used as biomarker for detection of Pca itself when it is founded in tissues. There is a relationship among TMPRSS2-ERG and aggressive Pca [41,42]. But some others reports not found these type of relations [43,44]. Beside the possible advantage of TMPRSS2-ERG and PCA3, these are currently used complementary to PSA and researches are being performed to check the result of these tests in the absence of PSA.
α-Methylacyl-Coenzyme A Racemase
Enzyme AMACR is founded by RNA expression profiling and AMACR can be used as a diagnostic biomarker for detection of Pca due to high level of sensitivities (approx. 90%) [45]. Low expression of AMACR gene is related with metastasis. Low expression of AMACR gene is also related with biochemical recurrence of Pca [46]. AMACR is not specific to Pca because AMACR can also be detected in nodular hyperplasia, adenosis and atrophy. It is not suitable for noninvasive detection in urine [47,48].
Future Prospective
Zinc-citrate relationship gives an opportunity to develop new approaches to the treatment of Pca. Consequently, we would propose that citrate oxidation inhibition would result in arrest or destroy malignant prostate cells and small adverse effects on nonmalignant prostate epithelial cells. If high prostate zinc levels can be restored in malignant cells of prostate, then citrate oxidation will be inhibited due to m-aconitase activity inhibition, which could stop the beginning of malignancy and further development. As already discussed, that unavailability of Zinc is not the cause of low amount of Zinc in Pca tissue. Efforts have to be made to increase uptake of Zinc in mitochondria of malignant prostate tissue. An additional agent which will increase Zinc uptake in prostate cells might be essential. Both testosterone and prolactin increase zinc accumulation in lateral prostate cells of rat, which is homologous to human lateral prostate which is primary origin of malignancy. Consequently, malignant prostate cells might exhibit the hormonal responses described for L-type cells. In support of this, as described above, we have demonstrated that prolactin treatment markedly increases zinc accumulation in LNCaP cells. These relationships bring us to the proposal that the administration of an agent to increase the cellular uptake of zinc, such as the elevation of circulating levels of prolactin coupled with an increased dietary intake of zinc, might increase the accumulation of zinc in malignant prostate cells and arrest malignancy. Because the prostate gland accumulates the high amount of circulating zinc and prolactin effect is specific for cells of prostate, there should be minimum effects elsewhere in the body. In addition, prolactin might inhibit the synthesis and level of m-aconitase, as it does in rat lateral prostate cells which would further decrease m-aconitase activity. So we need to find a method to increase the circulating level of prolactin because an injectable form of prolactin for human use is not available till now. An alternative but less desirable approach would be to enhance the pituitary release of prolactin by agents such as estrogen or perphenazine [49]. Provided that such agents do not have direct effects on inhibiting zinc uptake by the malignant cells and are not contraindicated by other adverse effects. This approach is supported by some preliminary studies in which we observed that the treatment of human Pca cell lines (LNCaP and PC-3 cells) with zinc resulted in a dose-response inhibition of proliferation of both cell lines. When coupled with our reports that LNCaP and PC-3 cells remain responsive to prolactin, these preliminary studies provide important support for this provocative proposal for the therapeutic use of zinc coupled with prolactin treatment. In addition to Zinc-citrate relationship there are needs to use biomarkers other than PSA to easy and early detection of Pca. Biomarkers like TMPRSS2-ERG, PCA3 and exosomes can prove very important for early and easy detection due to their specificity and sensitivity. Researches should be done in this lacked but very important area. While this presentation is highly speculative, it provides a rational basis for various latest perspective to control and elimination of Pca. Hopefully this review will serve to expand interest of the basic and clinical scientific community in addressing these important areas of research.
Author Contributions
RR and RK Planning and preparation of the manuscript. NKG has guided for preparation of the final manuscript. All authors contributed significantly to the study and have approved the final manuscript.
Acknowledgments
We sincerely thank Sir Ganga Ram Hospital in providing all the necessary support.
Conflict of Interest
Authors have conflict of interest.
Funding
Nil
References
- Claus GR, Black LK. (2011) The economic burden of prostate cancer. Bjui Int 108:806-813.
- Capper CP, Rae JM, Auchus RJ.(2016) The Metabolism, Analysis, and Targeting of Steroid hormones in breast and prostate cancer. Horm and Cancer; 7:149-164.
- Lilja H, Ulmert D, Vickers AJ.(2008) Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat Rev Cancer; 8:268-278.
- Carlsson SV, Kattan MW.(2016) Personalized risk-stratified screening or abandoning it altogether? Nat Rev Clin Oncol; 13:140-142.
- Kumar D, Gupta A, Nath K.(2016) NMR-based metabolomics of prostate cancer: A protagonist in clinical diagnostics. Expert Rev Mol Diagn; 16: 651-661.
- Ferenc Gyorkey, Kyung WM, JA Huff, Phyllis Gyorkey.(1967) Zinc and magnesium in human prostate gland: normal, hyperplastic, and neoplastic. Cancer Res; 27:1348-1353.
- NK Dhar, TC Goel, PC Dube, AR Chowdhury, Amiya BK et al. (1973) Distribution and concentration of zinc in the subcellular fractions of benign hyperplastic and malignant neoplastic human prostate. Exp Mol Pathol; 19:139-1142.
- Costello LC, Franklin RB.(1981) Aconitase activity, citrate oxidation, and zinc inhibition in rat ventral prostate. Enzyme; 26:281-287.
- Dakubo GD, Parr RL, Costello LC, Franklin RB, Thayer RE et al. (2005) Altered metabolism and mitochondrial genome in prostate cancer. J Clin Pathol;59:10-16.
- Costello L, Feng P, Milon B, Tan M, Franklin RB et al. (2004) Role of zinc in the pathogenesis and treatment of prostate cancer: Critical issues to resolve. Prostate cancer and Prostatic Dis; 7:111-117.
- Leslie C. Costello, Franklin RB.(2016) A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys; 611:110-112.
- Harkonen, P.(1981) Androgenic control of glycolysis, the pentose cycle and pyruvate dehydrogenase in the rat ventral prostate. Journal of Steroid Biochemistry; 14:1075-1084.
- Harkonen P, Kostian ML, Santti RS.(1981) Indirect androgenic control of citrate accumulation in Rat ventral prostate. Archives of Andrology Journal of Reproductive Systems; 8:107-116.
- Müntzing J, Varkarakis MJ, Saroff J, Murphy GP.(1975) Comparison and significance of respiration and glycolysis of prostatic tissue from various species. J Med Primatol; 4:245-251.
- Sidney Wein H, Otto Warburg, Dean Burk, Arthur L. Schade. (1956) On respiratory impairment in cancer cells. Science; 124:267-272.
- Fantin VR, St-Pierre J, Leder P.(2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell; 9:425- 434.
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB.(2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab; 7:11-20.
- Gatenby RA, Gillies RJ.(2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer;4:891-899.
- Hsu PP, Sabatini DM.(2004) Cancer cell metabolism: warburg and beyond. Cell 2008; 134:703-707.
- Gambhir SS.(2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer; 2:683-696.
- Sadeghi RN, Karami-Tehrani F, Salami S.(2014) Targeting prostate cancer cell metabolism: Impact of hexokinase and CPT-1 enzymes. Tumor Biol; 36:2893-2905.
- Twum-Ampofo J, Fu D-X, Passaniti A, Hussain A, Siddiqui MM et al. (2016) Metabolic targets for potential prostate cancer therapeutics. Curr Opin Oncol; 28:241-247.
- Dueregger A, Schöpf B, Eder T, Höfer J, Gnaiger E et al. (2015) Differential utilization of dietary fatty acids in benign and malignant cells of the prostate. Plos One; 10.
- Costello LC, Liu Y, Franklin RB.(1995) Testosterone stimulates the biosynthesis of m-aconitase and citrate oxidation in prostate epithelial cells. Mol Cell Endocrinol; 112:45-51.
- Liu Y, Costello LC, Franklin RB.(1996) Prolactin specifically regulates citrate oxidation and m-aconitase of rat prostate epithelial cells. Metabol; 4:442-449.
- Liu Y, Costello LC, Franklin RB.(1997) Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. The Prostate; 30:26-32.
- Wolf AM, Wender RC, Etzioni RB, Thompson IM, Amico AVD et al. (2010) American cancer society guideline for the early detection of prostate cancer: update 2010. Cancer J Clin; 60:70-98.
- Balk SP, Ko YJ, Bubley GJ.(2003) Biology of prostate-specific antigen. J Clin Oncol; 21:383-391.
- M. Scott Lucia, Amy K. Darke, Phyllis J. Goodman, Francisco G. La Rosa, Howard L. Parnes et al. (2008) Pathologic characteristics of cancers detected in the prostate cancer prevention trial: Implications for prostate cancer detection and chemoprevention. Cancer Prev Res; 1:167-173.
- Ian M. Thompson, Donna K. Pauler, Phyllis J. Goodman, Catherine M. Tangen, M. Scott Lucia et al. (2004) Prevalence of prostate cancer among men with a prostate-specific antigen level ≤ 4.0 ng per milliliter. N Engl J Med; 350:2239-2246.
- Bussemakers MGJ, Adrie van Bokhoven, Gerald W. Verhaegh, Frank P. Smit, Herbert F. M. Karthaus et al. (1999) DD3:A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res; 59:5975-5985.
- Jacques B. de Kok, Gerald W. Verhaegh, Rian W. Roelofs, Daphne Hessels, Lambertus A. Kiemeney et al. (2002) DD3PCA3, a very sensitive and specific marker to detect prostate tumors. Cancer Res; 62:2695-2698.
- Daphne Hessels, Jacqueline MT Klein Gunnewiek, Ingevan Oort, Herbert F.M.Karthaus, Geert JL van Leenders et al. (2003) DD3PCA3-based molecular urine analysis for the diagnosis of prostate Cancer. Eur Urol; 44:8-16.
- Kelly A. Landers, Michelle J. Burger, Michelle A. Tebay, David M. Purdie, Betty Scells et al.(2005) Use of multiple biomarkers for a molecular diagnosis of prostate cancer. Int J Cancer; 114: 950-956.
- Uta Schmidt, Susanne Fuessel, Rainer Koch, Gustavo B. Baretton, Andrea Lohse et al. (2006) Quantitative multiâ€gene expression profiling of primary prostate cancer. The Prostate; 66:1521-1534.
- Bialkowska-Hobrzanska H, Driman DK, Fletcher R, Harry V, Razvi H et al.(2006) Expression of human telomerase reverse transcriptase, Survivin, DD3 and PCGEM1 messenger RNA in archival prostate carcinoma tissue. Europe PMC; 13:2967-2974.
- Popa I, Fradet Y, Beaudry G, Hovington H, Beaudry G et al.(2007) Identification of PCA3 (DD3) in prostatic carcinoma by in situ hybridization. Mod Pathol; 20:1121-1127.
- D Hessels, JA Schalken.(2009) The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol;6:255-261.
- D Hessels, FP Smit, GW Verhaegh, JA Witjes, EB Cornel et al.(2007) Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin Cancer Res; 13:5103-5108.
- SA Tomlins, SM Aubin, J Siddiqui, RJ Lonigro, L Sefton-Miller et al.(2011) Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med; 3.
- F Demichelis, K Fall, S Perner, O Andrén, F Schmidt et al. (2007) TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene; 26:4596-4599.
- G Attard, J Clark, L Ambroisine, G Fisher, G Kovacs et al. (2007) Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene; 27:253-263.
- SW Fine, A Gopalan, MA Leversha, HA Al-Ahmadie, SK Tickoo et al.(2010) TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high-grade morphological features. Mod Pathol; 23:1325-1333.
- Anuradha Gopalan, Margaret A Leversha, Jaya M. Satagopan, Qin Zhou, Hikmat A Al-Ahmadie et al.(2009) TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res; 69:1400-1406.
- MA Rubin, M Zhou, SM Dhanasekaran, S Varambally, TR Barrette et al.(2002) α-methylacyl coenzyme a racemase as a tissue biomarker for prostate cancer. JAMA; 287:1662-1670.
- Mark A Rubin, Tarek A Bismar, Ove Andrén, Lorelei Mucci, Robert Kim et al.(2005) Decreased α-methylacyl coa racemase expression in localized prostate cancer is associated with an increased rate of biochemical recurrence and cancer-specific death. Cancer Epidemol Biomarkers Prev; 14:1424-1432.
- Z Jiang, GR Fanger, BA Woda, BF Banner, P Algate et al.(2003) Expression of α-methylacyl-coa racemase (p504s) in various malignant neoplasms and normal tissues: A study of 761 cases. Hum Pathol; 34:792-796.
- Bharathi Laxman, David S Morris, Jianjun Yu, Javed Siddiqui, Jie Cao et al.(2008) A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res; 68:645-649.
- Rosoff B, Diamond EJ (1982) Effect of perphenazine on growth and zinc-65 uptake of the rat prostatic adenocarcinoma, R 3327. The Prostate; 3:615-622.
Citation: Rana R, Kant R, Ganguly NK, et al. (2020) Role of Mitochondrial Zinc in Progression of Prostate Cancer: A Diagnostic Biomarker for Prostate Cancer. J Oncol Res Treat 5: 152. DOI: 10.4172/aot.1000153
Copyright: © 2020 Rana R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language