Salivary Duct Carcinoma in Minor Salivary Glands: Report of Two Cases with Different Clinical Behavior
Received: 16-Jan-2014 / Accepted Date: 14-Mar-2014 / Published Date: 19-Mar-2014 DOI: 10.4172/2161-0681.1000164
Abstract
Salivary duct carcinoma (SDC) is a malignant epithelial tumor extremely rare in minor salivary glands. This manuscript describes two cases of SDC of the minor salivary glands with different clinical behaviors. The first patient was an 80-year-old man who had SDC (T1N0M0) in the upper buccal fornix and the second patient was a 64-yearold man with a tumor (T2N2cM0) in the soft palate. Patients were treated with radical surgical resection and none patient underwent postoperative radiotherapy or chemotherapy. Histopathologically, the lesions showed proliferation of ductal cells with varying degrees of nuclear pleomorphism arranged in solid and cribriform structures and prominent comedonecrosis. The patient with SDC in the soft palate developed metastases and died shortly after diagnosis while the patient with SDC in upper alveolar ridge is alive without disease for over 6 years of follow-up. Although SDC is aggressive, anatomical site and precocious diagnosis are relevant factors for better prognosis.
Keywords: Salivary duct carcinoma; Minor salivary gland; Alveolar ridge; Soft palate
313506Introduction
Salivary duct carcinoma was first described in 1968 by Kleinsasser et al., [1] and it was recognized in the World Health Organization classification of salivary gland tumors in 1991 [2]. SDC is an uncommon and high-grade malignant tumor occurring predominantly in major salivary glands [3,4] with predilection for elderly men [5,6]. SDC is infrequent in minor salivary glands having resemblance to the ductal carcinoma of breast. SDC arises de novo or develops as the malignant component of carcinoma ex pleomorphic adenoma. SDC has invasive growth resulting in early regional and distant metastasis including lungs, liver and bones [4,7], and nearly 50% of the patients die of the disease within 4 to 5 years [8,9].
We report two cases of SDC arising of the minor salivary glands with different clinical behaviors.
Case reports
Case 1
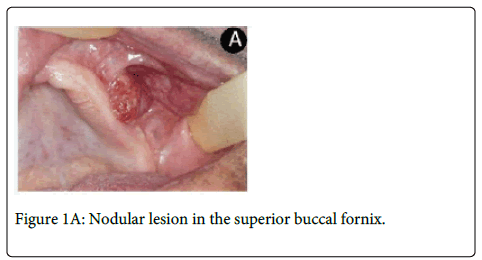
An 80-year-old man was referred to the Oral Diagnostic Clinic for evaluation of a lesion in left upper buccal fornix with two months of evolution. On clinical examination, it was observed a painless ulcerated nodule with 2.0 x 1.0 cm that impeded the proper use of upper prosthesis (Figure1A).
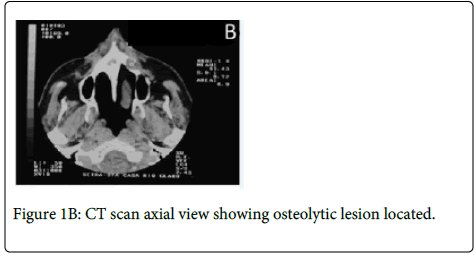
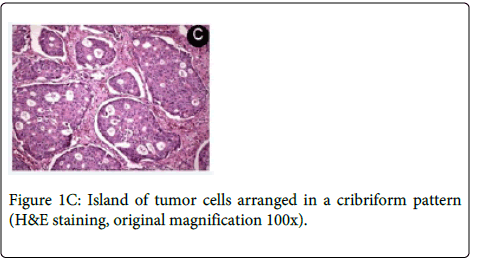
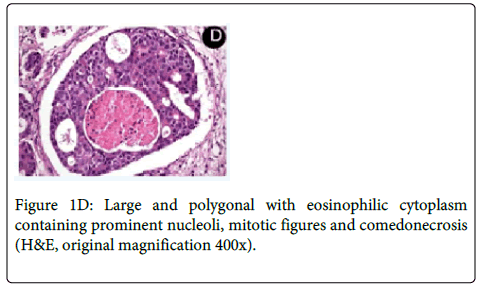
Medical history revealed that the patient had a prostate cancer surgically removed 8 years ago. Computed Tomography (CT) revealed a large osteolytic lesion without defined limits located in the left posterior maxilla without involvement of maxillary sinus (Figure 1B). The patient underwent incisional biopsy and the histopathological examination showed large and polygonal cells with eosinophilic cytoplasm containing prominent nucleoli arranged in cribriform and papillary pattern. Mitotic figures and prominent comedonecrosis were also noted highly suggestive of salivary duct carcinoma (Figure 1C and 1D).
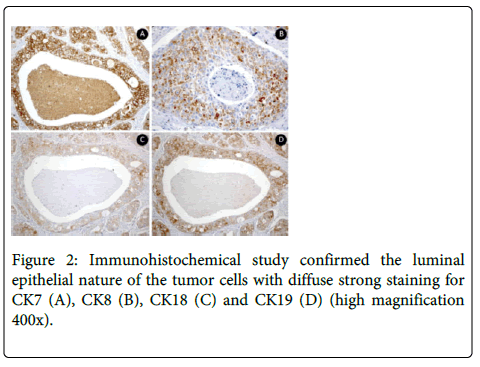
Absence of hemorrhage and perineural and vascular invasion were observed. No invasion to surrounding tissue was found. Immunohistochemical study confirmed the luminal epithelial nature of the tumor cells with strong staining for CK7, CK8, CK18, CK19 and PSA (Figure 2).
It was observed negativity for p63. The Ki-67 index in the tumoral cells was 5%.
The patient was referred to the head and neck surgeon who requested chest x-ray, gastrointestinal endoscopy, prostate ultrasound, bone scintigraphy and laboratory tests (PSA), which ruled out the possibility of metastasis or other primary tumors. The patient was staged as T1N0M0 and a maxillectomy was performed. The specimen measured 1.8x1.0 cm and the histopathologic analysis of the surgical specimen confirmed the diagnosis of SDC and showed free surgical margins. The tumor was well delimited. At six years of follow-up, the patient is alive without evidence of recurrence or metastasis.
Case 2
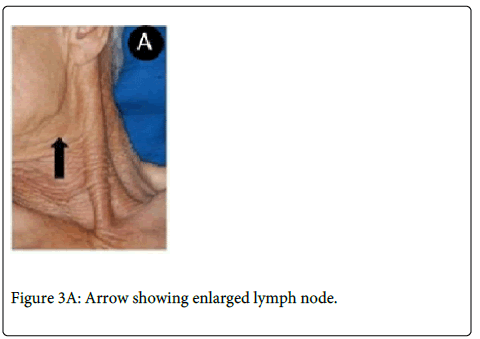
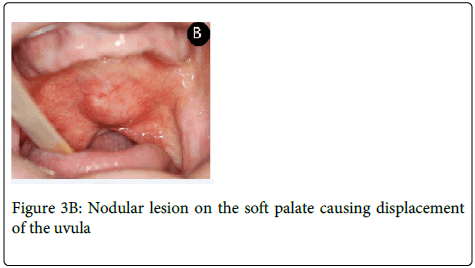
A 64-year-old man was referred complaining of a painless nodule in the palate for five months. Extraoral evaluation revealed cervical lymph nodes bilaterally suggestive of metastasis. On intraoral examination it was observed a nodular lesion involving the soft palate, measuring approximately 3.0x2.5 cm displacing the uvula to the right side (Figure 3A and 3B).
The patient underwent incisional biopsy and the microscopical findings showed a proliferation of carcinomatous islands and scattered individual cells immersed in a highly hyalinized connective tissue. The neoplastic cells formed solid islands in a ductal pattern. The luminal cells were eosinophilic with hyperchromatic nuclei and conspicuous central nucleoli. The diagnosis was of invasive adenocarcinoma.
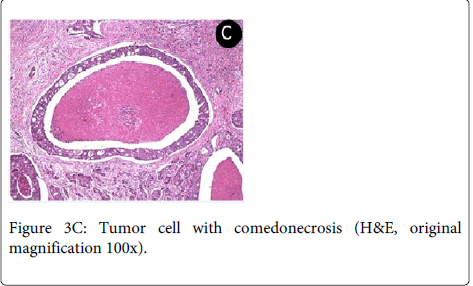
The patient was referred to head and neck surgeon, who staged the patient as T2N2cM0 (TNM system) through image examination (CT). The surgeon performed a radical tumor resection with bilateral neck dissection. The histopathological analysis of surgical specimen revealed that the tumor consisted of solid and cribriform cell nests with ductal structures, comedonecrosis, frequent mitoses and intense neural and vascular invasion allowing the final diagnosis of SDC (Figure 3C).
The immunohistochemical findings were the same case 1: positivity for CK7, CK8, CK18, CK19 and PSA, negativity for p63 and Ki-67 index in the tumoral cells was 10%.
However, the patient died after one month.
Discussion
Salivary duct carcinoma is uncommon and aggressive adenocarcinoma that occurs almost exclusively in the major salivary glands. Reports of SDCs involving minor salivary glands of the oral cavity are rare (Table 1) [1,5,6,10-29].
| Author | Age/Gender | Site | Size (cm) | Clinical status (UICC) | Treatment | Follow-Up |
|---|---|---|---|---|---|---|
| Kleinsasser et al. [1] | 57/M | Hard palate | “Hazelnut in size” | NI | Extirpation | NI |
| Chen [10] | 60/F | Tongue | “Lump” | NI | Wide excision | NED 5 years |
| Pesce et al. [11] | 62/F | Oral vestibule | 1.5 | NI | Excision | NI |
| Zohar et al. [12] | 47/M | Upper lip | 1 | NI | Wide excision | NED 2 years |
| Watatani et al. [13] | 60/F | Tongue | 0.4 | T1N0M0 | Wide excision | NED 4 years |
| Kumar et al. [14] | NI (2 cases) | Maxilla | NI | NI | NI | NI |
| Delgado et al. [15] | 42/M | Hard palate | 3 | T2N0M0 | Maxillectomy | DOD 5.2 years |
| Epivatianos et al. [16] | 80/M | Upper vestibular sulcus | 2.5 | T2N0M0 | Maxillectomy | NI |
| 74/M | Lower vestibular sulcus | 4 | T2N2cM0 | Wide excision-ND | DOD 2 years | |
| 58/M | Hard palate | 4 | T2N2bM0 | RT | DOD 6 months | |
| 60/M | Buccal sulcus | 3 | T2N0M0 | Maxillectomy-ND-RT | NED 4 years | |
| Yoshimura et al. [5] | 62/M | Buccal mucosa | 3.1 | T2N2bM0 | Radical surgery-RT | Alive after 1.6 years with multiple metastases |
| Tatemoto et al. [6] | 58/F | Hard palate | 1 | T1N0M0 | Local resection | NED 2.5 years |
| Guzzo et al. [17] | NI | Cheek | NI | TxN0M0 | Enucleated | Alive with disease (54 months) |
| Suzuki & Hashimoto [18] | 56/M | Mandible | 4 | NI | Excision | NED 5 years |
| Lopes et al. [19] | 63/M | Hard palate | 6 | T4N2bM0 | Hemimaxillectomy-ND-RT | DOD 11 months |
| Huh et al. [20] | 61/M | Hard palate | 5 | T3N0M0 | Hemimaxillectomy-ND-RT | NED 9 months |
| 54/M | Hard palate | 5 | T3N0M0 | Hemimaxillectomy | NED 15 months | |
| 23/F | Hard Palate | 4 | T4N0M1 | Patient denied further treatment | DOD 7 months | |
| Van Heerden et al. [21] | 53/F | Hard palate and right alveolar ridge | 14 | T4N-M0 | Patient refused treatment | Lost to follow-up |
| 71/M | Hard palate and buccal sulcus | 5 | T4N-M0 | Patient refused treatment | Lost to follow-up | |
| 57/M | Hard palate | 6 | T4N-M0 | Patient refused | Lost to follow-up | |
| treatment | ||||||
| 63/F | Hard palate | 7 | T4N-M0 | Hemimaxillectomy-ND-RT | NED 10 Months | |
| 47/M | Hard palate | 5 | T4N-M0 | Patient still considering surgical treatment | NI | |
| Cheuk et al. [22] | 44/F | Buccal mucosa | 1.2 | T1N0M0 | Local excision | NED 4 years |
| Ide et al. [23] | 45/F | Retromolar gingiva | 1.5 | T1N0M0 | Local excision | NED 11 years |
| Jaehne et al. [24] | NI (5 cases) | NI | NI | stage II (n=1), stage IV(n=4) | NI | NED (20%), DOD (80%) |
| Ponniah et al. [25] | 26/M | Hard palate | 3 | T2N0M0 | Local excision | NED 4 years |
| Suzuki et al. [26] | 64/F | Tongue | 1.5 | T1N0M0 | Partial glossectomy | NED 2.5 years |
| Kikuchi et al. [27] | 62/M | Mandible | ND | stage III | Hemimandibulectomy-ND-RT-ChT | NED 5 years |
| Dhanuthai et al. [28] | NI | Alveolar mucosa | NI | NI | NI | NI |
| Thamilselvi et al. [29] | 35/F | Soft palate | 5.5 | T3N0M0 | Local excision | NI |
| Present cases | 80/M | Buccal fornix | 1 | T1N0M0 | Hemimaxillectomy | NED 6 years |
| 64/M | Soft palate | 3 | T2N2cM0 | Radical tumor resection-ND | DOD 1 month |
ChT, chemotherapy; DOD, died of disease; F, female; M, male; N/A, not available; ND, neck dissection; NED, no evidence of disease; RT, radiotherapy; NI, no information.
Table 1: Salivary duct carcinoma in minor salivary gland in the English-language literature
To the best of our knowledge, only 37 cases of SDC originating in the intraoral minor salivary glands have been reported in the English-language literature. The most frequent site is the hard palate (14 cases). We related the first case of SDC in the buccal fornix and the second case of SDC in the soft palate. SDC most frequently affects older male patients in the fifth or sixth decade of life [30]. The mean age of the current patients was 72 years (64 and 80 years) and both patients were male.
Salivary duct carcinoma can occur de novo or as the malignant component of carcinoma ex pleomorphic adenoma [31]. SDC can be classified into three subtypes, according to intraductal or infiltrative predominance: 1) predominantly intraductal, where 90% of the tumor is intraductal; 2) predominantly infiltrative, when less than 20% of the tumor is intraductal; or 3) infiltrative, when the tumor is entirely infiltrative [15]. After histopathological analysis of the 2 presented cases, we noted that both cases were predominantly intraductal corresponding to the more frequent subtype.
Salivary duct carcinoma shows a remarkable resemblance to ductal carcinoma of the breast [15,32,33]. Because of this similarity, after the biopsy in the current cases and given a diagnosis of SDC, the possibility of metastatic breast carcinoma was excluded because the current patients were male. In female patients, studying estrogen, progesterone receptors and immunohistochemical analysis for HER-2 protein may be contributive [34]. In addition, SDC must be differentiated from other malignant salivary gland tumors, including high-grade mucoepidermoid carcinoma, adenoid cystic carcinoma and oncocytic carcinoma [19,32,35].
The main microscopic finding includes an intraductal component, comprising proliferating ductal cells with varying degrees of nuclear pleomorphism arranged in architectural patterns including solid, ‘‘Roman bridge’’, papillary and cribriform structures, often with prominent comedonecrosis [33]. The stroma is densely fibrous or desmoplastic. Angiolymphatic, perineural and bone invasion are common [4,36,37].
Immunohistochemical studies can confirm the luminal epithelial nature of the tumor cells [38]. SDC is immunoreactive for low- and high-molecular-weight cytokeratin. It was shown by immunohistochemical analysis performed in case 1 of the current study. Tumor cells had diffuse strong reactivity for cytokeratin CK7, CK8, CK18 and CK19. In addition, SDC is immunoreactive for Carcinoembryonic Antigen (CEA), Epithelial Membrane Antigen (EMA) [4,15], strong nuclear reactivity for Androgen Receptors (AR) [32] and high proliferative activity for MIB1 (Ki-67) [3,8]. SDC cells are negative for S-100 protein, myoepithelial markers, estrogen and progesterone receptors. Most SDC cells have positive distinct membrane staining for HER-2/neu protein [39,40] and show variable expression for prostatic markers [41].
The invasive nature of salivary duct carcinoma requires radical surgical treatment. Adjuvant radiotherapy or chemotherapy may be indicated and is based particularly on the postoperative pathologic findings such as grade of malignancy, bone or perineural invasion [42]. Insufficient resection with positive margins, perineural invasion, lymphatic embolism, lymphatic invasion, local or regional recurrence and metastasis are factors of poor prognosis [24,43-45]. Local recurrence occurs in 35-66% of patients and distant metastasis in 50-70%, [4,17] and the more common sites are lungs, bone, brain, skin, liver and thyroid gland [46-48]. Approximately 50% of the patients die of the disease within 4 to 5 years [8,9,49]. In the case 2, the patient developed bilateral cervical lymph node metastasis probably because the size and the site of primary tumor affecting the soft palate close to the midline. These facts favored the tumor spread and consequently a poor prognosis.
Carcinomas of minor salivary glands are staged (TNM system) according to their anatomic site of origin, similar to other carcinomas [50]. Spiro et al. (1991) [51] have applied the criteria used for squamous cell carcinoma to mucoepidermoid carcinoma of minor salivary glands. Thus, the patients reported were classified as Stage I or T1N0M0 (case 1) and Stage IVA or T2N2cM0 (case 2).
In the current study, both patients were treated with radical surgical excision. The patient of the case 1 diagnosed with SDC in upper buccal fornix had no local or distant metastases and is free of disease with more than 6 years of follow-up. The patient of the case 2 who had SDC in the soft palate, besides the resection of the primary tumor, also underwent bilateral neck dissection with confirmed lymph node metastasis. No adjuvant therapy was performed because the patient died shortly.
Local invasion, frequent lymphatic and hematogenous metastasis, and poor prognosis characterize the biologic behavior of salivary duct carcinoma. Several studies have described some correlation of prognosis to tumor size (less than 3 cm indicated a better prognosis) [2]. Zohar et al. (1988) showed the highly aggressive biological behavior of the tumor when occurring in the major salivary glands, in contrast to the benign course of the salivary duct carcinoma in the minor salivary gland.
In summary, SDC is an uncommon tumor in minor salivary glands. Although, it is aggressive and has high possibility of developing local and distant metastasis, besides several factors, anatomical location and clinical stage of the tumor are relevant and may interfere with the clinical course of the tumor.
Acknowledgment
The authors thank Fabiana F. Casarotti (University of Campinas) for the immunohistochemistry assistance.
References
- Kleinsasser O, Klein HJ, Hübner G (1968) Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma. Arch Klin Exp Ohren Nasen Kehlkopfheilkd 192: 100-105.
- Seifert G, Sobin LH (1991) Histological Typing of Salivary Gland Tumors. (2ndedn). World Health Organization International Histological Classification of Tumours. Springer-Verlag, New York, USA.
- Barnes L, Rao U, Krause J, Contis L, Schwartz A, et al. (1994) Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol 78: 64-73.
- Lewis JE, McKinney BC, Weiland LH, Ferreiro JA, Olsen KD (1996) Salivary duct carcinoma. Clinicopathologic and immunohistochemical review of 26 cases. Cancer 77: 223-230.
- Yoshimura Y, Tawara K, Yoshigi J, Nagaoka S (1995) Concomitant salivary duct carcinoma of a minor buccal salivary gland and papillary cystadenomalymphomatosum of a cervical lymph node: Report of a case and review of the literature. J Oral MaxillofacSurg 53:448-453.
- Tatemoto Y, Ohno A, Osaki T (1996) Low malignant intraductal carcinoma on the hard palate: a variant of salivary duct carcinoma? Eur J Cancer B Oral Oncol 32B: 275-277.
- McHugh JB, Visscher DW, Barnes EL (2009) Update on selected salivary gland neoplasms. Arch Pathol Lab Med 133: 1763-1774.
- Wee DT, Thomas AA, Bradley PJ (2012) Salivary duct carcinoma: what is already known, and can we improve survival? J LaryngolOtol 126 Suppl 2: S2-7.
- Grenko RT, Gemryd P, Tytor M, Lundqvist PG, Boeryd B (1995) Salivary duct carcinoma. Histopathology 26: 261-266.
- Chen KT (1983) Intraductal carcinoma of the minor salivary gland. J LaryngolOtol 97: 189-191.
- Pesce C, Colacino R, Buffa P (1986) Duct carcinoma of the minor salivary glands: a case report. J LaryngolOtol 100: 611-613.
- Zohar Y, Shem-Tov Y, Gal R (1988) Salivary duct carcinoma in major and minor salivary glands. A clinicopathological analysis of four cases. J CraniomaxillofacSurg 16: 320-323.
- Watatani K, Shirasuna K, Aikawa T, Matsuya T (1991) Intraductal carcinoma of the tongue. Report of a case. Int J Oral MaxillofacSurg 20: 175-176.
- Kumar RV, Kini L, Bhargava AK, Mukherjee G, Hazarika D, et al. (1993) Salivary duct carcinoma. J SurgOncol 54: 193-198.
- Delgado R, Vuitch F, Albores-Saavedra J (1993) Salivary duct carcinoma. Cancer 72: 1503-1512.
- Epivatianos A, Dimitrakopoulos J, Trigonidis G (1995) Intraoral salivary duct carcinoma: a clinicopathological study of four cases and review of the literature. Ann Dent 54: 36-40.
- Guzzo M, Di Palma S, Grandi C, Molinari R (1997) Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck 19: 126-133.
- Suzuki H, Hashimoto K (1999) Salivary duct carcinoma in the mandible: report of a case with immunohistochemical studies. Br J Oral MaxillofacSurg 37: 67-69.
- Lopes MA, de Abreu Alves F, Levy BA, de Almeida OP, Kowalski LP (2001) Intraoral salivary duct carcinoma: case report with immunohistochemical observations. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 91: 689-692.
- Huh KH, Heo MS, Lee SS, Choi SC (2003) Three new cases of salivary duct carcinoma in the palate: a radiologic investigation and review of the literature. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 95: 752-760.
- van Heerden WF, Raubenheimer EJ, Swart TJ, Boy SC (2003) Intraoral salivary duct carcinoma: a report of 5 cases. J Oral MaxillofacSurg 61: 126-131.
- Cheuk W, Miliauskas JR, Chan JK (2004) Intraductal carcinoma of the oral cavity: a case report and a reappraisal of the concept of pure ductal carcinoma in situ in salivary duct carcinoma. Am J SurgPathol 28: 266-270.
- Ide F, Mishima K, Saito I (2004) Circumscribed salivary duct carcinoma of the palate: a non-threatening variant. Histopathology 45: 89-91.
- Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, et al. (2005) Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer 103: 2526-2533.
- Ponniah I, Murali GM, SureshKumar P, Kumaran MG, Shaheen A (2005) Salivary duct carcinoma of the palate. Indian J Dent Res 16: 167-170.
- Suzuki M, Suzukawa K, Ogawa M, Suzuki T (2006) Salivary duct carcinoma with comedonecrosis in the mobile portion of the tongue. J LaryngolOtol 120: e13.
- Kikuchi Y, Hirota M, Iwai T, Aoki S, Chikumaru H, et al. (2007) Salivary duct carcinoma in the mandible: a case report. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 103: e41-46.
- Dhanuthai K, Boonadulyarat M, Jaengjongdee T, Jiruedee K (2009) A clinico-pathologic study of 311 intra-oral salivary gland tumors in Thais. J Oral Pathol Med 38: 495-500.
- Thamilselvi R, Subramaniam PM, Shivarudrappa AS, Venugeethan A, Sinha P (2010) Sarcomatoid salivary duct carcinoma of minor salivary gland: a rare case. Indian J PatholMicrobiol 53: 808-810.
- Hosal AS, Fan C, Barnes L, Myers EN (2003) Salivary duct carcinoma. Otolaryngol Head Neck Surg 129: 720-725.
- Lewis JE, Olsen KD, Sebo TJ (2001) Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol 32: 596-604.
- Simpson RH, Prasad AR, Lewis JE, Skálová A, David L (2003) Mucin-rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four cases. Am J SurgPathol 27: 1070-1079.
- Simpson RH, Desai S, Di Palma S (2008) Salivary duct carcinoma in situ of the parotid gland. Histopathology 53: 416-425.
- Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS (2007) Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck 29: 907-912.
- Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, et al. (2004) Low-grade salivary duct carcinoma: description of 16 cases. Am J SurgPathol 28: 1040-1044.
- Murrah VA, Batsakis JG (1994) Salivary duct carcinoma. Ann OtolRhinolLaryngol 103: 244-247.
- Brandwein MS, Jagirdar J, Patil J, Biller H, Kaneko M (1990) Salivary duct carcinoma (cribriform salivary carcinoma of excretory ducts). A clinicopathologic and immunohistochemical study of 12 cases. Cancer65:2307-2314.
- Wick MR, Ockner DM, Mills SE, Ritter JH, Swanson PE (1998) Homologous carcinomas of the breasts, skin, and salivary glands. A histologic and immunohistochemical comparison of ductal mammary carcinoma, ductal sweat gland carcinoma, and salivary duct carcinoma. Am J ClinPathol109:75-84.
- Skálová A, Stárek, Kucerová V, Szépe P, Plank L (2001) Salivary duct carcinoma--a highly aggressive salivary gland tumor with HER-2/neu oncoprotein overexpression. Pathol Res Pract 197: 621-626.
- Skálová A, Stárek I, Vanecek T, Kucerová V, Plank L, et al. (2003) Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology 42: 348-356.
- Fan CY, Wang J, Barnes EL (2000) Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J SurgPathol 24: 579-586.
- Jamal AM, Sun ZJ, Chen XM, Zhao YF (2008) Salivary duct carcinoma of the parotid gland: case report and review of the literature. J Oral MaxillofacSurg 66: 1708-1713.
- BenJelloun H, Maazouzi A, Benchakroun N, Acharki A, Tawfiq N, et al. (2004) [Salivary duct carcinoma of the parotid gland: report of two cases and literature review]. Cancer Radiother 8: 383-386.
- Sato K, Shimode Y, Itoi A, Ueda Y, Katsuda S (2007) Salivary duct carcinoma of the parotid gland presenting KIT (CD117) overexpression. Histopathology 51: 114-115.
- Yamamoto H, Uryu H, Segawa Y, Tsuneyoshi M (2008) Aggressive invasive micropapillary salivary duct carcinoma of the parotid gland. PatholInt 58: 322-326.
- Aygit AC, Top H, Cakir B, Yalcn O (2005) Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol 27: 48-50.
- Valeri RM, Hadjileontis C, Skordalaki A, Pandidou A, Vahtsevanos C, et al. (2005) Salivary duct carcinoma of the parotid gland: report of a rare case with a comparative study of aspiration cytology and histomorphology. ActaCytol 49: 61-64.
- Bhalla R, Parker DC, Tadros TS (2006) Salivary duct carcinoma metastatic to inguinal lymph node: a case report of salivary duct carcinoma with distant metastasis diagnosed by fine-needle aspiration. DiagnCytopathol 34: 41-44.
- Colecchia M, Frigo B, Leopardi OM (1997) Salivary duct carcinoma of the parotid gland. Report of a case with cytologic and immunocytochemical findings on fine needle aspiration biopsy. ActaCytol 41: 593-597.
- Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, et al. (2002) American Joint Committee on Cancer.Cancer Staging Manual. Springer, New York, USA.
- Spiro RH, Thaler HT, Hicks WF, Kher UA, Huvos AH, et al. (1991) The importance of clinical staging of minor salivary gland carcinoma. Am J Surg 162: 330-336.
Citation: Gondak RO, Mariano FV, Alves FA, Almeida OP, Lopes MA (2014) Salivary Duct Carcinoma in Minor Salivary Glands: Report of Two Cases with Different Clinical Behavior. J Clin Exp Pathol 4:164. DOI: 10.4172/2161-0681.1000164
Copyright: © 2014 Gondak RO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16775
- [From(publication date): 3-2014 - Jul 04, 2025]
- Breakdown by view type
- HTML page views: 12042
- PDF downloads: 4733