Research Article Open Access
Seasonal and Spatial Patterns of Phase-Segregated Atmospheric Polycyclic Aromatic Hydrocarbons (PAHs) in North Western Part of Turkey Analysed by GC-MS
Emin Taylan and Semra Tuncel G*
Department of Chemistry, Middle East Technical University, 06800 Ankara, Turkey
- *Corresponding Author:
- Semra Tuncel G
Department of Chemistry
Middle East Technical University
06800 Ankara
Turkey
Tel: 903122103195
Fax: 903122013200
E-mail: semratun@metu.edu.tr
Received date: August 10, 2017; Accepted date: August 28, 2017; Published date: August 31, 2017
Citation: Taylan E, Tuncel SG (2017) Seasonal and Spatial Patterns of Phase- Segregated Atmospheric Polycyclic Aromatic Hydrocarbons (PAHs) in North Western Part of Turkey Analysed by GC-MS. J Anal Bioanal Tech 8: 377. doi: 10.4172/2155-9872.1000377
Copyright: © 2017 Taylan E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Seasonal and spatial variation and sources of phase-distributed polycyclic aromatic hydrocarbons (PAHs) in Northwestern part of Turkey was studied for pollution level and sources appointment. Daily sampling of air was done for one year in two sites; Bal�?±kesir city and the dam lake. Samples were analyzed by using GC-MS. The sum of the total concentration of PAHs was 517 ± 425 ng/ml for city and 153 ± 108 ng/ml dam lake respectively. Seasonal variations were observed sharply, for the city winter highest of 700 ng/ml. Highest observed concentration for Dam Lake was 160 ng/ml in summer season. Gas to particle phase partitioning was calculated and dam lake data was more favoring for the study. In summer gas/particle ratio was 38. We have observed in city low molecular weight PAHs were in the gas phase. In winter Gas/particle ratio was in between 2-5. Diagnostic ratio analysis and factor analysis indicated combustion and vehicular emission as the major sources for PAHs pollution.
Keywords
PAHs; GC-MS; Gas/Particle ratio; Diagnostic ratio; Factor analysis
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are group of chemicals that are formed during the incomplete combustion of fossil and biofuels. They are notorious for their serious health concern in the world [1,2]. They are the main carcinogenic constituent of ambient aerosols which have also genotoxic effects on health [3]. These compounds are mainly products of anthropogenic activities such as incomplete combustion and the pyrolysis of fossil fuels or wood. However natural processes can also be a source of their production. PAHs are mostly accumulated in densely populated urban areas such as city center as opposed to rural areas for obvious reasons. Because of the health concerns, number of PAH studies carried out in rural and urban areas enormously increased [2,4].
Once they enter into the atmosphere, PAHs are distributed between gas and particle phases depending on. Different factors; temperature, relative humidity, elemental and organic carbon content, and PAH sub cooled liquid vapor pressure [5-8]. Gas and particle partition of PAHs is an important issue for determining atmospheric processes and estimating its effects on environment and human health.
Partitioning at a given site are expected to vary during the year as meteorological and sunlight conditions change. It is therefore important to examine the seasonal variation of PAHs. Many studies have shown the effects of these parameters on PAH partitioning [7,9-11]. Detailed studies of the seasonal and spatial variation of atmospheric levels of PAHs, in both gas and particle phases are important for evaluation of their effects on atmospheric transport and human exposure. Despite the fact that there are some studies reported in Turkey dealing with gasto- particle partitioning and sources of the PAHs in Turkey [12-14]. There is very little research had been conducted on spatial and temporal distributions for the PAHs in the atmosphere of the Northeastern part of the Turkey, especially in Balikesir atmosphere.
One year long investigation of spatial and seasonal variation of 19 PAH compounds, describes that PAHS Are dangerous and identified as priority pollutants by US Environmental Protection Agency (EPA). Both sampling sites; in urban and rural part of Balikesir province of Northwestern Turkey showed high level of PAHs. In addition, the source apportionment was done to identify different contributing sources for PAHs concentration for those sites.
Materials and Methods
All chemicals used during the study were of analytical grade. The PAH-mix 68 (100 mg/L in cyclohexane) and standard reference materials; SRM 1597a (complex mixture of polycyclic aromatic hydrocarbons from coal tar prepared in DCM), were bought from National Institute of Standards and Technology, USA. Analytical standards used for the calibration curve were prepared in HPLC grade hexane (Merck). The GC-MS surrogate solution mix which includes acenaphtene- D10 (Ace-D10), phenanthrene-D10 (Phe-D10), chrysene-D12 (Chr-D12) and perylene-D12 (Per-D12) (1000 mg/L, in acetone) was bought from Dr. Ehrenstorfer- Germany. Anhydrous sodium sulphate (Na2SO3, extra pure, was purchased from Merck. High purity nitrogen (99.999), helium (99.999) gas were used for GC-MS analyses
Anhydrous sodium sulphate was used for the removal of the water from extracted samples. The PUF and glass fiber filters were used for sample collection. High purity glass wool was used during the extraction of samples. Anhydrous sodium sulphate and glass-wool were pre-cleaned using hexane:acetone in a large glass column (1 L capacity or larger). Anhydrous sodium sulphate was activated at 400°C for 4 h. Glass-wool was conditioned by heating overnight at 225°C in an oven. All glassware was cleaned with Alconox detergent powder (Supelco Cat.No. 1104) in hot water and rinsed with deionized water and acetone. Rinsed glassware was dried in an oven for overnight.
Heidolph rotary evaporator (laborota 4000 efficient) and mini-vap evaporator with six ports (Catalog No. 22970, 192 Cat. No. 22971) were used for solvent evaporation and Bransonic ultrasonic bath (Model B-2200 E4, 205 W, and 220 V) for the sample extractions. Deionized water (ultra-filtered type 1 water) was obtained from y Barnstead nanopure ultrapure water system.
Two sampling sites were considered for the study; Ikizcetepeler Dam Lake as Rural site and the urban site was the garden of the education faculty of Balikesir University. The Balikesir University located in city center. Ikizcetepeler Dam Lake was built in 1986- 1991, for multi purposes such as irrigation, drinking water, and flood prevention. The surface area of dam lake is 9.6 km2 and located at 52.0 m above the sea level. The distance between both sites; Dam lake and Balikesir University is 25 km. Ikizcetepeler dam lake sites is under the influence of the urban pollution and dense traffic emissions from these cities Balikesir-Izmir-Bursa. The high traffic route near dam lake is one of the other reason for our study of Dam lake and balikesir university located in the city. The map of the sampling site is given in Figure 1.
Sample collection
Atmospheric sampling of the gas and particle phase of PAHs were performed with Thermo Andersen high volume sampler (GPS II). Particle phase pollutants were collected on glass fiber filter with 90 mm diameter. Gas phase PAHs were collected on polyurethane foams (PUF) with an air flow of 0.225 m3/min for 24-hour.
Glass fiber samples extraction
At first, glass fiber filters were heated to clean in a furnace at 400°C for 5 hours. Then, they were weighed and placed in petri dishes separately and preserved in the desiccator until extraction. Samples were extracted with ultrasonic bath. The filter sample was spiked with 1 mg/L surrogate solution and extracted in a 50 ml beaker for about 15 minutes with a 20 ml of DCM and acetone mixture (1:1, v/v). After extraction solvent was evaporated with rotary evaporator and final volume was completed to 1 ml with hexane. Four surrogates were used and each surrogate was assigned to the different set of PAHs according to number of rings. Acenaphtene-D10 (ace-D10) and phenanthrene-D10 (phe-D10) were used for the recovery calculations of 3 rings PAHs, chrysene-D12 (chr- D12) for 4 rings PAHs and perylene-D12 (per-D12) for the 5-6 rings PAHs. Calculated average recoveries of surrogates in glass fiber filter samples were 68% in the average (41%, 60%, 85%, 86% for ace-D10, phe-D10, chr-D12 and per-D12 correspondingly).
Polyurethane Foam (PUF) samples extraction
PUF cartridges were cleaned with soxhlet apparatus for 16 hours in pure acetone for sampling. At gas phase sampling, PUF cartridges were covered with aluminum foil and stored in refrigerator at -18°C until extraction is done. Soxhlet apparatus is used for the extraction of Polyurethane foam (PUF) samples it is performed for 24 hours in 750 ml ¼ v/v dichloromethane-petroleum ether. Surrogate standard was added to all samples during the extractions. and recovery for PAHs was found as 73% ± 19 for ace-D10, 83% ± 21 for phe-D10, 83% ± 16 for chr-D12 and 88% ± 19 per-D12 for PUF samples. Rotary evaporator was used to evaporate solvents and Anhydrous sodium sulphate was used to remove moisture. The clean-up process was done by florisil coloums. The florisil columns were 8 cm long, 0.5 cm in diameter and filled with 15:85% of magnesium oxide, silicon dioxide (magnesium silicate) mixture. The florisil columns were activated with 5 ml of n-hexane and 20 ml ¼ v/v n-hexane - toluene mixture was used as an eluent. Final volume was obtained by evaporated until dryness with rotary evaporator and completed to 1 ml solution with n-hexane.
Instrumental Analysis
All the PAHs samples were analysed with Hewlett-Packard (HP) 6890 GC system equipped with a 5973 mass selective detector. GCMS conditions were as follows: injection port was in split less mode and maintained at 280°C. The column was HP-5; 5% phenyl methyl siloxane, 30.0 m in length, having a film thickness of 0.25 µm and internal diameter of 0.32 mm. The oven temperature program was held at 50°C at 2 min, ramped with 8°C/min to 280°C and held at that temperature for 12 min. The MS source temperature and MS quadruple temperature was adjusted to 230°C and 150°C respectively. Injection volume of 1 µl was used with the flow rate of 1.5 mL/min.
Quality Control and Quality Assurance
A strict QC/QA procedure was applied during the analyses. Blank PUF cartridges and filters were routinely placed in the field to determine if there was any contamination during sample handling and preparation. Extraction and analysis methods were verified using NIST SRM 1597. The percent recoveries of the PAHs in SRM 1597a are between 53.1 and 150. Filter and puf samples, spiked with surrogates, were analyzed with high precision ranging from 1 to 5%. The standard curves were obtained with 10-1000 µg/L PAH standards and the response curves for 16 PAHs were linear. The range of r values is 0.997- 1.000. The method detection limits ranged from 0.012-0.390 µg/L. The method detection limits were determined based on the concentration (or amount) of an analyte which gave a signal three times of the background noise.
Results and Discussion
Measured PAH concentrations
Averages and standard deviations of the measured concentrations for two phases; gas phase (GP) and particle phase (PP) PAHs for two sampling sites are shown in Table 1. Standard deviations were calculated using all the measured concentrations for the entire period for both sites. The sum of the vapor and particle phase concentrations were also indicated in Table. For the city atmosphere, the average total (GP+PP) PAH concentration (517 ng/m3) was higher than the dam lake site (153 ng/m3).
| PAHs | City-Gas Conc. (ng/m3) |
Range | Stdev | City- Particulate Con.(ng/m3) |
Range | Stdev | City-Total Conc. (ng/m3) |
Stdev | DamLake-Gas Conc. (ng/m3) |
Range | Stdev | Dam Lake- Particulate Conc.(ng/m3) |
Range | Stdev | Dam Lake- Total Conc. (ng/m3) |
Stdev |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nap | 34.9 | 0.0790-244 | 50.9 | 0.335 | 0.0500-2.31 | 0.433 | 35.3 | 51.3 | 32.2 | 0.0550-153 | 42.2 | 0.147 | 0.0374-2.36 | 0.438 | 32.4 | 42.6 |
| Acy | 45.9 | 0.0690-273 | 62.9 | 0.311 | 0.000-2.91 | 0.517 | 46.2 | 63.4 | 10.1 | 0.0305-255 | 40.2 | 0.174 | 0.00700-7.87 | 1.090 | 10.2 | 41.3 |
| Ace | 4.23 | 0.304-21.6 | 5.45 | 0.0215 | 0.00300-0.201 | 0.0514 | 4.25 | 5.50 | 1.28 | 0.0360-20.4 | 3.22 | 0.0376 | 0.0310-0.769 | 0.151 | 1.32 | 3.38 |
| Fle | 44.4 | 0.634-257 | 59.0 | 0.473 | 0.00100-4.82 | 0.818 | 44.9 | 59.9 | 20.0 | 0.227-426 | 66.6 | 0.150 | 0.0170-6.62 | 0.917 | 20.1 | 67.5 |
| Phe | 149 | 4.14-512 | 122 | 5.01 | 0.0320-25.5 | 6.30 | 154 | 128 | 32.5 | 1.12-435 | 68.1 | 0.878 | 0.157-8.94 | 1.319 | 33.4 | 69.4 |
| Ant | 33.0 | 1.30-204 | 40.4 | 0.984 | 0.0480-3.70 | 0.894 | 34.0 | 41.3 | 8.31 | 0.512-56.8 | 10.4 | 0.529 | 0.0376-6.25 | 0.889 | 8.84 | 11.3 |
| Fla | 26.2 | 0.507-98.0 | 20.6 | 13.8 | 0.0490-72.5 | 17.2 | 40.0 | 37.8 | 5.82 | 0.0450-39.1 | 7.15 | 1.4018 | 0.0117-6.73 | 1.529 | 7.22 | 8.68 |
| Pyr | 22.4 | 0.0100-144 | 28.5 | 14.8 | 0.0770-69.1 | 17.7 | 37.2 | 46.2 | 4.26 | 0.0290-26.9 | 4.97 | 1.6526 | 0.0716-7.57 | 1.643 | 5.91 | 6.61 |
| CPP | 1.01 | 0.0280-8.99 | 1.65 | 6.34 | 0.00700-37.7 | 8.49 | 7.35 | 10.1 | 0.621 | 0.0900-13.7 | 2.20 | 0.472 | 0.00450-7.27 | 1.038 | 1.09 | 3.24 |
| BaA | 7.32 | 0.0850-207 | 35.4 | 12.5 | 0.141-50.9 | 12.7 | 19.8 | 48.2 | 1.21 | 0.0940-11.6 | 2.44 | 0.820 | 0.0501-4.01 | 0.840 | 2.03 | 3.28 |
| Chr | 2.15 | 0.0150-46.5 | 7.88 | 5.87 | 0.0570-23.2 | 5.79 | 8.02 | 13.7 | 0.561 | 0.0370-6.02 | 0.990 | 0.481 | 0.0218-2.28 | 0.450 | 1.04 | 1.44 |
| BbF | 0.236 | 0.00900-1.68 | 0.417 | 8.40 | 0.0200-38.6 | 8.99 | 8.63 | 9.40 | 3.44 | 0.0510-27.8 | 6.68 | 0.436 | 0.00300-2.24 | 0.446 | 3.88 | 7.13 |
| BkF | 0.186 | 0.00900-1.27 | 0.314 | 7.12 | 0.0150-29.8 | 7.25 | 7.30 | 7.57 | 3.32 | 0.0560-24.0 | 6.32 | 0.349 | 0.00400-1.67 | 0.339 | 3.67 | 6.65 |
| BaP | 0.606 | 0.0100-11.6 | 2.04 | 15.7 | 0.104-75.6 | 18.3 | 16.3 | 20.3 | 13.6 | 0.254-192 | 40.9 | 0.883 | 0.0410-4.04 | 0.872 | 14.5 | 41.8 |
| BeP | 0.152 | 0.00500-1.44 | 0.313 | 10.9 | 0.011-42.88 | 11.7 | 11.0 | 12.0 | 2.89 | 0.0630-48.0 | 9.24 | 0.302 | 0.0173-1.59 | 0.318 | 3.19 | 9.56 |
| IcP | 1.18 | 0.000-33.7 | 5.79 | 13.0 | 0.027-61.02 | 13.8 | 14.2 | 19.5 | 0.614 | 0.00700-11.1 | 1.99 | 0.698 | 0.00700-4.09 | 0.939 | 1.31 | 2.93 |
| DaA | 0.138 | 0.000-2.38 | 0.468 | 5.80 | 0.066-34.26 | 8.31 | 5.93 | 8.78 | 0.318 | 0.0330-7.99 | 1.30 | 0.069 | 0.0070-0.640 | 0.133 | 0.387 | 1.44 |
| BgP | 0.327 | 0.0100-3.97 | 0.963 | 11.0 | 0.0240-49.3 | 12.6 | 11.3 | 13.6 | 0.654 | 0.0200-11.4 | 2.05 | 0.784 | 0.00650-4.01 | 0.897 | 1.44 | 2.95 |
| Att | 1.70 | 0.156-48.3 | 8.29 | 9.79 | 0.156-38.0 | 10.5 | 11.5 | 18.8 | 0.665 | 0.653-10.6 | 1.93 | 0.274 | 0.640-3.58 | 0.765 | 0.938 | 2.69 |
| Toplam PAH | 375 | 8.859-1234.9 | 280 | 142 | 0.805-537 | 145 | 517 | 425 | 142 | 16.0-1231 | 198 | 10.5 | 0.159-43.5 | 9.87 | 153 | 208 |
Table 1: Average concentration (ng/m-3) of PAHs for gas and particle phases.
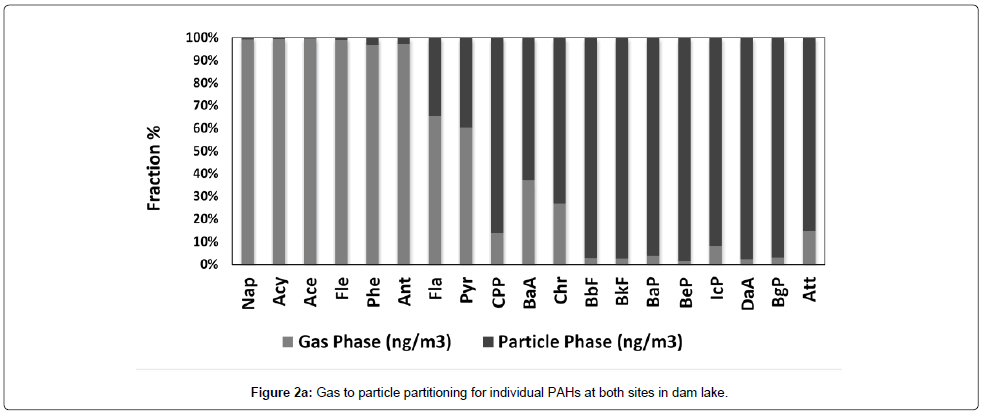
The city total PAH gas phase concentration is 375.0 ± 280.0 ng/ m3 with range of 8.86-123 ng/m3. For dam lake, total gas phase PAHs is 142.0 ± 198.0 ng/m3 and ranged from 16.00 to 1231 ng/m3. The city total PAH particulate phase (142.0 ± 145.0) concentrations was nearly the half of the city total PAH gas phase concentration (375 ng/m3) with a concentration range of 0.8050-537.6 ng/m3. This amount is lower than the city gas phase total PAH concentration. The dam lake total PAH particulate concentration was calculated as 10.50 ± 9.870 ng/m3 which is very low compared to dam lake gas phase concentration. It is also lower than the city particulate phase concentration with a range of 0.1595-43.50 ng/m3. The particle /gas ratio for total PAHs is 2.64 for city and 13.5 for dam lake. Obviously, gas phase concentrations are higher in the lake atmosphere. Figure 2a and 2b show gas to particle partitioning for individual PAHs at both sites. As can be seen from the Figure 2a and 2b, volatile PAHs including naphtalene (Nap), acenaphtane (Ace), fluorene (Flu), and phenanthrene (Phe) accounts 90% of the total PAH mass in the gas phase for both sites. But for dam lake atmosphere all PAHs are higher in the gas phase. For city center starting from Fla to Att, medium and high molecular weight PAHs are in the particulate phase. Especially high molecular weight PAHs like Bbf, BaP, DaA and BgP are almost entirely in the particle phase.
Seasonal and spatial variations in total PAH concentrations
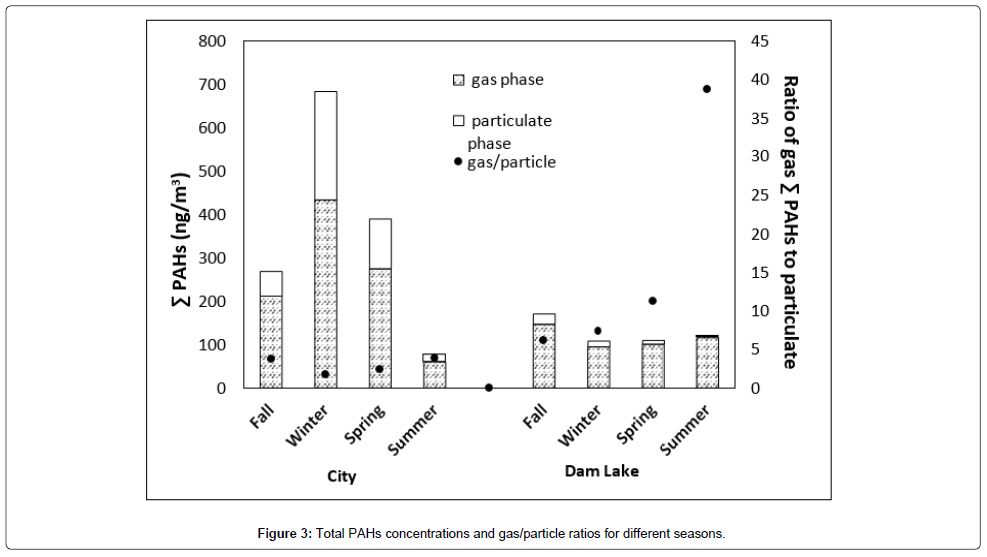
Total PAHs concentrations and gas/particle ratios for different seasons are given in Figure 3. All the seasons except summer total PAHs are high in concentration for the city. In general, for all seasons city concentrations are considerably higher than dam lake atmosphere except for summer. The highest for city was observed for winter season with a total concentration of 700 ng/m3 of which 430 ng/m3 was in the gas phase. Winter is followed by spring and fall. Since the temperatures in fall and spring do not differ significantly, concentrations are almost the same. Lowest concentration was observed in summer as expected due to decreased domestic heating. On the other hand, for Dam Lake highest total concentration was observed for fall season. For winter, spring and summer concentrations were comparable. Summer total in Dam Lake is higher than city. If we look at the gas/particle ratio; for city, it was in between 2 to 5 indicating comparable partitioning in between two phases for all seasons. For Dam Lake, the same ratio was in between 7-38. Highest gas/particle ratio (38) being for summer season. For summer total PAH concentration was almost entirely in the gas phase. This is probably due to increased recreational activities in the lake region during the summer season. On the other hand, city is affected by domestic heating and transportation, therefore highest total concentration of PAHs was observed during the winter season, but almost similar gas/particle ratio was found for all the other seasons.
Statistical tests for source apportionment diagnostic ratio analysis
Atmospheric PAH profiles can be affected by meteorological variables such as solar radiation and temperature, and their sources mainly come from the incomplete combustion. Despite these facts, the ratios (also called diagnostic ratios) between some of these compounds were considered as the “fingerprint “of emission sources [15,16], as they exhibited the characteristics of the specific sources. Diagnostic ratios can give a preliminary idea about the contributing sources. Among these ratios Fla/(Fla+Pyr), BaA/(BaA+ChR), IcP/(IcP+BgP) and Ant/ (Phe+Ant) were the conventional ones to characterize the potential emission sources of PAHs [17]. It is reported that diagnostic ratio of Fla/(Fla+Pyr) was 0.44 for the motor vehicle exhaust with catalytic converter. The IcP/(IcP+Bgp) ratios of particles from gasoline and diesel vehicle, and coal- fired plants were 0.18, 0.37 and 0.56, respectively. Another ratio; Phe/ (Phe+Ant) is used to differentiate coal combustion (0.85) from oil combustion due to traffic (0.93). The ratio for diesel emissions is 0.73. A comparison between the various diagnostic ratios was obtained in the literature [18]. In this study before performing factor analysis diagnostic ratios were also calculated. Four ratios; Phe/ (Phe+Ant), Fla/(Fla+Pyr) and BaA/(BaA+Chr), IcP/(IcP+BgP) were calculated for both sampling sites for two seasons; summer (s) and winter (w). Calculations were performed for both phases; particle and gas phase. Accordingly, sources affecting different locations at different seasons could be evaluated. These results are listed in Table 2.
| Bal�?±kesir Atmosphere | Bal�?±kesir Atmosphere (Particulate) | Dam Lake Atmosphere | Dam Lake Atmosphere (Particulate) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Ratios | (Gas) | S1 | W2 | S | W | S | W | S | W | |
| Fla/(Fla+Pyr) | <0.4 | Petrogenic | 0.54 | 0.54 | 0.45 | 0.48 | 0.42 | 0.46 | 0.42 | 0.46 |
| 0.4-0.5 | Fossil fuel combustion | |||||||||
| >0.5 | Grass, wood and coal combustion | |||||||||
| BaA/(BaA+Chr) | 0.2-0.35 | Coal combustion | 0.81 | 0.62 | 0.64 | 0.68 | 0.69 | 0.62 | 0.69 | 0.62 |
| >0.35 | Vehicular emission | |||||||||
| <0.2 | Petrogenic | |||||||||
| Fle/(Fle+Pyr) | <0.5 | Petrol emission | 0.72 | 0.66 | 0.01 | 0.03 | 0.04 | 0.09 | 0.04 | 0.09 |
| >0.5 | Diesel emission | |||||||||
| Grass, Wood,Coal combustion | Grass, Wood,Coal combustion | Fossil fuel combustion | Fossil fuel combustion | Fossil fuel combustion | Fossil fuel combustion | Fossil fuel combustion | Fossil fuel combustion | |||
| Vehicular emission | Vehicularemission | Vehicularemission | Vehicular emission | Vehicular emission | Vehicular emission | Vehicular emission | Vehicular emission | |||
| Dieselemission | Dieselemission | Petrolemission | Petrolemission | Petrolemission | Petrolemission | Petrolemission | Petrol emission | |||
Table 2: Diagnostic ratio analysis.
As can be seen from Table 2, for city gas samples in summer PAH source is oil combustion due to traffic (diag. ratio is 0.93) but, for winter the source is wood combustion due to heating with a ratio of 0.82. For the rest of the samples (city particulate s-w, dam lake gas s-w, dam lake particulate s-w) PAH source is mainly traffic in summer and in winter both traffic (% 50) and coal combustion (% 50).
Fla/(Fla+Pyr) ratio is used to differentiate sources whether it is any combustion for heating or vehicular emission. All the found ratios showed that main source of PAHs in summer is vehicular emission and it is mainly domestic heating such as natural gas combustion, coal burning during winter.
Another ratio, BaA/(BaA+Chr), was also used and it was found that for city atmosphere and dam lake atmosphere main PAH source is diesel emission for all seasons (winter and summer) and for all phases (gas and particulate). The last ratio used was IcP/(IcP+BgP) equally distributed between coal and wood combustion (50%, 50%). This is expected due to heating activities during winter. On the other hand, summer ratios also surprisingly reveal, that the main source is coal and wood combustion. This result for summer may be explained by recreational activities (barbeque) or long range transport of PAHs.
Factor analysis
Factor analysis is a multivariate analysis which groups the variables with the same variance in the same factor (principal components). The factors with eigenvalues over 1 were extracted according to Kaiser- Meyer-Olkin (KMO) and Bartlett’s test of sphericity and were rotated using the Varimax method. Each extracted factor is assigned as source. The assumption is, the variables with the same variance are assumed to be originating from the same source [19]. Factor numbers were identified with reasonable communalities.
Factor analysis was performed using SPSS 17.0 package program on the combined concentrations of particulate and gas phase for both sampling sites. For the city atmosphere three sources were identified by looking at the composition of the factors. These sources are; coal combustion (37%), mixed (21%) and traffic (16%) (Table 3). Numbers in the parentheses are the variance explained by each factor [20]. Factor 1 is heavily loaded with Pyr, BaP, IcP and BgP which are HMW PAHs and represents coal combustion. Factor 2 represents mixed source and contains heavily Chr. On the other hand, last factor (Factor 3) is mainly dominated by Acy, Ace which are LMW PAHs and fingerprint for traffic emission.
| Gas Phase+Particulate Phase | ||||||
|---|---|---|---|---|---|---|
| Factors | ||||||
| Bal�?±kesir City | Dam Lake | |||||
| PAHs | 1 | 2 | 3 | 1 | 2 | 3 |
| Nap | 0.633 | -0.134 | -0.077 | -0.256 | -0.174 | 0.095 |
| Acy | 0.654 | 0.115 | 0.046 | 0.121 | -0.27 | 0.337 |
| Ace | 0.686 | 0.028 | -0.005 | 0.327 | -0.23 | -0.064 |
| Fle | 0.711 | -0.234 | 0.016 | -0.137 | -0.385 | 0.36 |
| Phe | 0.783 | -0.059 | 0.223 | -0.156 | 0.001 | 0.859 |
| Ant | 0.614 | 0.278 | 0.369 | 0.053 | 0.092 | 0.784 |
| Fla | 0.725 | 0.103 | 0.162 | 0.684 | 0.101 | 0.289 |
| Pyr | 0.508 | -0.277 | 0.086 | 0.773 | -0.189 | 0.16 |
| CP | -0.187 | -0.033 | -0.363 | -0.016 | 0.634 | -0.187 |
| BaA | -0.126 | 0.1 | -0.764 | 0.343 | 0.255 | 0.011 |
| Chr | -0.137 | 0.057 | -0.777 | 0.562 | 0.425 | -0.12 |
| BbF | -0.119 | 0.763 | 0.215 | 0.342 | 0.722 | 0.153 |
| BkF | -0.204 | 0.703 | -0.178 | 0.045 | 0.811 | -0.07 |
| BaP | 0.038 | 0.655 | 0.448 | 0.77 | -0.114 | 0.177 |
| BeP | 0.609 | -0.024 | -0.003 | -0.263 | 0.597 | 0.301 |
| IcP | -0.297 | -0.546 | 0.4 | 0.118 | 0.067 | 0.419 |
| DaA | -0.21 | 0.622 | 0.449 | -0.365 | -0.42 | 0.115 |
| BgP | -0.026 | 0.251 | 0.642 | 0.56 | 0.108 | -0.078 |
| Att | -0.29 | -0.515 | 0.385 | -0.009 | -0.323 | -0.158 |
| % Variance | 23 | 15 | 15 | 15 | 15 | 11 |
| Source | Coal | Vehicle | Vehicle | Biomass | Vehicle | Coal |
Table 3: Factor analysis for both sampling sites.
According to factor analysis results of Dam Lake; main sources are; coal combustion (27%), traffic (20%) and mixed (19%) (Table 4).
| BaP-TEQ Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAHs | TEF Values | Bal�?±kesirDam Lake | Bal�?±kesirCity Center | Spain [7] | Rome [9] | China [21] | |||||
| Gas | Particle | Gas | Particle | Gas | Particle | Gas | Particle | Gas | Particle | ||
| Nap | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acy | 0.001 | 0.0101 | 0.000174 | 0.0458 | 0.000311 | 0 | 0 | 0.0390 | 0.00460 | 0.0112 | 0.000200 |
| Ace | 0.001 | 0.00128 | 0.000038 | 0.00423 | 0.000021 | 0 | 0 | 0.0570 | 0.00220 | 0.00250 | 0 |
| Fle | 0.001 | 0.0199 | 0.000150 | 0.0444 | 0.000473 | 0 | 0 | 0.0180 | 0 | 0.0237 | 0.000300 |
| Phe | 0.001 | 0.0325 | 0.000878 | 0.149 | 0.00501 | 0.00220 | 0.000129 | 0.0710 | 0.000900 | 0.0431 | 0.00460 |
| Ant | 0.01 | 0.0831 | 0.00529 | 0.330 | 0.00984 | 0.00450 | 0 | 0.0560 | 0.00500 | 0.0730 | 0.00500 |
| Fla | 0.001 | 0.00582 | 0.00140 | 0.0262 | 0.0138 | 0.000840 | 0.000230 | 0.0180 | 0.00350 | 0.0152 | 0.0123 |
| Pyr | 0.001 | 0.00426 | 0.00165 | 0.0224 | 0.0148 | 0.000820 | 0.000310 | 0.00760 | 0.00920 | 0.00990 | 0.0105 |
| BaA | 0.1 | 0.121 | 0.0820 | 0.732 | 1.25 | 0.00650 | 0.0270 | 0.0400 | 0.140 | 0.0400 | 1.19 |
| Chr | 0.001 | 0.000561 | 0.000481 | 0.00215 | 0.00587 | 0.000130 | 0.000290 | 0.000500 | 0.00390 | 0.000800 | 0.0115 |
| BbF | 0.1 | 0.344 | 0.0436 | 0.0236 | 0.839 | 0.00180 | 0.0460 | 0.0700 | 0.680 | 0.0100 | 1.74 |
| BkF | 0.001 | 0.00332 | 0.000349 | 0.000186 | 0.00712 | 0.00000500 | 0.000120 | 0.000700 | 0.00680 | 0.000100 | 0.00840 |
| BaP | 1 | 13.6 | 0.883 | 0.606 | 15.7 | 0.00400 | 0.290 | 0.300 | 2.40 | 0.100 | 9.20 |
| IcP | 0.1 | 0.0614 | 0.0698 | 0.1182 | 1.29 | 0.000400 | 0.110 | 0 | 0.160 | 0 | 0.240 |
| DaA | 1 | 0.318 | 0.0690 | 0.138 | 5.79 | 0.00400 | 1.10 | 0 | 0 | 0 | 7.70 |
| BgP | 0.01 | 0.00654 | 0.00784 | 0.00327 | 0.109 | 0.0000200 | 0.0100 | 0.00500 | 0.0240 | 0 | 0 |
| (BaP-TEQ)Σ16PAH | 14.6 | 1.17 | 2.25 | 25.0 | 0.0252 | 1.58 | 0.683 | 3.44 | 0.330 | 20.1 | |
Table 4: Comparison of BAP-TEQ values with literature.
Low Molecular weight PAHs (Acy, Ace, Fle, Phe, Ant) are accumulated in Factor 1 and these PAHs represents traffic as a source.
High Molecular weight PAHs (BbF, Bkf, BaP, BeP) are collected in Factor 2 and this factor represents coal combustion as a source. In factor 3; Fla, Pyr, CPP, BaA are accumulated and these PAHs represent mixed source (traffic + coal combustion). Since coal combustion is greater than traffic (27>20) it is the main source and traffic is the second dominant source in the dam lake area. An intercity highway passing nearby the dam lake could act as a traffic source in the area. Picnic activities may also contribute the coal combustion source, which is mainly originated from domestic heating [21]. Another reason for traffic emission is the cars belong to people coming recreational purposes. Coal component in the mixed source is probably due to picnic activities. Domestic heating in the city may also be transported to lake area.
As can be seen from Tables 3 and 4 source resolutions for city and dam lake atmosphere are almost the same. Coal combustion and traffic emissions are the two major sources. The only difference is coal combustion is the primary source for city atmosphere. On the other hand, dam lake area traffic emission is the dominant source. This is a reasonable result as no heating and industrial activities on dam lake area. The observed coal component is probably due to transported air masses from the city.
Literature comparison of BaP- toxic equivalency quotient (TEQ) values
Polycyclic aromatic hydrocarbons (PAHs) are known with having a cancerogenic effect. Among them benzo(a)pyrene (BaP) is identified as the most dangerous one. Little is known about the rest of the PAHs. For that reason, while toxicity level of PAH was investigated BAP is taken as a reference and BAP equivalent toxicity is calculated. To obtain BaPTEQ values listed TEF (toxic equivalency factor) values are multiplied with PAH concentrations and added to each other. The found BAPTEQ values were compared with other countries such as Spain, Rome and China. Comparison resulted that Balikesir dam-lake gas phase and city particulate samples has the highest BAP-TEQ values (Table 4) [22].
Conclusion
A total of 19 PAH compounds in Balikesir city and nearby Dam Lake (Ikizcetepeler) atmosphere were investigated. In general, both for city atmosphere and dam lake atmosphere, gas phase pollutants are higher mainly with low molecular weight (LMW) PAHs. However, particle phase pollutants highly enriched with high molecular weight (HMW) PAHs. The total PAH concentrations in city atmosphere is higher than the dam lake atmosphere as expected due to the nonexistence of heating activities around the dam lake which is a rural site. Phase distribution analysis revealed that although in the city gas to particle distribution is close to each other, in Dam Lake most of the PAHs were present in the gas phase. At city atmosphere, gas phase is dominated by low molecular weight (LMW) PAHs like; Ace, Fle, Phe and Ant. For dam lake atmosphere, almost all PAHs are in the gas phase.
The found BAP-TEQ values were compared with other countries such as Spain, Rome and China and comparison resulted that Balikesir dam-lake gas phase and City center particulate samples has the highest BAP-TEQ values. Factor analysis and diagnostic ratio analysis resulted that coal combustion and traffic are the main sources in both sampling site. For gas phase pollutants, coal is the dominant source, on the other hand for the particle phase pollutants main source is the traffic.
Highlights
• Gas phase pollutants are enriched with LMW PAHs, particle phase pollutants are enriched with HMW PAHs.
• PAH concentrations in city atmosphere is higher than the dam lake atmosphere.
• In urban areas, during winter because of the heating activities higher concentration of PAHs were observed.
• In rural areas, summer PAH concentrations are higher than the winter PAH concentrations due to picnic activities.
• In Balikesir, coal combustion (in gas phase) and traffic (in particulate phase) are the main sources of PAH pollution.
References
- Gaspari AA, Sauder DN (2003) Immunotherapy of basal cell carcinoma: evolving approaches. Dermatol Surg 29: 1027-1034.
- Zhang YX, Tao S, Shen HZ, Ma JM (2009) Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. P Natl Acad Sci USA 106: 21063-21067.
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, et al. (2006) Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet-Oncology 7: 977-978.
- Cheng H, Deng Z, Chakraborty P, Liu D, Zhang R, et al. (2013) A comparison study of atmospheric polycyclic aromatic hydrocarbons in three Indian cities using PUF disk passive air samplers. Atmos Environ 73: 16-21.
- Mader BT, Pankow JF (2001) Gas/Solid Partitioning of Semi-volatile Organic Compounds (SOCs) to Air Filters. 2: Partitioning of Polychlorinated Dibenzodioxins, Polychlorinated Dibenzofurans, and Polycyclic Aromatic Hydrocarbons to Quartz Fiber Filters. Atmos Environ 35: 1217-1223.
- Offenberg JH, Baker JE (2002) The Influence of Aerosol Size and Organic Carbon Content on Gas/Particle Partitioning of Polycyclic Aromatic Hydrocarbons (PAHs). Atmos Environ 36: 1205-1220
- Bae SY, Yi SM, Kim YP (2002) Temporal and Spatial Variations of the Particle Size Distribution of PAHs and Their Dry Deposition Fluxes in Korea. Atmos Environ 34: 5491-5500.
- Griffin RJ, Nguyen K, Dabdub D, Seinfeld JH (2003) A Coupled Hydrophobic-Hydrophilic Model for Predicting Secondary Organic Aerosol Formation. J Atmos Chem 44: 171-190.
- Callen MS, Cruz MT, Lopez JM, Murillo R, Navarro MV, et al. (2008) Some inferences on the mechanism of atmospheric gas/particle partitioning of polycyclic aromatic hydrocarbons (PAH) at Zaragoza (Spain). Chemosphere 73: 1357-1365.
- Possanzini M, Di Palo V, Gigliucci P, Sciano MCT, Cecinato A (2004) Determination of phase- distributed PAH in Rome ambient air by denuder/GC-MS method. Atmos Environ 38: 1727-1734.
- Gustafson KE, Dickhut RM (1997) Particle/Gas Concentrations and Distributions of PAHs in the Atmosphere of Southern Chesapeake Bay. Environ Sci Technol 31: 140-147.
- Gaga EO, Ari A (2011) Gas��?particle partitioning of polycyclic aromatic hydrocarbons (PAHs) in an urban traffic site in Eskisehir, Turkey. Atmos Res 99: 207-216.
- Tasdemir Y, Esen F (2007) Dry deposition fluxes and deposition velocities of PAHs at an urban site in Turkey. Atmos Environ 41: 1288-1301.
- Sofuoglu A, Odabasi M, Tasdemir Y, Khalili NR, Holsen TM (2001) Temperature Dependence of Gas-Phase Polycyclic Aromatic Hydrocarbon and Organochlorine Pesticide Concentrations in Chicago Air. Atmos Environ 35: 6503-6510.
- Kavouras IG, Koutrakis P, Tsapakis M, Lagoudaki E, Stephanou EG, et al. (2001) Source apportionment of urban particulate Aliphatic and polynuclear aromatic hydrocarbons (PAHs) using multivariate methods. Environ Sci Technol 35: 2288-2294.
- Dickhut RM, Canuel EA, Gustafson KE, Liu K, Arzyus KM, et al. (2000) Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay Region. Environ Sci Technol 34: 4635-4640.
- Rogge WF, Mazurek MA, Hildemann LM, Cass GR, Simoneit BRT (1993) Quantification of Urban Organic Aerosols on a Molecular Level: Identification, Abundance and Seasonal Variation. Atmos Environ 27: 1309-1330.
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell H, Goyette D, et al. (2002) PAHs in the Fraser River basin: a critical appraisal of PAHs ratios as indicators of PAH source and composition. Org Geochem 33: 489-515.
- Kim E, Hopke PK, Larson TV, Maykut NN, Lewtas J (2004) Factor Analysis of Seattle Fine Particles. Aerosol Sci Tech 38: 724-738.
- Khalili NR, Scheff PA, Holsen TM (1995) PAH Source Fingerprints for Coke Ovens, Diesel and Gasoline Engines, Highway Tunnels, and Wood Combustion Emissions. Atmos Environ 4: 533-542.
- Ma WL, De Zhi Sun DZ, She WG, Yang Y, Qi H, et al. (2011) Atmospheric concentrations, sources and gas-particle partitioning of PAHs in Beijing after the 29th Olympic Games. Environ Pollut 159: 1794-1801.
- Zhao J, Zhang F, Xu L, Chen J, Xu Y (2011) Spatial and temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere of Xiamen, China. Sci Total Environ 409: 5318-5327.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3847
- [From(publication date):
August-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 3002
- PDF downloads : 845