Side of Donation or Side of Implantation: Which One Could be a More Valuable Determinant in Kidney Transplantation?
Received: 18-Jun-2017 / Accepted Date: 19-Jul-2017 / Published Date: 25-Jul-2017 DOI: 10.4172/2475-7640.1000116
Abstract
Purpose: The aim of this single center study was to determine if the outcomes after kidney transplant were influenced by side of kidney donation and/or side of implantation (left versus right).
Materials and methods: All kidney transplantations in our department from June 1993 to November 2015 were included. Data was prospectively gathered and used for analysis. Serum creatinine of postoperative days during hospitalization and then at follow up visits was used for determination of kidney function.
Results: A total of 3334 transplantations were investigated during the study period. Mean serum creatinine was highest at the 3rd postoperative day and gradually decreased to 1.4 ± 0.7 mg/dl in the 3rd postoperative month and remained rather stable up to 12 months after transplantation. Recipients’ 7th day postoperative creatinine for right donated kidneys were 1.99 ± 1.80 mg/dL and 1.63 ± 1.64 for left donated kidneys (p<0.001). Recipients’ 7th day postoperative creatinine from right donated kidneys were 1.85 ± 1.49 mg/dL when donated kidneys were implanted in the right side of the recipients versus 2.17 ± 2.10 mg/dL when donated kidneys were implanted in the left side of the recipients (p=0.05). This statistically significant difference was no longer observed in the 1st month after operation and thereafter. Complications in recipients according to the Clavien-Dindo categories of 0, 2, 3b, 4a, 4b and 5 were observed in 89, 0, 1.1, 7.8, 0.6 and 1.7% of left implantations and 93, 0.3, 0.8, 2.7, 0.8, and 2.3% of right implantations (p=0.54)
Conclusion: Our data suggest that side of donor or recipient surgery in kidney transplantation is not important in one year follow-up. One-week outcome of right donor kidneys were inferior to left donated kidneys. Also one year complications for side of donation or transplantation were not significantly different.
Keywords: Kidney transplantation; Kidney donor side; Deceased donor; Living donor; Kidney recipient side
16319Introduction
The left kidney has an elongated renal vein which accelerates the grafting procedure in living donor transplantation. However the inferior vena cava and aorta are manageable via the right-side surgery in the recipient. Age, sex, body mass index, cause of demise, last renal function, co-morbidities and non-beating heart in cadaveric donor may ominously sway renal transplant results [1-4]. A few studies propose that donor kidney side (left or right) may meaningfully effect ensuing kidney transplant fallouts for first 3-12 months, probably associated to operational technical hitches up-and-coming from the inequality in the sizes of the left and right renal veins but 2, 3 and 4 years transplant survival were akin [5-8]. However contemporary studies bared that even in first year, complications, delayed graft function, compromised early and medium-term renal allograft function in right kidney is an as good as left kidney recipient [9,10].
On the other hand in the recipient usually transplant surgeons implant graft in the opposite side. This procedure make pelvis and ureter come medial and ventral to hilum and be more accessible for ureterovesical anastomosis and also facilitate management of possible ureteral complications. Because iliac vessels are abysmal in left side which may complicate operation, we decided to compare outcomes of right kidney which implanted in right side with right kidney which implanted in left side. Our aim was to evaluate the effect of side of kidney donation and also side of transplantation in the recipient on allograft consequence in an Iranian population of living or deceased donor, by observing early and late allograft outcome measures.
The aim of this study was to determine if the outcomes after kidney transplant were influenced by side of kidney donation and/or side of implantation (left versus right).
Materials and Methods
This study was directed from June 1993 to November 2015 in the Department of Urology at Labbafinejad university hospital (Referral Center, Shahid Beheshti University of Medical Sciences) in Tehran, Iran. Our center is in collaboration with the Collaborative Transplant Study (CTS) which data of transplant patients, donors and their annual follow-ups was used for this study.
To assess the recipient, we narrowed our donor laboratory tests to ABO compatibility and introductory cross-matching. These patients experienced renal ultrasound, voiding cystourethrography (if needed), chest X-ray, ear-nose-throat examination, dental examination, complete blood count, blood coagulation tests, stool examination, venereal disease research laboratory, human immune deficiency antibody, human T-lymphotropic virus-1 antibody, hepatitis B surface antigen, hepatitis C virus antibody, urinalysis, urine culture, and sometimes renal biopsy. Other tests like gastrointestinal endoscopy were done when needed.
Our technique was typical retroperitoneal flank approach for open donor nephrectomy until 1997. Since 1997, the standard method in our department has been left side laparoscopic donor nephrectomy but in some cases right side nephrectomy and same side or inverted kidney transplantation has been performed based on patients’ situations. The allograft transplant was accomplished by anastomosis of the renal artery to the internal iliac artery or to the external or common iliac arteries when the internal was not appropriate. The renal vein in just about all patients was anastomosed to the external iliac vein. Aorta and inferior vena cava were the locations of vascular anastomosis in small pediatric recipients. Suture material was prolene 6-0 and 5-0 for vascular anastomosis. Ureteral anastomosis was done within modified Lich technique using ureteral stent. All transplantations were implemented by the team ran by three transplantation urologists (N.S., A.B. and A.T.).
Immunosuppression was similar and patients received Calcineurin inhibitor-based immunosuppression.
Serum creatinine was measured at postoperative days during hospitalization and then at follow up appointments. Serum creatinine >4 mg/dL and first week dialysis were defined as directories describing graft failure or delayed graft function. Serum creatinine was used for comparing the efficiency of side of donor and recipient graft results.
We categorized our patient’s one-year follow-up complications according to Clavien-Dindo surgical grading system. Patients in grade 1 need no pharmacologic or surgical intervention to complication management. In grade 2, patients need medical treatment or blood transfusion due to surgery. Patients requiring surgery for relieving complication with regional or general anesthesia are categorized in grade 3a or 3b respectively. Life threatening complication with single or multiple organ failure is categorized in grade 4a or 4b respectively and death is considered a grade 5 complication.
Statistical analysis
All data is collected prospectively in our center for transplantations and deposited in computerized software. This data was moved to the Statistical Package for Social Sciences package (SPSS) ver. 19.0 software (Chicago, IL) and used for analysis. Chi square test was engaged to compare the rate of graft failures over dichotomous predictor variables. Mantel-Hantzel statistic was used to adjust the odds ratio over level of one confounding variable. Comparison of Continuous variables over categorical variables was completed by independent samples t-test. Ln transformation was employed to make creatinine distribution closer to normal distribution. Linear regression was implemented to evaluate the influence of donor side and recipient side on 7th day creatinine after introduction of potential confounding variables into the model.
Results
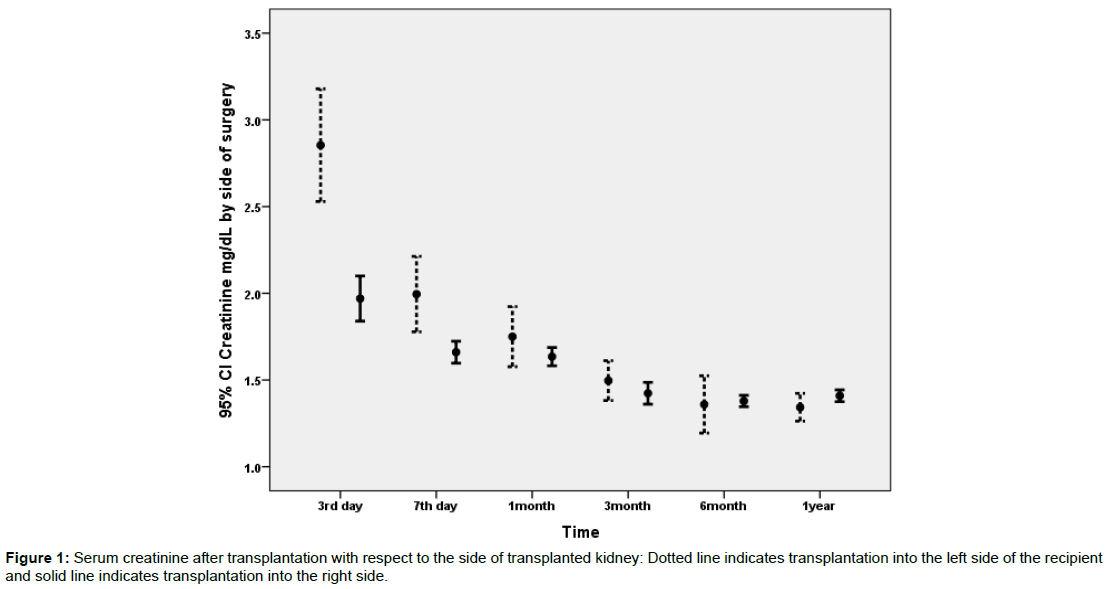
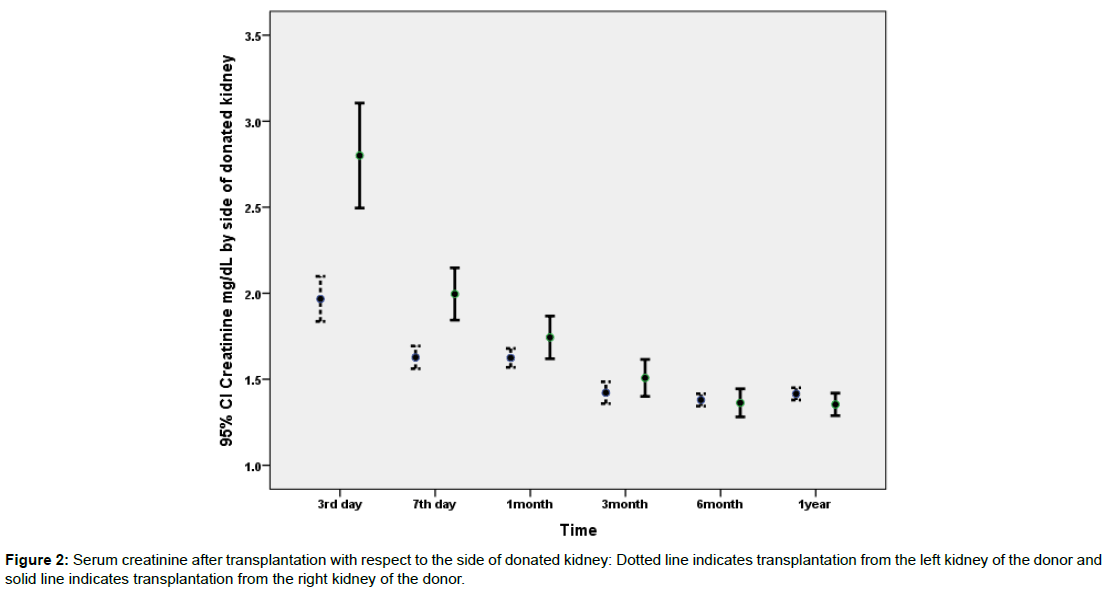
3334 patients were included during the study period. Detail of demographic data of the recipients and donors is presented in Table 1. Comorbidities were observed in 1043 patients and included: diabetes mellitus (N=21), hypertension (N=898), polycystic kidney disease (N=40), neurogenic bladder (N=25), hepatitis B infection (N=41), and others (N=18). Serum creatinine data was available for the 3rd and 7th postoperative days and then at 1, 3, 6 and 12 months after transplantation. As it is evident in Table 1 mean serum creatinine was highest at the 3rd postoperative day and gradually decreased to 1.4 ± 0.7 mg/dl in the 3rd postoperative month and remained rather stable in follow-up till 12 months after transplantation. Figures 1 and 2 depict the changes of serum creatinine with respect to the side of transplantation on the recipient or side of donated kidney (left versus right).
| Variable Donors | Number | Mean Serum Creatinine |
|---|---|---|
| Age, years; mean ± SD | 3248 | 28.6 ± 6.8 |
| Side (L/R) | 3252 | 2627/625 |
| Type (cadaver/living) | 3326 | 358/2968 |
| Gender (Male/Female) | 3201 | 2584/617 |
| Recipients | ||
| Age, years; mean ± SD | 3325 | 36.2 ± 15.8 |
| Side (L/R) | 3240 | 372/2868 |
| Gender (Male/Female) | 3326 | 2112/1214 |
| BMI; mean ± SD | 1572 | 24.2 ± 9.9 |
| 3rd day creatinine, mg/dL; mean ± SD | 781 | 2.16 ± 1.66 |
| 7th day creatinine, mg/dL; mean ± SD | 3006 | 1.70 ± 1.67 |
| 1 month creatinine, mg/dL; mean ± SD | 2589 | 1.65 ± 1.29 |
| 3 month creatinine, mg/dL; mean ± SD | 679 | 1.44 ± 0.68 |
| 6 month creatinine, mg/dL; mean ± SD | 1741 | 1.38 ± 0.70 |
| 12 month creatinine, mg/dL; mean ± SD | 2117 | 1.41 ± 0.74 |
| 7th day creatinine ≥4 mg/dL; N (%) | 3006 | 193 (6) |
| 7th day creatinine ≥3 mg/dL; N (%) | 3006 | 283 (9) |
Table 1: Demographic data on recipients and donors.
Data on the number of transplantations for each recipient was available on 2598 patients. In 2431 patients, transplantation was performed for the first time, in 155 patients for the second time, and in 12 patients for the third time. 93%, 50%, and 83% of first, second and third transplantations were performed on the right side of the recipient. Ninety seven percent of left donor kidneys were transplanted into the right side of the recipient. For right donor kidney, 53.5% were transplanted into the right side of the recipient and 46.5% into the left side.
Data on weight of donated kidneys were available only in 635 patients. There was a statistically significant inverse association between donor kidney weight and 7th day postoperative creatinine (r=-0.104, p=0.009). The mean ± SD weight of left and right donated kidneys were 193 ± 29 g versus 185 ± 25 g respectively (p=0.027). As data on weight of donated kidneys were missing on many patients, this variable was removed from multivariate analysis.
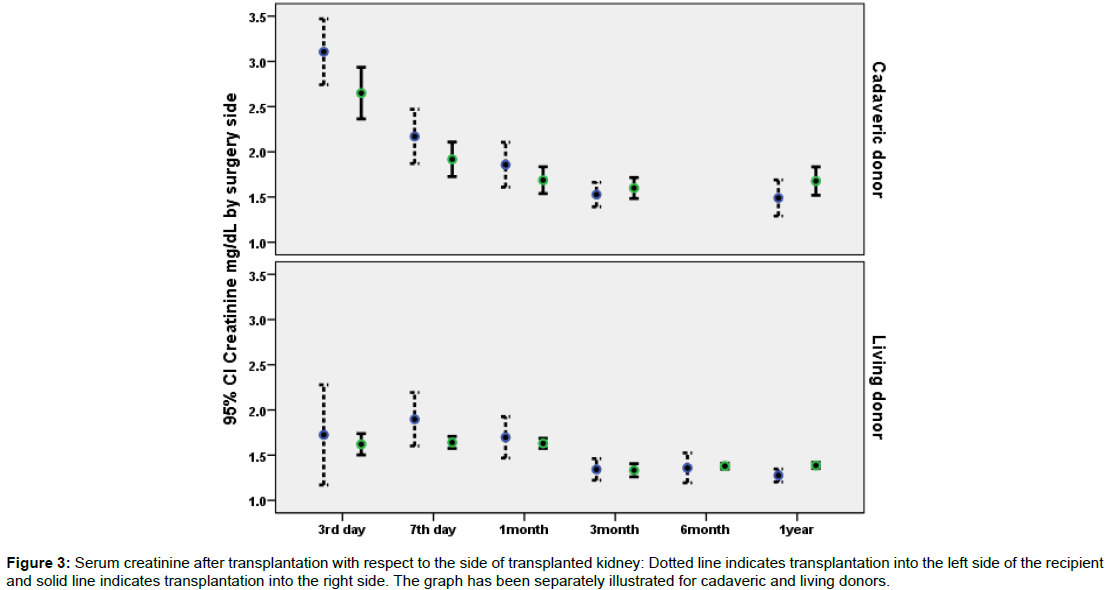
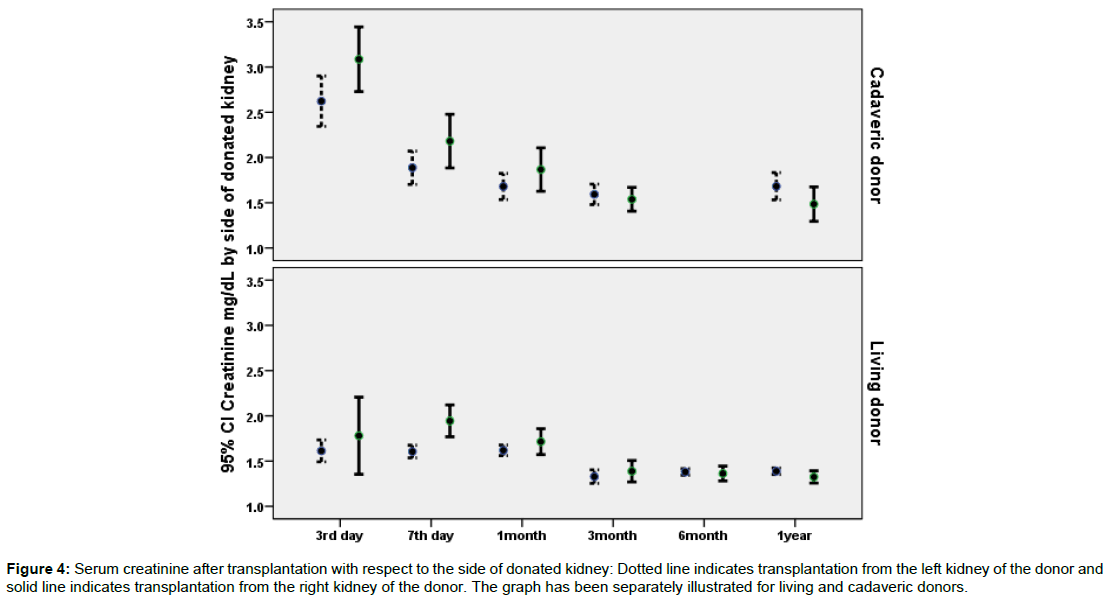
Data on the association of potential predictor variables and recipients 7th day creatinine has been illustrated in Table 2. According to the uni-variable statistical analysis presented in Table 2; donor type (cadaveric versus living), donor age, recipient age, side of implantation, side of donation, and sequence of implantation were statistically significant predictors of 7th day postoperative creatinine. However, type of arterial anastomosis and recipients’ comorbidities were not related to 7th day postoperative creatinine. Figure 1 illustrates postoperative creatinine after transplantation with respect to the side of implantation. Figure 2 illustrates postoperative creatinine after transplantation and in follow up with respect to the side of donation. Figures 3 and 4 illustrate the findings of Figures 1 and 2 separately for donor type (living versus cadaveric). As the age of donors and age of recipients was higher in cadaveric donors versus living donors, we performed multivariable linear regression to remove the confounding effect of donor and recipient age, the association of donor type (cadaveric versus living) with 7th day postoperative creatinine was no longer statistically significant in this regression model.
| Variable | Number | 7th day creatinine; mg/dL | P-value | |
|---|---|---|---|---|
| Gender of recipient | Male | 1904 | 1.73 ± 1.41 | 0.13 |
| Female | 1102 | 1.63 ± 2.04 | - | |
| Donor type | Cadaveric | 346 | 1.99 ± 1.44 | <0.001 |
| Living | 2660 | 1.66 ± 1.69 | - | |
| Donor age | ≤30 years | 2050 | 1.60 ± 1.37 | 0.001 |
| >30 years | 879 | 1.86 ± 2.22 | - | |
| Recipient age | ≤40 years | 1757 | 1.64 ± 1.79 | 0.02 |
| >40 years | 1249 | 1.78 ± 1.48 | - | |
| Side of implantation | Right | 2609 | 1.66 ± 1.64 | 0.004 |
| Left | 309 | 1.99 ± 1.94 | - | |
| Donor side | Right | 541 | 2.00 ± 1.80 | <0.001 |
| Left | 2388 | 1.63 ± 1.64 | - | |
| Living donor nephrectomy | Laparoscopy | 1302 | 1.67 ± 1.92 | 0.86 |
| Open | 935 | 1.69 ± 1.52 | - | |
| Sequence of transplantation | 1st | 2165 | 1.71 ± 0.04 | 0.002 |
| 2nd | 135 | 2.11 ± 0.18 | - | |
| 3rd | 12 | 2.64 ± 0.76 | - | |
| Artery anastomosis | End to End | 633 | 1.78 ± 1.43 | 0.53 |
| End to side | 225 | 1.75 ± 1.42 | - | |
| Other | 38 | 2.04 ± 2.63 | - | |
| Comorbidity | Yes | 924 | 1.63 ± 1.41 | 0.28 |
| No | 1349 | 1.71 ± 1.95 | - |
Table 2: Association of donor and recipient factors with 7th day postoperative creatinine.
Figure 3: Serum creatinine after transplantation with respect to the side of transplanted kidney: Dotted line indicates transplantation into the left side of the recipient and solid line indicates transplantation into the right side. The graph has been separately illustrated for cadaveric and living donors.
Figure 4: Serum creatinine after transplantation with respect to the side of donated kidney: Dotted line indicates transplantation from the left kidney of the donor and solid line indicates transplantation from the right kidney of the donor. The graph has been separately illustrated for living and cadaveric donors.
For transplantations from the left kidney of the donor, no statistically significant difference was observed between 3rd day, 7th day and one month serum creatinine when transplantation was performed on the right side or the left side of the recipient.
For transplantations from the right side of the donor, serum creatinine in the 3rd and 7th postoperative days were higher when kidney was transplanted into the left side of the recipient (P<0.001 and P=0.05 respectively). This statistically significant difference in 3rd and 7th day postoperative creatinine with respect to transplantation side was no longer observed in the 1st month after operation and thereafter. However, the frequency of transplantations from living donors was 99% when the kidney was implanted in the right side of the recipient versus only 61% living donors when the kidney was implanted in the left side of the recipient (p<0.001).
As the sequence of 1st, 2nd and 3rd transplantations was different between right and left implantations; and also to remove the potential confounding effects of donor and recipient age; we performed a linear regression analysis with enrolling potential confounders including donor age, recipient age, and sequence of transplantation with side of implantation into the model. The results of the regression model outlined in Table 3, indicates the lack of significance of side of surgery after enrolment of the above mentioned confounders in the model. A second regression analysis was performed for donor side after enrollment of donor age, recipient age, and sequence of transplantation into the model, in this model the influence of donor side on 7th day postoperative creatinine was still statistically significant after enrollment of the above mentioned variables. Briefly, left donated kidneys were associated with better 7th day postoperative creatinine in comparison with right donated kidneys. Due to co-linearity between donor side and side if implantation, we could not perform one regression taking both donor side and recipient side simultaneously into one model and separate models were used for each of them.
| Model Number | Variables | B | SE of B | P-value |
|---|---|---|---|---|
| 1 | Donor age | 0.006 | 0.002 | 0.003 |
| Recipient age | 0.006 | 0.001 | <0.001 | |
| Sequence of transplantation | 0.134 | 0.045 | 0.003 | |
| Side of implantation | -0.002 | 0.046 | 0.97 | |
| Comorbidity | -0.03 | 0.024 | 0.21 | |
| 2 | Donor age | 0.006 | 0.002 | 0.003 |
| Recipient age | 0.006 | 0.001 | <0.001 | |
| Sequence of transplantation | 0.126 | 0.042 | 0.003 | |
| Side of donation | 0.135 | 0.03 | <0.001 | |
| Comorbidity | -0.02 | 0.024 | 0.4 |
Table 3: Results of the multivariable regression analyses for investigation of the influence of donor side and side of implantation on the 7th day postoperative creatinine.
Complications were observed in 170 patients and included: Death in 53 patients, rejection in 71 patients, hematoma in 3 patients, need for ureteral re-implantation in 19 patients, vein thrombosis in 17 patients, reactivation of tuberculosis in 2 patients, arterial thrombosis in 3 patients, lymphocele formation in 1 patient and kidney cancer in one patient. Table 2 summarized the frequency of complications based on the side of donated kidney, side of kidney implantation, and side of kidney implantation for right kidney donations. Complications in recipients according to the Clavien-Dindo categories (19) of 0, 2, 3b, 4a, 4b and 5 were observed in 89, 0, 1.1, 7.8, 0.6 and 1.7% of left implantations and 93, 0.3, 0.8, 2.7, 0.8, and 2.3% of right implantations (p=0.007). Clavien-Dindo grades 0, 2, 3b, 4a, 4b and 5 were observed in 91.4, 0.3, 0.8, 3.4, 1.0 and 3.1% of transplantations from left donor kidneys and in 87.5, 0.2, 1.1, 5.7, 1.9, and 3.6% of transplantations from right donor kidneys (p=0.07). It is noteworthy that failure of graft which terminated in recipient need for dialysis was considered as a grade 4a complication in our grading of Clavien-Dindo complications.
Discussion
We appraised the aftermath of right and left kidneys procured from the deceased and living donors. Recipients of left donated kidneys revealed better allograft short term function in terms of 7th day postoperative creatinine in the crude data. Recipients of right kidneys that transplanted in left side were more likely to experience low graft function within the first week after transplantation but after one month their outcome became similar to recipients of right kidney transplanted in right side. Because our data was inhomogeneous in age, type of donor (cadaveric or living, open nephrectomy or Laparoscopic donor nephrectomy) and sequence of transplantation, to remove mentioned confounding factors in first week effect, we performed regression analysis including all proved confounders in univariate analysis into the regression model. The result of this model revealed that donor side of kidney is yet statistically significant of 7th day postoperative creatinine while the side of implantation was not.
Vacher-Coponata et al. reported that recipients of right-sided kidneys obtained from heart-beating brain-dead donors are at higher risk of emerging delayed graft function, poorer graft function and higher risk of graft loss in the first year after transplantation.
The lengthier vein of the left kidney allows easier implantation without additional procedure. Nonetheless, right kidneys have longer arteries and give the impression to suffer risk of kinking. Withal shorter right renal vein may complicate venous anastomosis especially in obese recipients with deeper iliac vessels [7,11]. Also the frequency of anatomical variations in right kidney vessels is higher than the left, and these vessels are usually smaller in size which makes it prone it to thrombotic complications [12,13] Many surgeons especially in open donor nephrectomy obtain a patch of inferior vena cava if possible that can be more challenging in laparoscopic donor nephrectomies although results of right laparoscopic donor nephrectomy in overall is same in comparison with the left side.
Johnson et al. reported 201 renal implantations and Salehipour et al. reported 60 recipients with a comparable delayed graft function proportion for right and left kidneys although the number of their patients is rather small. Similar findings have also been reported for laparoscopic living donor renal transplant operations.
Khalil et al. evaluated 58 599 living donor transplants and reported more delayed graft function and more vessel thrombosis in right kidney recipients with a hazard ratio of 1.38 and 1.48 although graft survival difference was little. They showed that laparoscopic conversion to open was more in right donor nephrectomy. While our study yields higher postoperative creatinine just in first week for right kidney transplantation in either side of recipient however becomes similar in first year. Lechevallier et al. in a retrospective study of 257 patients, advocated that delayed graft function is more prevalent in right kidney recipients [9,14-18,20,21].
A review article by Phelan et al. revealed that delayed graft function were much higher during the 1990s at 25–30% but its rate gradually decreased to 16%. Up-to-date advances in kidney transplant management may have amended any shortfall accompanying with right-sided allografts and cleared different long-term results in current studies.
Although the exact reason cannot be proven, the anatomical differences between left and right kidney prejudice to a more difficult surgery, more anastomosis duration and extended warm ischemia time in case of transplantation from right kidneys. This phenomenon may explain our study results about inferior one-week outcome of right sided donated kidneys. Our crude results as explained in the results section reveals that when the right kidney in implanted in the right side of the recipient the outcome is superior than when it is implanted as suggested in the left side of the recipient. This could mean that in addition to difficulties with donor kidney explained above; recipient left side more is associated with a more difficult operation because of deeper iliac vessels. Interestingly for the left donor kidney, the crude data indicate that side of implantation was not associated with any difference in the postoperative function of implanted kidneys. This may because of longer left kidney vein and its easier anastomosis. Another possibility could be that the larger size of left kidney (146 cm3) versus right kidney (1343) may compensate operational insults in the recovery period sooner [10,14,21]. Nonetheless, if right kidney transplanted in left side of recipient outcome will be similar with other groups in one year follow-up after first week inferiority (Table 4).
| Complication for side of kidney implantation | Left; N (%) | Right; N (%) | P-value |
|---|---|---|---|
| Death | 3 (0.009) | 50 (0.017) | 0.54 |
| Rejection | 14 (0.40) | 57 (0.020) | |
| Hematoma | 0 (0) | 3 (0.001) | |
| Ureteral re-implantation | 2 (0.006) | 17 (0.006) | |
| Vein thrombosis | 1 (0.003) | 16 (0.006) | |
| Tuberculosis re-activation | 0 (0) | 2 (0.001) | |
| Arterial thrombosis | 0 (0) | 3 (0.001) | |
| Lymphocele | 0 (0) | 1 (0.0003) | |
| Kidney cancer | 0 (0) | 1 (0.0003) | |
| For side of kidney donation | |||
| Death | 65 (0.025) | 17 (0.027) | 0.52 |
| Rejection | 69 (0.026) | 27 (0.043) | |
| Hematoma | 3 (0.001) | 0 (0) | |
| Ureteral re-implantation | 16 (0.006) | 5 (0.008) | |
| Vein thrombosis | 13 (0.005) | 6 (0.010) | |
| Tuberculosis re-activation | 2 (0.001) | 0 (0) | |
| Arterial thrombosis | 3 (0.001) | 3 (0.005) | |
| Lymphocele | 1 (0.0004) | 0 (0) | |
| Kidney cancer | 1 (0.0004) | 0 (0) | |
| Complications of right donated kidneys based on side of implantation | |||
| Death | 2 (0.007) | 5 (0.015) | 0.83 |
| Rejection | 8 (0.027) | 14 (0.042) | |
| Implantation | 1 (0.003) | 4 (0.012) | |
| Vein thrombosis | 1 (0.003) | 4 (0.012) | |
Table 4: Complications with respect to side of donated kidney, side of kidney implantation and side of implantation for right donated kidneys.
There are some limitations in this study. First, we accept the characteristic flaws of any single-center study. However narrowing the study to one center lessens the confounding effects of numerous peri-operative practices. We performed a linear regression analysis with enrolling potentially confounding variables including donor age, recipient age, comorbidities, and sequence of transplantation with either side of implantation or side of donations into two models to increase the validity of the conclusion. Second, the operating surgeons might have selected left and right kidneys based on patient situations. Although cold ischemia times in living donor groups were comparable, deceased donors may confound outcome. Although arithmetical modifications were prepared, the likelihood of enduring confounding cannot be totally omitted. Lastly, estimated glomerular filtration rate is more valuable than creatinine which we practiced in comparing result.
Conclusion
Our data suggest that side of donor or recipient surgery in kidney transplantation is not important in one year follow-up. However, right donor kidneys were associated with higher 7th day postoperative creatinine in the recipients. Complications for side of donation or transplantation were not significantly different.
References
- Feduska NJ (1993) Donor factors in cadaveric renal transplantation. Clin Transpl pp: 351-357.
- Kim SJ, Lee HH, Lee DS, Lee KW, Joh JW, et al. (2004) Prognostic factors affecting graft and patient survival in cadaveric and living kidney transplantation. Transplant Proc 36: 2038-2039
- Weiss-Salz I, Mandel M, Galai N, Nave R, Boner G, et al. (2004) Factors associated with primary and secondary graft failure following cadaveric kidney transplant. Clin Transpl 18: 571-575
- Campbell SB, Hothersall E, Preston J, Brown AM, Hawley CM, et al. (2003) Frequency and severity of acute rejection in live- versus cadaveric-donor renal transplants. Transplantation 76: 1452-1457
- Gjertson DW (1992) Multifactorial analysis of renal transplants reported to the United Network for Organ Sharing Registry. Clin Transpl pp: 299-317.
- Janschek EC, Rothe AU, Holzenbein TJ, Langer F, Brugger PC, et al. (2004) Anatomic basis of right renal vein extension for cadaveric kidney transplantation. Urology 63: 660-664.
- Satyapal KS, Kalideen JM, Singh B, Haffejee AA, Robbs JV (2003) Why we use the donor left kidney in live related transplantation. S Afr J Surg 41: 24-26.
- Johnson DW, Mudge DW, Kaisar MO, Campbell SB, Hawley CM, et al. (2006) Deceased donor renal transplantation—does side matter? Nephrol Dial Transplant 21: 2583-2588
- Phelan PJ, Shields W, O’Kelly P, Pendergrass M, Holian J, et al. (2009) Left versus right deceased donor renal allograft outcome. Transpl Int 22: 1159-1163
- Santangelo M, Spinosa G, Grassia Clemente SM, Caggiano M, et al. (2008) In situ elongation patch in right kidney transplantation. Transplant Proc 40: 1871-1872.
- Hern´andez D, Rufino M, Armas S, González A, Gutiérrez P, et al. (2006) Retrospective analysis of surgical complications following cadaveric kidney transplantation in the modern transplant era. Nephrol Dial Transplant 21: 2908-2915
- Kang KY, Lee YJ, Park SC, Yang CW, Kim YS, et al. (2007) A comparative study of methods of estimating kidney length in kidney transplantation donors. Nephrol Dial Transplant 22: 2322-2327.
- Vacher-Coponat H, McDonald S, Clayton P, Loundou A, Allenand RDM, et al. (2013) Inferior early posttransplant outcomes for recipients of right versus left deceased donor kidneys: An ANZDATA registry analysis. Am J Transplant 13: 399-405
- Salehipour M, Bahador A, Jalaeian H, Salahi H, Nikeghbalian S, et al. (2008) Comparison of right and left grafts in renal transplantation. Saudi J Kidney Dis Transpl 19: 222-226.
- Lechevallier E, Dussol B, Luccioni A, Thirion X, Vacher-Copomat H, et al. (1998) Post-transplantation acute tubular necrosis: Risk factors and implications for graft survival. Am J Kidney Dis 32: 984-991.
- Swartz DE, Cho E, Flowers JL, Dunkin BJ, Ramey JR, et al. (2001) Laparoscopic right donor nephrectomy. Surg Endosc 15: 1390-1394
- Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240: 205-213.
- Khalil A, Mujtaba MA, Taber TE, Yaqub MS, Goggins W, et al. (2015) Trends and outcomes in right vs. left living donor nephrectomy: an analysis of the OPTN/UNOS database of donor and recipient outcomes–should we be doing more right-sided nephrectomies? Clin Transplant 30: 145-153.
- Kashiwadate T, Tokodai K, Amada N, Haga I, Takayama T (2015) Right versus left retroperitoneoscopic living-donor nephrectomy. Int Urol Nephrol 47: 1117-1121.
- Emamian SA,Nielsen MB, Pedersen JF, Ytte L (1993) Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR 160: 83-86.
Citation: Rad HM, Basiri A, Simforoosh N, Tabibi A, Kashi AH (2017) Side of Donation or Side of Implantation: Which One Could be a More Valuable Determinant in Kidney Transplantation? J Clin Exp Transplant 2: 116. DOI: 10.4172/2475-7640.1000116
Copyright: © 2017 Rad HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7547
- [From(publication date): 0-2017 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 6654
- PDF downloads: 893