Scintigraphy in Functional Neuroendocrine Tumors with Review of Literature
Received: 15-Oct-2018 / Accepted Date: 17-Oct-2018 / Published Date: 30-Oct-2018
Abstract
Neuroendocrine neoplasms can arise at multitude of sites as they originate from neuroendocrine cells of diffuse endocrine system. Tumor markers aid in suspecting this entity and immunohistochemistry provides definitive diagnosis. All imaging modalities have their own place. Computed tomography and MRI are helpful in anatomical localisation of these tumors. However, many a times these tumors are small and slow growing and hence localisation is difficult. Scintigraphy provides functional characteristics of the tumors increasing the specificity in addition to sensitivity with whole body scans. Radionuclide methods have evolved over the years with development of new radiotracer molecules. Present series illustrates the evaluation of NET group of tumors using SPECT as well as PET imaging agents as they became available in clinical practice. Attempt has been made to present as much data as possible including histological photomicrographs in several cases.
Keywords: NET; Pheochromocytoma; Carcinoid; Gastrinoma; Insulinoma; DOTA; Somatostatin receptor analogue
Introduction
Neuroendocrine tumours (NET) are neoplasms that are derived from neuroendocrine cells of diffuse endocrine system located in various organs [1]. These comprise of cells with dual properties i.e., the neuro property by virtue of dense core granules and the endocrine property based on the property of these cells to secrete monoamines [2]. These may originate in endocrine glands such as adrenal medulla, pituitary, parathyroid gland or endocrine islet cells of thyroid, pancreas, GI tract, liver, respiratory tract. For ease of understanding these may be classified according to their origin i.e., foregut, midgut and hindgut tumor [3]. Foregut tumors could arise in thymus, lung, esophagus, stomach, duodenum and pancreas. Midgut tumors arise in appendix, ileum, caecum and ascending colon. Hind gut tumors could be in distal colon and rectum. Lung and GI tract is the most common site of NET [4]. They secrete serotonin, catecholamine, histamine (amines) as well as somatostatin and gastrin (polypeptide hormones). Functional hormones such as insulin, glucagon, calcitonin, proinsulin, pancreatic polypeptide, vasoactive intestinal peptide, C-peptide, 5 hydroxyindole acetic acid (5 HIAA) chromogranin A are various other hormones secreted by NET [5,6]. Majority of NET express somatostatin receptors and can be visualised with radio-labelled somatostatin analogues [7]. There are 1 to 5 SSTR with 2SSTR being most frequent followed by SSTR1 and SSTR 5 and then by SSTR3 and SSTR4 in that order of frequency [8]. Both single photon emitters (Technetium-99m; Indium-111; Iodine-123) and positron emitters have been labelled with somatostatin analogue for imaging. Somatostatin is a 14 chain amino acid peptide indigenously produced in hypothalamus where it regulates secretion of growth hormone, as well as pancreas where it regulates secretion of insulin, glucagon and gastrin. Two naturally occurring bioactive forms of somatostatins are SMS-14 and SMS 28 [9]. Primary action of these peptide hormones is:
1. Inhibition of hormone secretion.
2. Modulation of neurotransmission and cell proliferation through specific membrane bound G protein-coupled receptors.
Octreotide is a synthetic analogue of somatostatin comprising of 8 amino acids with a half-life of 2-3 hours [10]. It is relatively resistant to enzyme degradation. It has been shown to bind to somatostatin receptors both in vivo and in vitro. Certain radioactive tracers can be labelled with octreotide analogues to enable imaging in NET (called Somatostatin receptor scintigraphy - SRS). Present atlas illustrates various functional NET studies performed at our clinic over past few years. F18-FDG PET CT has proved ineffective in well differentiated NET [11]. However, it is useful in high grade neuroendocrine carcinomas [12].
Specific radiotracer compounds are available for evaluating functional neuroendocrine tumors. Iodine131- MIBG (Metaiodobenzyle Guanidine), C11-ephedrine, F18-DOPA for pheochromocytoma. Ga68-GLP1R (Glucagon like peptide receptor 1) for insulinoma. Functional NET are not encountered very frequently in clinical practice. When suspected clinically the practicing as well as referring physician must know the merits and limitations of radioisotope studies. The present atlas illustrates various functional NET studies performed at our clinic will over past few years and it will hopefully be useful to the practicing physician.
Pheochromocytoma
Pheochromocytomas are catecholamine producing tumors that when oxidized on immersion with chromate salt, turn brown (Phaios-dusky brown; Chromo-color; cytoma-tumor). This is called as chromaffin reaction. Pheochromocytomas can be adrenal or extraadrenal. Functional triad comprises of palpitation, sweating and headache associated with hypertension (paroxysmal or constant). Rule of 10% include: 10% are bilateral, 10% familial, 10% occur in children, 10% not associated with hypertension, 10% are extra-adrenal, 10% malignant and 10% contain calcification [13]. Imaging can be performed with specific or nonspecific agents.
Specific SPECT agents
Radiolabelled ligands that enter the catecholamine synthesis, uptake or storage pathway are classified as specific agents whereas those that target metabolism or somatostatin receptor expression are grouped as nonspecific [14].
I-131-MIBG is a catecholamine precursor taken by tumor cells by human norepinephrine transporter pathway. It is sequestered in storage granules of chromaffin cells.
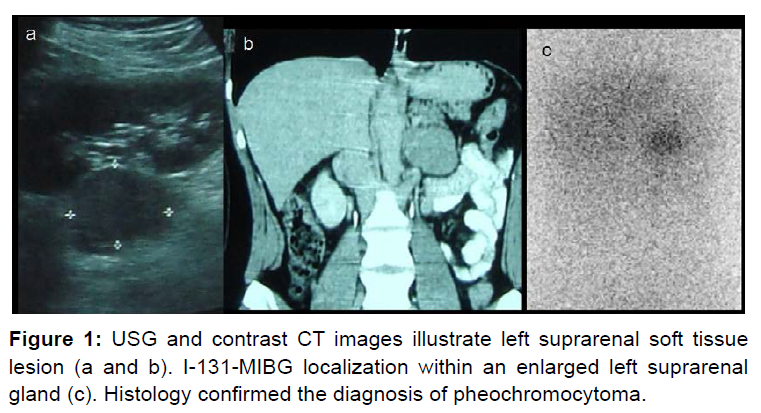
I-131-MIBG has sensitivity of 88% (79-91%) and specificity of 95% (88-99%) [15]. Sensitivity is superior for adrenal pheochromocytoma than extra-adrenal paragangliomas [16]. I-123 MIBG is devoid of beta radiation and has higher sensitivity. However, it has limited availability. Figure 1 illustrates a case of left adrenal pheochromocytoma diagnosed on I131-MIBG scan.
Nonspecific SPECT agents
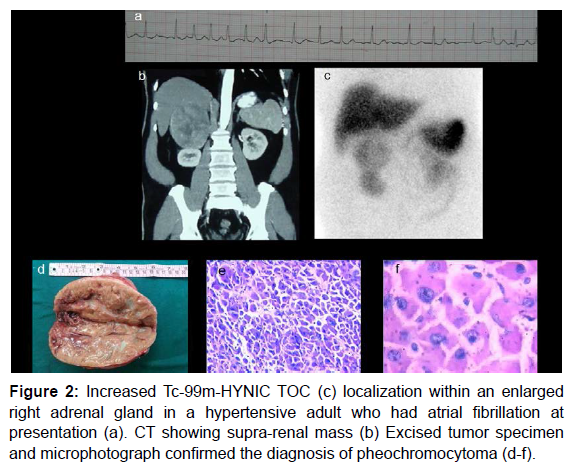
A high density of somatostatin receptors is found in many neuroendocrine tumors [17,18]. In vivo demonstration of somatostatin receptor positive tumor was done initially using radio iodinated somatostatin analogue [19]. Octreotide is a synthetic analogue of somatostatin (8 Amino Acid as against 14) that can be labelled with Indium 111 and was found to be superior to I123-MIBG in head and neck paragangliomas (81% versus 61%) [20]. In111- octreotide is expensive and not readily available in our country. Technetium- 99m- hydrazine-nicotinyl- tyr3-octeotide (HYNIC TOC) is another radiotracer that localizes on somatostatin receptors. It has been shown to be superior to I-131-MIBG in detection of extra-adrenal pheochromocytoma [21]. Figure 2 demonstrates a case showing utility of Tc-99m- HYNIC TOC in adrenal pheochromocytoma.
Specific PET molecules
Ephedrine is a catecholamine that resembles norepinephrine and is a substrate for monoamine oxidase and catechol-O methyl transferase. C11-Ephedrine has a half-life of 20 minutes and can be used for imaging pheochromocytoma [22]. Dopamine is a catecholamine precursor and when labelled with Fluorine-18 (half-life 110 minutes) has been found to be superior to MIBG for detection of pheochromocytoma [23].
DOPA (dihydroxyphenylalanine) is converted to dopamine and is transported through human norepinephrine transporter. This can be labelled with F18 (F18-DOPA) with excellent results [24]. Both these radiotracer compounds are not available readily.
Nonspecific PET molecules
Pheochromocytomas that are negative on specific imaging modalities may be further evaluated with nonspecific imaging molecules such as F18-FDG (fluorodeoxyglucose) and discordant results have been reported earlier [25]. Pheochromocytomas and paragangliomas express somatostatin receptors [2,3,5]. Gallium68-Somatostatin has been shown to have high sensitivity for pheochromocytomas [26].
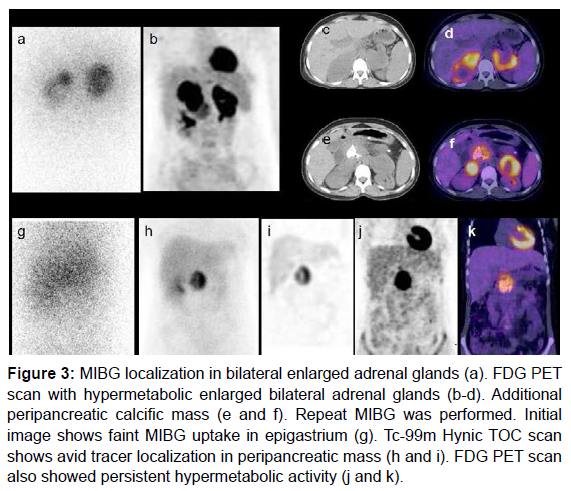
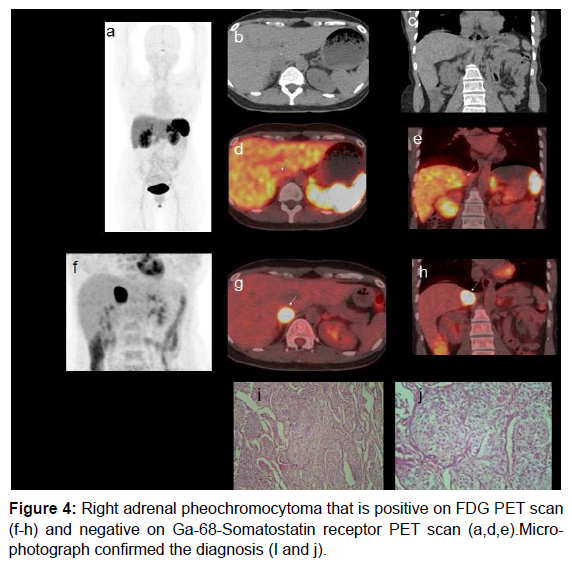
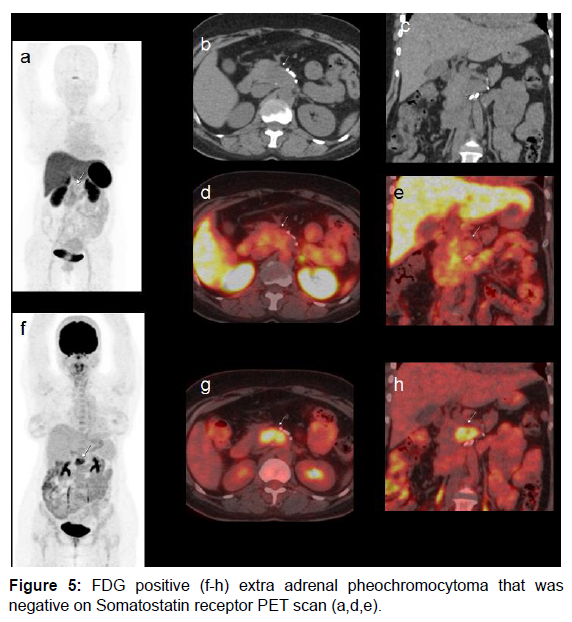
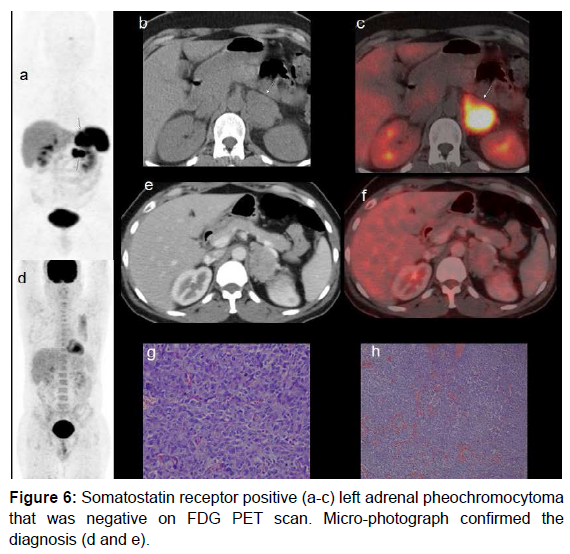
In our case there was discordance observed between F18-FDG and Ga68-Somatostatin receptor PET molecules (both nonspecific agents). Figure 3 shows utility of multiple radiotracers (I131-MIBG, F18-FDG and Tc99-m-HynicTOC) in diagnosis of adrenal and extra-adrenal pheochromocytoma. Ga68-DOTA TOC scan was not available in this period. Figures 4 and 5 show cases with adrenal and extra-adrenal pheochromocytomas, which were positive on FDG and negative on somatostatin receptor PET scan. Whereas case in Figure 6 shows the reverse (Flip-Flop phenomenon).
Figure 3: MIBG localization in bilateral enlarged adrenal glands (a). FDG PET scan with hypermetabolic enlarged bilateral adrenal glands (b-d). Additional peripancreatic calcific mass (e and f). Repeat MIBG was performed. Initial image shows faint MIBG uptake in epigastrium (g). Tc-99m Hynic TOC scan shows avid tracer localization in peripancreatic mass (h and i). FDG PET scan also showed persistent hypermetabolic activity (j and k).
Carcinoid
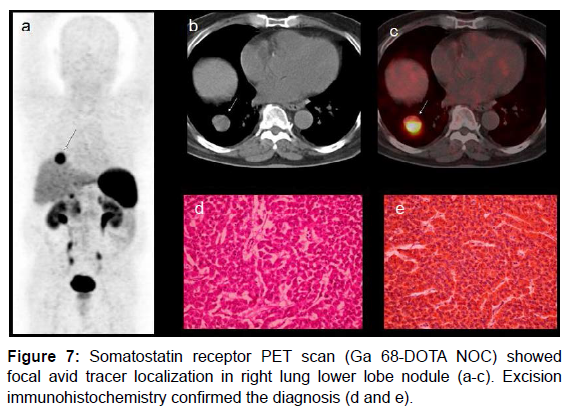
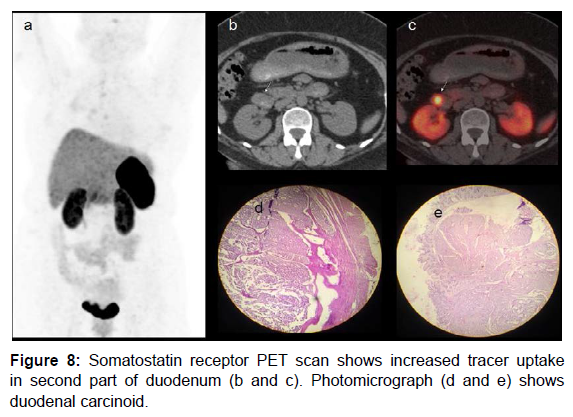
Carcinoid tumor was initially described as “karzinoide” because of less aggressive nature than gastrointestinal carcinomas [27]. Carcinoid tumors secrete serotonin that is metabolised to 5HIAA (hydroxylindole- acetic acid) and Chromogranin (both are tumor markers). Bronchial carcinoids may produce paraneoplastic manifestations by secreting ACTH (Cushing’s syndrome). 75% of carcinoids are seen in abdomen and 25% occur in lung [28]. Carcinoid tumors express high concentration of somatostatin receptors. Carcinoid syndrome comprises of flushing, diarrhoea and hyperdynamic circulation. Carcinoids are frequently small and may be detected by USG /CT only when they metastasize to liver [29]. Age related predilection is observed as follows: carcinoid of cervix fourth decade, small intestine and lung in sixth- early seventh decade and rectal carcinoids in late seventh decade [30]. CT scan has limited value in detecting small bowel carcinoids [31]. CT enteroclysis has demonstrated overall accuracy of 84% [32]. Post Gadolinium enhanced fat suppressed MR has shown to detect small gut carcinoids [33]. Duodenal carcinoids could be G cell origin (gastrin producing), D cell origin (somatostatin producing) or rarely enterochromaffin cell origin (serotonin producing) [34]. Our case presented with secretary diarrhoea and was detected with right lung carcinoid on PET scan. Somatostatin receptor PET scan (Ga 68- DOTA NOC) (Figure 7) showed right lung carcinoid. Another case of a 45 yrs. woman with gastritis in whom endoscopic biopsy confirmed carcinoid tumor. Somatostatin receptor PET scan shows increased tracer uptake in second part of duodenum (Figure 8).
Gastrinoma
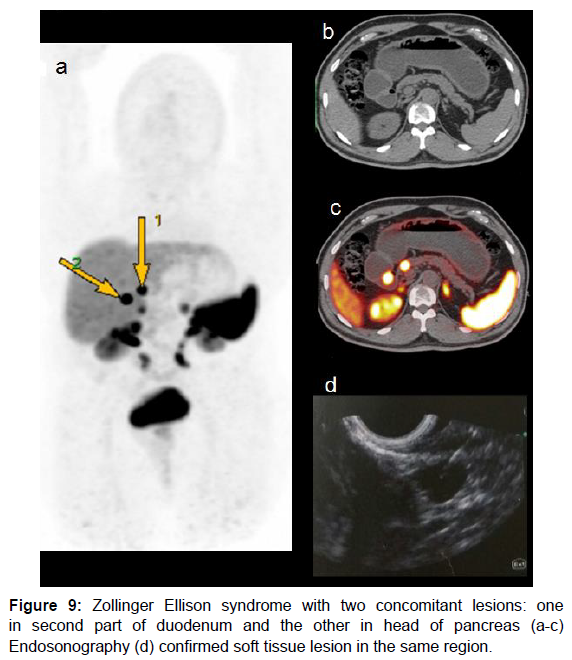
These are the second most common pancreatic NETs. 50-60% may present with liver metastasis [35]. Majority of these tumors are located in gastrinoma triangle formed as follows: superiorly by the junction of cystic duct and common bile duct, inferiorly by second and third part of duodenum and medially by the junction of neck and body of pancreas. These tumors are usually small 0.3 to 3cm and more often extra-pancreatic as compared to insulinoma [35]. Tumors in gastrinoma triangle are supposed to origin from stem cells of ventral pancreatic bud that become embedded in duodenal wall during normal embryonic rotation of ventral pancreas [36]. Our case presented with features of Zollinger Ellison syndrome and has two concomitant lesions: one in second parts of duodenum and the other in head of pancreas (Figure 9).
Insulinoma
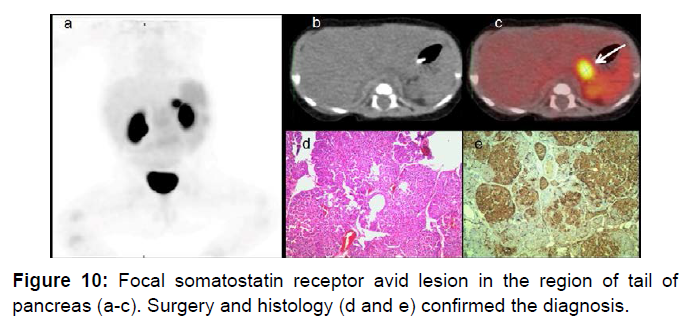
Insulinoma is the most common NET of pancreas. Nesidioblastosis (nesidion- islet; blastos- germ), islet cell dysmaturation syndrome, persistent hyperinsulinemic hypoglycaemia of infancy (PHHI), congenital hyperinsulinism are synonyms. Nesidioblastosis is classified in focal or diffuse type [37]. Whipple’s triad is typical of insulinoma: 1) hypoglycemia <40 mg/dL; 2) high serum insulin level; 3) relief of symptoms with glucose administration. These tumors are evenly distributed one third each in head- uncinate process, body and tail of pancreas [38]. Majority (about 90%) of Insulinomas are smaller than 2 cm [39]. These could be sporadic or in association with MEN 1 syndrome. Somatostatin receptor expression is variable in insulinomas and several studies have shown discouraging results [40]. Selective pancreatic angiography is no longer used due to its unpredictable results [41]. Figure 10 illustrates an infant with focal pancreatic tail insulinoma. Ga68-DOTATOC has been shown to localize in SSTE2 receptor on mRNA thereby proving its tendency to be specific for neuroendocrine tumors that are known to express these. Ga68-DOTATATE was shown to be superior to FDG PET for bronchial carcinoids [42].
Discussion
NET (neuroendocrine tumors) is characterized by their endocrine metabolism and distinct histology pattern. Previously they were classified as APUDOMAs (amine precursor uptake and decarboxylation). Largely these tumors are slow growing and carry good prognosis. Cytotoxic chemotherapy and external beam radiotherapy require high tumor proliferation rate to be effective and hence are redundant in these cases Table 1.
| Definition of grades | ||
|---|---|---|
| Well differentiated NENs | Mitotic index(10/HPF) | Ki67index* |
| Neuroendocrine tumour (NET) G1 | <2% | <2% |
| Neuroendocrine tumour (NET) G2 | 2-20% | 2-20% |
| Neuroendocrine tumour (NET) G3 | 2-20% | 2-20% |
| Poor definition of grades | ||
| Neuroendocrine carcinoma (NEC) G3 | >20% | >20% |
| Small cell type | ||
| Large cell type | ||
Table 1: WHO grading for Pancreatic Neuroendocrine Neoplasms 2017.
Mixed Neuroendocrine-Nonneuroendocrine Neoplasm (MiNEN)
The term mixed adenoneuroendocrine carcinoma (MANEC) was introduced by the World Health Organization in 2010 for a neoplasm with both adenocarcinomatous and neuroendocrine differentiation, with each component representing at least 30% of the entire tumor. Before 2010, these tumors were reported as mixed or composite tumors [43]. Hyperplasia is preneoplastic condition recognised as presence of more than double the normal number of enterochromaffin cells and it can undergo dysplasia and neoplasia as described by Solcia et al. [44]. Neuroendocrine tumors may be functional or non-functional. Accordingly presentation of tumors can be nonspecific in nonfunctional tumors depending on the location of the tumor, and in case of functional tumors it depends upon the type of the primary tumor as well as the effect of the secreted hormones. Various modalities are used in diagnosis of NET depending on the presentation. CT, MRI and USG are common and initial imaging modalities used in diagnosis and treatment evaluation of the NET. Each modality has its own advantages and disadvantages. USG- has the advantage of easy availability, cost effectiveness with no radiation and is commonly used as a screening modality especially for patients with abdominal complains. Its disadvantage is operator dependence and limited sensitivity and specificity if performed without micro-bubble contrast. CT has a major advantage of high spatial resolution of liver and retroperitoneal structures and is useful for pre-surgical planning and disease staging. Its disadvantages are radiation exposure, requirement of intravenous contrast. MRI is a good modality for hepatic and pancreatic lesions with no radiation exposure with gadolinium being safer in renal dysfunction. Its major disadvantage is poor evaluation of gastrointestinal, lung and mesenteric lesions due to motion artefacts. A common disadvantage of all these modalities is they are not a functional in nature. This is overcome by PET and SPECT which are functional imaging modalities used in suspected NETs.
SPECT advantages
1) Staging and re-staging (study of primary tumours and detection of distant metastases.
2) Monitoring/ evaluation of treatment responses.
3) Selection of patients for treatment with “cold” or radiolabelled analogues.
4) Prognosis formulation based on lesion receptor expression.
5) Detection of cancers with unknown primary origin in patients with metastases or high levels of circulating markers.
6) Easy availability.
Disadvantages of SPECT
Disadvantages of SPECT - 1) Due to the physiological distribution of the radiopharmaceutical and its uptake in a variety of benign conditions and non-NETs. 2) Absence/paucity of anatomical landmarks and the limited spatial resolution in the case of small-sized lesions. This is overcome by SPECT CT.
Most of the immune tracers used in PET and SPECT have affinity for somatostatin receptors. Somatostatin receptors are G-protein-coupled membrane glycoproteins. Five subtypes of human somatostatin receptors have been identified viz. sst1-sst5 [45] Gastroentero- pancreatic NETs express somatostatin receptors in 80-100% of cases, although these receptor expression may be less in insulinomas (50-70%). Most abundant is sst2, followed by equal amounts of sst1 and sst5, lower amounts of sst3 and hardly any sst4 [46]. Overall 70- 90% of NETs express sst2 [47]. Somatostatin analogues have affinity for these receptors and hence can be used as a radiotracer in imaging of NETs. Somatostatin receptor scintigraphy has a sensitivity ranging from 75% to 100%.
Characteristics of good Immunotracer
a. It should be possible to pair the radionuclide with the antibody.
b. Physical half-life of radioisotope should be compatible with biologic half-life of tracer.
c. Stability of the compound. Octreotides are commonly used synthetic somatostatin analogues.
SPECT
Hynictoc is a common nonspecific SPECT agent used in suspected NETs. Its advantages are:
1) Prompt examination (i.e., within the first 4 hours of administration of the radiopharmaceutical.
2) Imaging by the gamma camera resulting in satisfactory image quality.
3) Favourable dosimetry for patients (the effective dose corresponds to 8.8-11.8 mSv for a patient weighing 70 kg)
4) Better patient compliance.
Disadvantages of examination with these radiopharmaceuticals are:
1) No possibility of delaying imaging of the lesions beyond 24-48 hours (possible with [111In] pentetreotide.
2) Reduction of diagnostic accuracy due to interference from abdominal background activity (indeed, some non-thoracic lesions can be imaged only with difficulty).
3) Absence (according to the few clinical studies published) of a significant clinical advantage over the consolidated [111In] pentetreotide approach.
PET
The major advantage of PET over SPECT are 1) Better Sensitivity and resolution. 2) The quantitative nature of PET helps to quantify the amount of tracer uptake expressed as standardized uptake values (SUVs). The SUVs are very useful in the planning of Peptide Receptor Radionuclide Therapy (PRRT) and may help in predicting prognosis [48]. Several studies have indicated that positron emitting tracers are better compared with the γ-emitting tracers, both in detection rates and clinical impact [49-51]. 1,4,7,10-tetraazacyclodecane- 1,4,7,10-tetraacetic acid (DOTA), a universal chelator capable of forming stable complexes with radiotracers of the metal group, such as 111In, 67Ga, 68Ga, 64Cu, 90Y and 177Lu [52]. Peptides labelled with 90Y and 177Lu are used for radionuclide therapy, while 68Ga is a most common radio-isotope used for somatostatin receptor imaging. The major advantage of this isotope is that it can be produced from a generator, and hence available without a cyclotron. The most frequently used modifications of octreotide are Tyr3-octreotide, Tyr3- octreotate and l-Nal3-octreotide. When combined with the DOTA chelator and 68Ga, they are called 68Ga-DOTA-Tyr3-octreotide (68Ga- DOTATOC), 68Ga-DOTA-Tyr3- octreotate (68Ga-DOTATATE) and 68Ga-DOTA- l-Nal3-octreotide (68Ga-DOTANOC). Tracer uptake in the uncinate process of the pancreas must be interpreted with caution as physiological uptake in the uncinate process is commonly seen. Distribution of somatostatin receptors in bronchial carcinoids was studied by Reubi and Waser.sst1 and sst2 were detected in 70% of tumors. sst2 had the highest density, sst3 and sst4 were virtually undetected and sst5 was found in 20% and with low density. This distribution might favour the use of DOTATATE, since it is the somatostatin analogue with the highest sst2 affinity [53]. Kayani et al. examined 18 pulmonary NET patients with 68Ga-DOTATATE and found a sensitivity of only 72%. However, the false-negative tumors were all high-grade tumors that were positive on 18F-fluorodeoxyglucose (FDG) scans, while the typical bronchial carcinoids had high and selective uptake [54]. This is flip-flop phenomenon. The “flip-flop” phenomenon of well differentiated NETs showing high DOTATATE and low FDG uptake while the opposite imaging phenotype in poorly differentiated tumors. Heterogeneity may be present with different grade tumors at different sites. This powers FDG and DOTATATE for better selection of management for an individual patient. This was seen in our case of pheochromocytoma which was positive on FDG and negative of GaDOTA. The pattern however is not universal as some highly differentiated tumors do not express somatostatin receptors (e.g., insulinomas) and not all fast growing tumors use glycolytic metabolism for growth.
Treatment with PRRT-A distinct advantage of SRS in NET is its close relationship with theranostics. If an entity is diagnosed with somatostatin receptor scan and is found to be inoperable, then a compound with similar properties /closely related agent may be used to treat the disease. As majority of NETs show somatostatin receptor expression, particularly SST2 receptors, they can be effectively treated with Beta-emitting radionuclide-somatostatin analogues targeted at tumor cells. This has led to increasingly utilize somatostatin receptor agonists for the treatment of patients with advanced metastatic NETs with PRRT [55] 90Y-DOTATOC and 177Lu-DOTATATE are commonly used radio-tagged somatostatin receptor agonists for radionuclide therapy.
Conclusion
Suspected neuroendocrine tumors should be subjected to nuclear imaging which helps in functional as well as anatomical characterisation. Radio-nuclide tracers are helpful in primary diagnosis as well as treatment along with monitoring of treatment response. Awareness about diagnosis, imaging and treatment will be helpful to the patient in long-term survival.
References
- Pearse AG (1977) The diffuse neuroendocrine system and the apud concept: related “endocrine†peptides in brain, intestine, pituitary, placenta, and anuran cutaneous glands. Med Biol. 55: 115-125.
- Basu B, Sirohi B, Corrie P (2010) Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr Relat Cancer 17: R75-90.
- Williams ED, Sandler M (1963) The classification of carcinoid tumours. Lancet 1: 238-239.
- Anaizi A, Rizvi-Toner A, Valestin J, Schey R (2015) Large cell neuroendocrine carcinoma of the lung presenting as pseudoachalasia: a case report. J Med Case Reports 9: 56.
- Modlin IM, Oberg K, Chung DC, Jensen RT, De Herder WW, et al. (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9: 61-72
- Oberg K, Eriksson B (2005) Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 19: 753-781.
- Patel YC (1999) Somatostatin and its receptor family. Front Neuroendocrinol 20: 157-198.
- Kwekkeboom DJ, Krenning EP, Scheidhauer K, Lewington V, Lebtahi R, et al. (2009) ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: somatostatin receptor imaging with (111)In-pentetreotide. Neuroendocrinology 90: 184-189.
- Pradayrol L, Jornvall H, Mutt V, A Ribet, et al. (1980) N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett 109: 55-58.
- Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, et al. (1982) Octreotide: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci 31: 1133-1140.
- Sundin A, Eriksson B, Bergström M, B Langstrom, K Oberg, et al. (2004) PET in the diagnosis of neuroendocrine tumors. Ann N Y Acad Sci 1014: 246-257.
- Pasquali C, Rubello D, Sperti C, P Gasparoni, G Liessi, et al. (1998) Neuroendocrine tumor imaging: can 18Ffluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg 22: 588-592.
- Zuber SM, Kantorovich V, Pacak K (2011) Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am 40: 295-311.
- Shulkin BL, Ilias I, Sisson JC, K Pacak, et al. (2006) Current trends in functional imaging of pheochromocytomas and paragangliomas. Ann N Y Acad Sci 1073: 374-382.
- Shapiro B, Sisson JC, Shulkin BL, MD Gross, S Zempel, et al. (1995) The current status of meta-iodobenzylguanidine and related agents for the diagnosis of neuro-endocrine tumors. Q J Nucl Med 39: 3-8.
- Wiseman GA, Pacak K, O' Dorisio MS, DR Neumann, AD Waxman, et al. (2009) Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. Journal of Nuclear Medicine 50: 1448-1454.
- Reubi JC, Krenning E, Lamberts SW, Kvols L (1992) In vitro detection of somatostatin receptors in human tumors. Metabolism 41: 104-110.
- Lamberts SW, Reubi JC, Krenning EP (1993) Validation of somatostatin receptor scintigraphy in localisation of neuroendocrine tumors. Acta Oncol 32: 167-170.
- Boy C, Heusner TA, Poeppel TD, Redmann-Bischofs A, Unger N, et al. (2011) Ga-68-DOTA TOC PET/CT and somatostatic receptor (sst1-sst5) expression in normal human tissue: correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging 38: 1224-1236.
- Milardovic R, Corssmit EP, Stokkel M (2010) Value of 123I-MIBG Scintigraphy in paraganglioma. Neuroendocrinology 91: 94-100.
- Gabriel M, Decristoforo C, Donnemiller E, Ulmer H, Watfah Rychlinski C, et al. (2003) An intrapatient comparison of 99mTc EDDA/HYNIC-TOC with 111In-DTPA-octreotide for diagnosis of somatostatin receptor-expressing tumors. Journal of nuclear medicine 44: 708-716.
- Shulkin BL, Wieland DM, Schwaiger M, Thompson NW, Francis IR, et al. (1992) PET scanning with hydroxyephedrine: an approach to the localization of pheochromocytoma. Journal of Nuclear medicine 33: 1125-1131.
- Ilias I, Yu J, Carrasquillo JA, Chen CC, Eisenhofer G, et al. (2003) Superiority of 6-[18F]-fluorodopamine positron emission tomography versus [131I]-metaiodobenzylguanidine scintigraphy in the localization of metastatic pheochromocytoma. J Clin Endocrinol Metab 88: 4083-4087.
- Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, et al. (2008) 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. J Nucl Med 49: 573-586.
- Mamede M, Carrasquillo JA, Chen CC, Del Corral P, Whatley M, et al. (2006) Discordant localization of 2-[18F]-fluoro-2-deoxy-D-glucose in 6-[18F]-fluorodopamine-and [123I]-metaiodobenzylguanidine-negative metastatic pheochromocytoma sites. Nucl Med Commun 27: 31-36.
- Duet M, Sauvaget E, Petelle B, Rizzo N, Guichard JP, et al. (2003) Clinical impact of somatostatin receptor scintigraphy in the management of paragangliomas of the head and neck. Journal of Nuclear Medicine. 44: 1767-1774.
- Oberndorfer S (1907) Carcinoid tumors of the small intestine Z Pathol. 1: 426-432.
- Modlin IM, Lye KD, Kidd M (2003) A 5â€decade analysis of 13,715 carcinoid tumors. Cancer. 97: 934-59.
- Woodard PK, Feldman JM, Paine SS, Baker ME (1994) Midgut carcinoid tumors: CT findings and biochemical profiles. J Comput Assist Tomogr. 19: 400-405.
- Newton JN, Swerdlow AJ, dos Santos Silva IM, Vessey MP, Grahame-Smith DG, et al. (1994) The epidemiology of carcinoid tumours in England and Scotland. British journal of cancer. 70:939.
- Sugimoto E, Lorelius LE, Eriksson B, Oberg K (1995) Midgut carcinoid tumours: CT appearance. ActaRadiologica. 36: 367-371.
- Pilleul F, Penigaud M, Milot L, S Jean-Christophe, C Jean-Alain, et al. (2006) Possible small-bowel neoplasms: contrast-enhanced and water-enhanced multidetector CT enteroclysis. Radiology. 241: 796-801.
- Bader TR, Semelka RC, Chiu VC, Armao DM, Woosley JT (2001) MRI of carcinoid tumors: spectrum of appearances in the gastrointestinal tract and liver. J Magn Reson Imaging. 14: 261-269
- Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB, et al. (1990) Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med. 114: 700-704.
- Halfdanarson TR, Rabe KG, Rubin J, Petersen GM (2008) Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 19: 1727-1733.
- Passaro Jr E, Howard TJ, Sawicki MP, Watt PC, Stabile BE, et al. (1998) The origin of sporadic gastrinomas within the gastrinoma triangle: a theory. Archives of Surgery. 133: 13-16.
- Taguchi T, Suita S, Hirose R (1991) Histological classification of nesidioblastosis: efficacy of immunohistochemical study of neuron-specific enolase. J Pediatr Surg. 26: 770-774.
- Howard TJ, Stabile BE, Zinner MJ, Chang S, Bhagavan BS, et al. (1990) Anatomic distribution of pancreatic endocrine tumors. The American Journal of Surgery. 159: 258-264.
- Berends FJ, Cuesta MA, Kazemier G, van Eijck CH, de Herder WW, et al. (2000) Laparoscopic detection and resection of insulinomas. Surgery. 2000 128: 386-91.
- Finlayson E, Clark OH (2004) Surgical treatment of insulinomas. Surg Clin North Am.84: 775-85.
- Grant CS (2005) Insulinoma. Best Pract Res Clin Gastroenterol. 19: 783-798.
- Kayani I, Conry BG, Groves AM, Win T, Dickson J, et al. (2009) A comparison of 68Ga-DOTATATE and 18FFDG PET/CT in pulmonary neuroendocrine tumors. Journal of Nuclear Medicine. 50: 1927-1932.
- Kitajima T, Kaida S, Lee S, Haruta S, Shinohara H, et al. (2013) Mixed adeno (neuro) endocrine carcinoma arising from the ectopic gastric mucosa of the upper thoracic esophagus. World J Surg Oncol.11: 218.
- Solcia E, Rindi G, Buffa R, Fiocca R, Capella C (2000) Gastric endocrine cells: types, function and growth. Regul Pept. 25;93: 31-35.
- Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, et al. (1995) Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 16: 86-88.
- Reubi J, Waser B, Schaer JC, Laissue JA (2001) Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 28: 836-846.
- Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, et al. (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 9: 61-72.
- Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, et al. (2010) Standardized uptake values of 68Ga- DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J Nucl Med.51: 353-359.
- Krausz Y, Freedman N, Rubinstein R, Lavie E, Orevi M, et al. (2011) 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with 111In-DTPA-octreotide (OctreoScan®). Mol Imaging Biol. 13: 583-593.
- Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, et al. (2007) Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 34: 1617-1626.
- Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, et al. (2010) The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPAoctreotide scintigraphy. J Nucl Med. 51: 875-882.
- Al-Nahhas A, Win Z, Szyszko T, Singh A, Nanni C, et al. (2007) Gallium-68 PET: a new frontier in receptor cancer imaging. Anticancer Res. 27: 4087-4094.
- Reubi JC, Waser B (2003) Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. European journal of nuclear medicine and molecular imaging. 30: 781-793.
- Kayani I, Conry BG, Groves AM, Win T, Dickson J, et al. (2009) A comparison of 68Ga-DOTATATE and 18FFDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med. 50:1927-1932.
- Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, et al. (2011) Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 38: 2125-2135.
Citation: Solav SV, Patil AM (2018) Scintigraphy in Functional Neuroendocrine Tumors with Review of Literature. J Neuroendocrinol Res 1: 102.
Copyright: © 2018 Solav SV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4384
- [From(publication date): 0-2017 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 3440
- PDF downloads: 944