Significant role of wild Genotypes of tomato trichomes for Tuta absoluta resistance
Received: 13-Apr-2018 / Accepted Date: 07-May-2018 / Published Date: 15-May-2018
Abstract
Tuta absoluta is a devastating pest of tomato. The use of host plant resistance to control pests is an interesting, potentially useful technique, but there is no known cultivated tomato resistant to T. absoluta. The objective of this resistance was to screen wild tomato accession and identify resistance mechanism against T. absoluta. Sixteen wild tomato accessions from six species were tested in two experiments. The tested parameters were damaged leaf area, oviposition rate, emerged larvae, adult and larvae survival, larvae and adult preference. Those parameters were correlated with the density of each trichome type and with LC-MS data. From no-choice test parameters accession LA1777, LA1718 and LA716 were the most resistant and LA1401 and LA1139 were the most susceptible, and all S. lycopersicum accessions were susceptible. From choice test, the accessions G1.1561, LA1718, LA716, LA1645, LA0483 and LA1408 were not preferred by the larvae, and the accessions LA1777 and G1.1561 were the least preferred by the adults. The accession LA1777 and LA716 were out of the most resistance. The resistance of this genotype was related to the presence of trichome Type I and IV.
Keywords: Tuta absoluta; Tomato; LA1777; LA716; Glandular Trichomes; LC-MS
Introduction
Tomato (Solanum lycopersicum, formerly, Lycopersicon esculentum Mill.) is the second most important vegetable crop after potato in the world [1] and the used as a model plant species for genetic studies to qualitative traits, and biotic and abiotic stress tolerance. In addition to being consumed as a fresh vegetable, it is also used as a salad, in spicy sauce, as a puree, a pickle, soups that is also a significant source of vitamin A and C as well as source of lycopene, a carotenoid pigment with antioxidant properties and in many other forms, depending up on the growing area [2]. It is estimated that 4.6 million ha of tomatoes are grown annually worldwide producing more than 126 million metric ton. In the U.S., it is grown in an area of 175,000 ha producing about 11.5 million Metric ton annually [1]. The tomato systematics of genus description was under debate and reorganized several times [3]. Nowadays, tomatoes are recognised as nested inside the genus Solanum [4]. Together with the cultivated tomato (S. lycopersicum) , there are 17 recognised wild species: S . cheesmaniae, S. galapagense, S. chilense, S. chmielewskii, S. lycopersicum, S. habrochaites, S. neorickii, S. pennellii, S. arcanum, S. corneliomulleri, S. huaylasense, S. peruvianum, S. pimpinellifolium, S. juglandifolium, S. lycopersicoides, S. ochranthum, S. sitiens [4]. All tomato species are diploid (2n=24) and exhibits great difference in morphological characters such as matting system, habitat preference, trichome densities and types, resistant to pest and diseases and other agronomic traits important for breeding [5].
Despite its importance for consumption and genetic studies tomato is highly challenged by different biotic and abiotic factor and among the biotic factors, insects like Tuta absoluta ranked the prioritized area.
Tuta absoluta was described in 1917 by Meyrick as Phthorimaea absoluta from specimens collected in Peru, and it classified under family Gelechiidae, order Lepidoptera and phylum Arthropoda [6]. After its first description, this insect has spread with the tomato producing areas of South America become commonly known as a tomato leaf miner [7]. In the year 2006, it was detected for the first time in Europe, Spain. Since then, it has spread rapidly to all Mediterranean countries, North Europe, Middle East and Africa [8,9].
Tomato is the main host of Tuta absoluta . In tomato plants, the female adults lay eggs on all above ground part of the plant (leaves, shoots and flowers as well on the fruits). Despite the clear preference of this insect on tomato species, it also affects common bean, potato, eggplant and tobacco [9,10]. It also has been using weeds as an alternative host such as; Lycium chilense, Solanum nigrum and Datura stramonium ; Datura ferox and Nicotiana glauca [11,12].
Tomato leaf minor is one of the major devastating pests of processing and fresh tomatoes, both in greenhouse and open field [9]. Tuta absoluta larvae can absolutely destroy the tomato canopy by excavating the leaves, stems and buds; and burrows into fruits causing the quality decline of fresh tomato and yield loss that range from 50% to 100%. Because of its biology and behaviour this insect is hard to control. Chemical control methods have been trusted, but the feeding habits of the larvae, the increasing number of resistant strains of this pest, together with the negative impact of the chemical into the environment makes the chemical control method not sustainable [13,14]. In addition, mating disruption methods (like sex pheromones) have been used to control T. absoluta , but this technique is a lot more expensive than pesticide applications. The biological control methods are still under development for further investigation to find it in an operative way [15].
Because of stated above, exploring wild accession of tomato for new sources of resistance is needed. Resistant to several herbivore insects have been described in wild relatives of the tomato. Resistant to Bemisia tabaci and Trialeurodes vaporariorum was described in S. galapagense [16]. Insect resistance in wild relatives of tomato was associated with the presence of glandular trichomes and secondary metabolites such as acyl sugars and methyl ketones [17-19]. Insect resistance is generally associated with the presence of trichome types and densities [20]. Trichomes are specialized structures on the epidermis [21] and based on the presence/absence of a glandular head trichomes can be classified as glandular (type I, IV, VI & VII) or nonglandular trichomes (types II, III, V). Glandular trichomes are the sites of synthesis and storage of secondary metabolites. This study screened resistant accessions from 16 genotypes of tomato against Tuta absolute and identified the mechanism involved in the resistance.
Materials and Methods
Plant materials
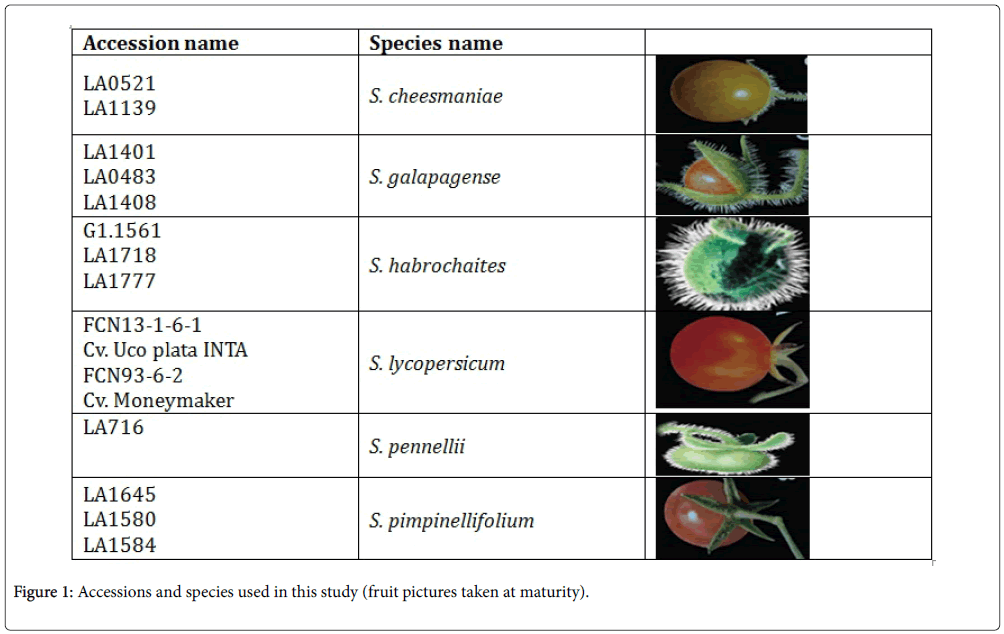
Seeds of sixteen tomato accessions were ordered from the Tomato Genetics Resource Centre (TGRC) as its name listed (Figure 1). To break seed dormancy, the tomato seeds were treated with 1% sodium hypochlorite for 30 minutes, washed and sown immediately. Ten seeds from each 16 accessions were sown on January 25, 2013 in a polyethylene pot with soil and grown for three weeks in mass. Then, five seedlings were transplanted individually to polyethylene pot and allowed for further growth of five weeks. And it was acclimated in a greenhouse (Unifarm, Wageningen UR) for a period of two months at 23°C and 77% relative humidity. After this period, plants moved into the insect proof greenhouse under 25°C and 75% relative humidity, where all the screening were carried out. Sixteen accessions were arranged in a randomized complete design with five replications. Afterwards, choice and no-choice experiments were implemented.
Tuta absoluta rearing
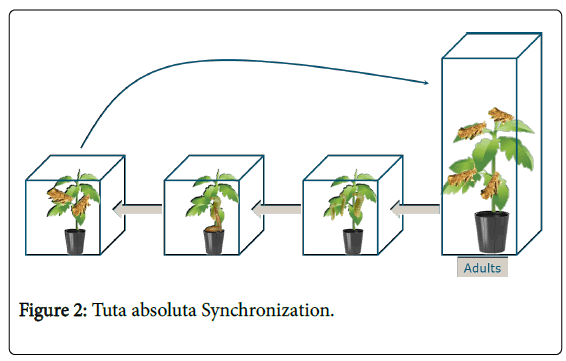
Tuta absoluta was reared in a climate room (Nergena, Wageningen UR) at 25°C, RH 75% and 16-8h day-night. It was synchronized two times a week and maintained by feeding the clean tomato plant, S. lycopersicum cv . Moneymaker. To get each stage of T. absoluta , four insect cage boxes were prepared, which contained adults, larvae, pupas and the mixture of all, the left-side box (Figure 2). Adults were laying eggs in the larger box on the right side. The plant which contained eggs from larger box moves to the next box to obtain larvae instar I and on the next time of synchronization, the plant that contained the larvae go to the third box to get larvae instars II-IV, as well pupa had been present. New adults have been found from the last box (left side) which can also contain other stages, and those adults trapped and released back to the larger box with new plant by using an insect trapper jar. To maintain eggs and available feed for larvae growth, new plants was placed in the first three right-side box.
Experiments
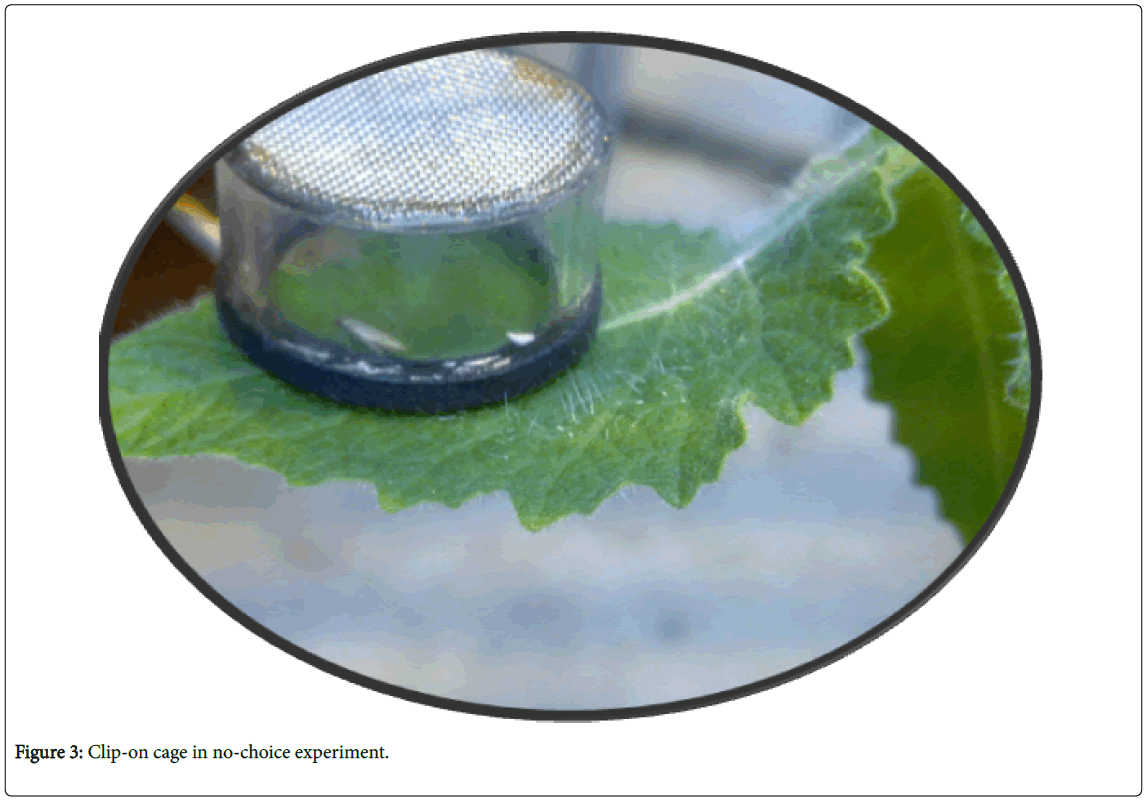
No-choice experiments: Clip-on cage test: Three months after sowing, a clip-on experiment was performed. Which contained one insect per clip-on cage (2.5 cm diameter and 1.0 cm height); one clipon per plant and five plants per accession were prepared for this test (Figure 3). The cages were placed on the first to third fully expanded leaf from the top of the plant. Three parameters were considered; adult survival (AS), oviposition rate (OR) and a ratio of emerged larvae from eggs (EL); following the formula described [22].
Adult survival (AS): one non-sexed T. absoluta adult was placed into a clip-on cage. The clip-on cage was placed on the second to third fully expanded leaf on the adaxial part of the leaflet. The cage was left in a period to seven days, and the survival of the insects was counted daily. Because in the seven days, there were not adults alive and then AS was calculated out only at the first two days, after inoculation.
Oviposition rate (OR): T. absolute adults were anaesthetized using CO2 and one female were placed per clip-on cage. One clip-on cage per plant and five plants per accession were used. Seven days after infestation, the number of death, live T. absolute and laid eggs were recorded on the last day.
Emerged larvae (EL): five days after counting eggs, the number of larvae per cage was counted and the ratio larva/eggs were calculated.
After all, one-way ANOVA was analysed for each parameter followed by the least significant difference.
Detached leaflet test: Five leaflets from the upper first and second leaf branches from each of the 16 accession were detached. Individual leaflets were put per petri-dish, which were prepared by dropping a little of agar water (1.5%) at the one end. Then two larvae per petridish were applied. The larvae were starved for a period of two hours before inoculation. One leaflet per petri-dish and five petri-dishes per accession were used. All 85 petri-dishes were wrapped with para film and set in the growing conditions. In this experiment, two variables were considered, damaged leaf area and larvae survival.
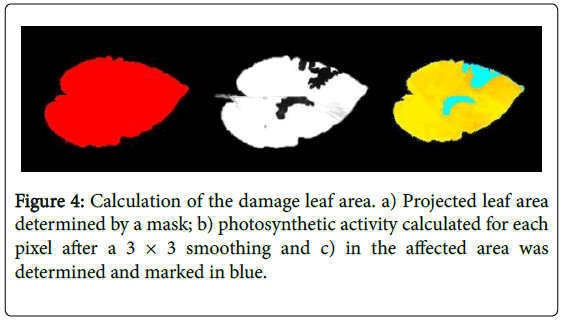
Damaged leaf area: The larvae were left to feed over a period of 24 hours. After that period, the cages were moved to a dark area for 15 minutes, and pictures were taken to measure damaged leaf area through chlorophyll fluorescence. The pictures were performed using mobile camera version 3 with an exposure of 800 microseconds and an LED pulse 2.5 (DC17). Scoring was performed using the CFII analysis software single pulse option. First, the data were retrieved in the software, then the projected leaf area was determined by a mask, thirdly the photosynthetic activity was calculated at each pixel after a 3 × 3 smoothing, and finally, the affected area was determined that marked and token out in blue colour (Figure 4). Then the damaged leaf area of the infected pixels transformed to Log2 (x + 1) and general analysis of variance was done using the total leaf area (mask pixel) as a covariate.
Larvae survival: forty-eight hours after inoculation, survived larvae were counted. The method of calculating larva survival was as proposed [22] and data were analysed in one-way ANOVA.
Choice experiment: Detached leaflet test: In choice test, each tomato accession was compared to the reference cultivar Moneymaker. This experiment was done to examine two parameters, larvae preference and adult settlement preference.
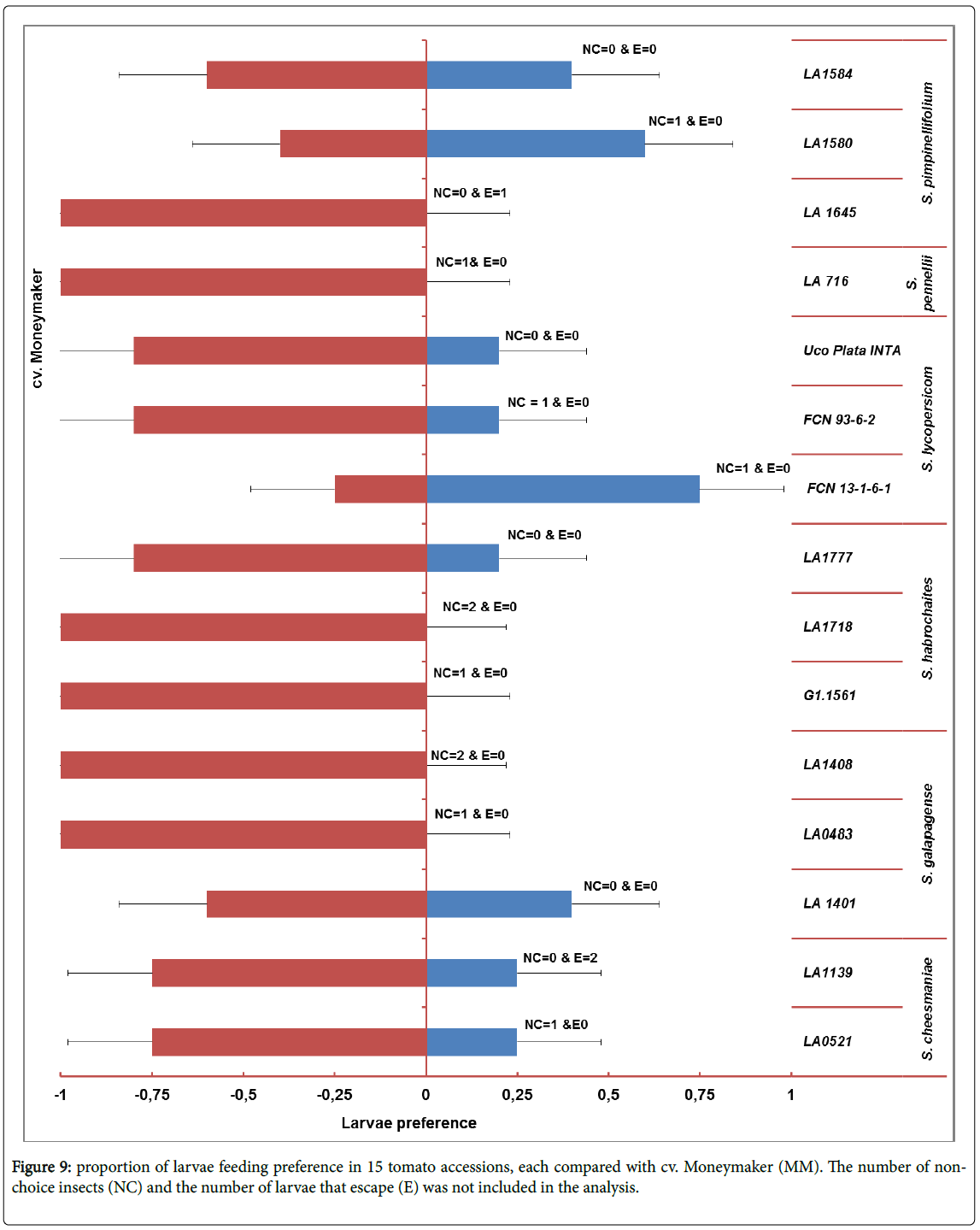
Larvae preference: One leaflet from the testing accession and one leaflet from the cv. Moneymakers (control tomato genotype, which is extensively preferred by the larvae) were interleaved on a rectangular petri-dish to protect the leave from drying little agar water (1.5%) in two opposite corners of the petri-dish was poured. Afterwards one larva was put in the middle of the petri-dish and five petri-dishes per accession were used. Then the larvae going to choice either control, Monymaker or other genotype to eat (Figure 3). Data was collected 24 hours after inoculation. The proportion of larvae between the two categories was calculated. Data were analysed by binomial distribution test. The larvae that did not make a choice or have escaped were excluded from the analysis.
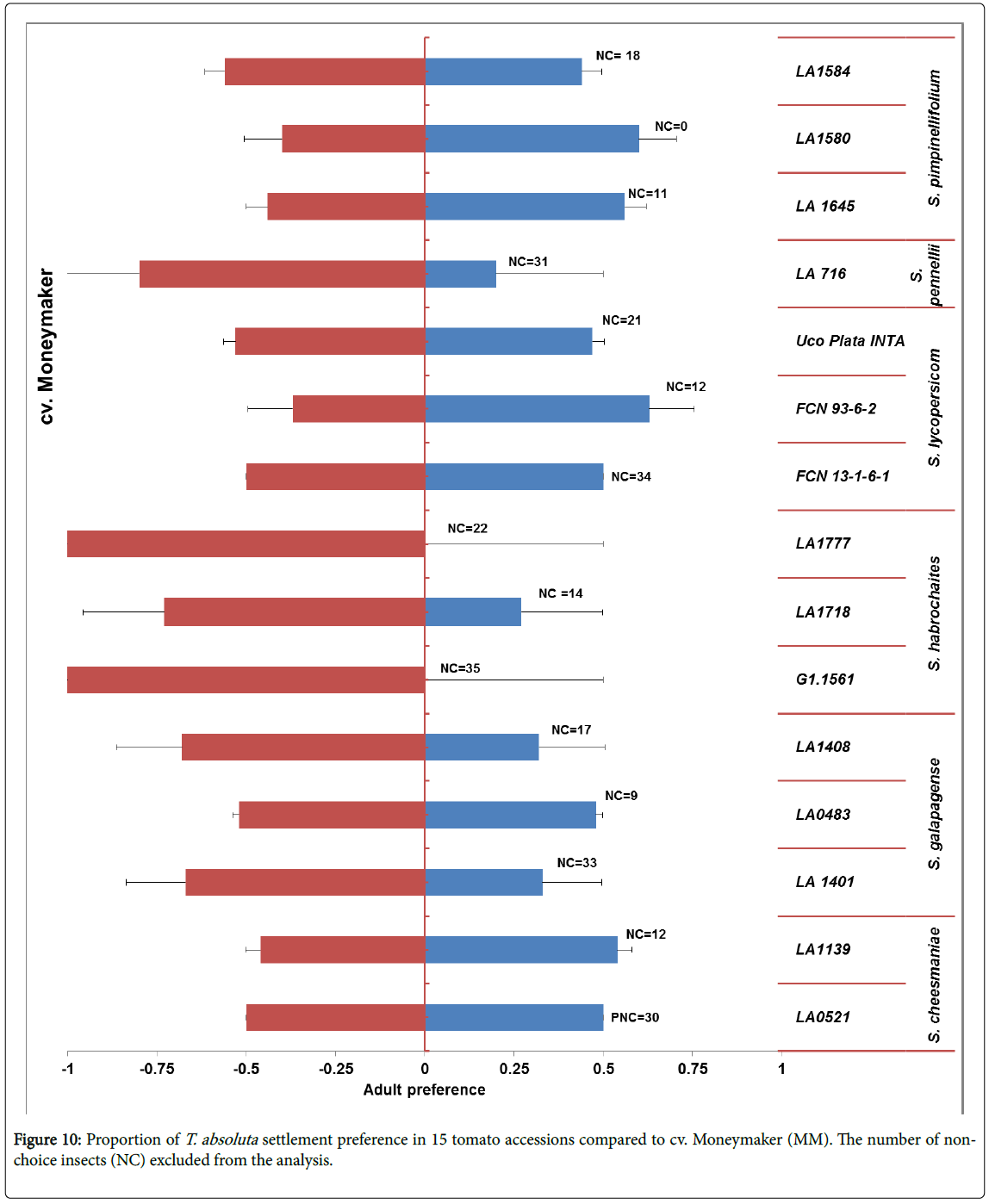
Adult settlement preference: In this choice test, three leaves from each of the 15 accession compared to three leaflets of the cv. Moneymaker (six leaves per tray) (Figure 5). Leaflets were put in wet Oasis floral foam, on a tray (5 cm height and 35 cm width) and arranged in a circle. Eighteen T. absoluta adults were placed in the centre and repeated two times in the 45-minute interval. The proportion of insects that made a choice was calculated, and data were analysed by a binomial distribution test. Insects that did not make a choice were not included into the analysis.
Trichome morphology: The type and density of trichomes were analysed per accession. Trichomes were counted by using a binocular microscope on adaxial and abaxial leaflets of each of the 16 accessions. Ten sample leaflets were detached from the second to third fully expanded leaves of each accession. Based on the identified morphology of trichome types [23], the densities of each trichome type were recorded from an area of 0.785 mm2 on the abaxial and adaxial sampled leaflets of each accession. The density of each trichome types was analysed by one-way ANOVA. Finally, densities of each trichome type were correlated to the choice and no-choice test parameters.
Correlation of acyl-sugar with density of trichome type, choice and no-choice test: The metabolite data of Liquid chromatography–mass spectrometry (LC-MS) used to find acyl sugars as discussed in Lucatti et al. (2013). Eight accessions were used for this correlation analysis. Those were S. lycopersicum accessions (FCN 13-1-6-1, FCN 93-6-2, cv. Uco Plata INTA and cv. Moneymaker), LA1584 (S. pimpinellifolium ), LA716 (S. pennellii ), LA1401 (S. galapagense ) and LA1777 (S. habrochaites ). The relative concentration of metabolites was transformed to Log2 (x + 1), and auto scaled to (X̅ and SD are the means and standard deviations of a memetabolite across accessions, respectively and Xi is the amount of a metabolite of a given accession). Auto scaled data were correlated with the transformed data of choice, no-choice tests and trichome density.
Result
No-choice experiments
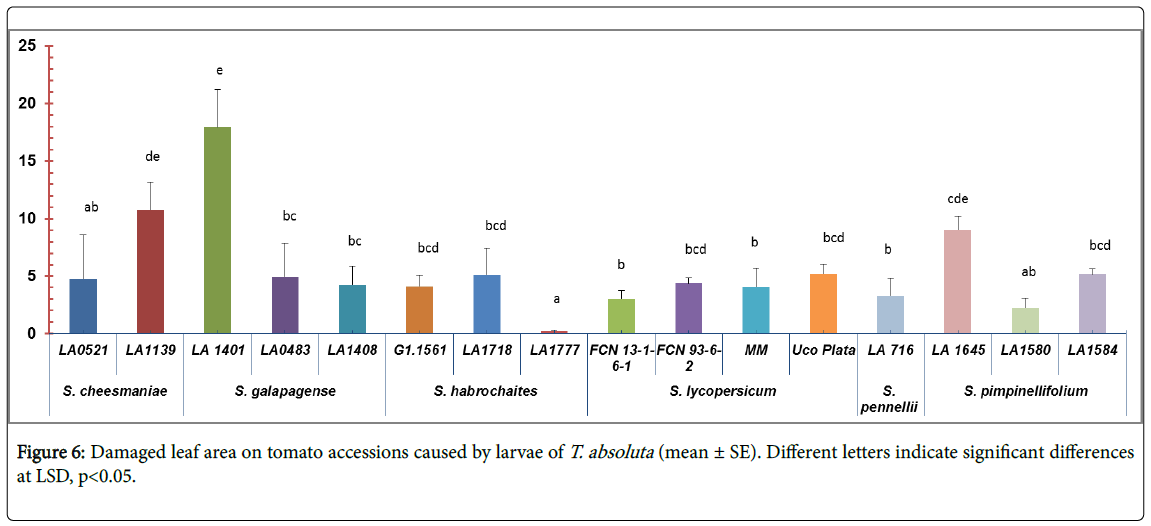
Resistance to larvae feeding: Significant differences were found among accessions in a damaged leaf area (F=4.28; df=15, 64; p<0.01) (Figure 6). The accession LA1777 (S. habrochaites) was the least affected (0.24 ± 0.19 mm2) (Table 1, appendix). The highest value of damage was observed on the accession LA1401 of S. galapagense (17.94 ± 7.34 mm2) (Figure 1, appendix).
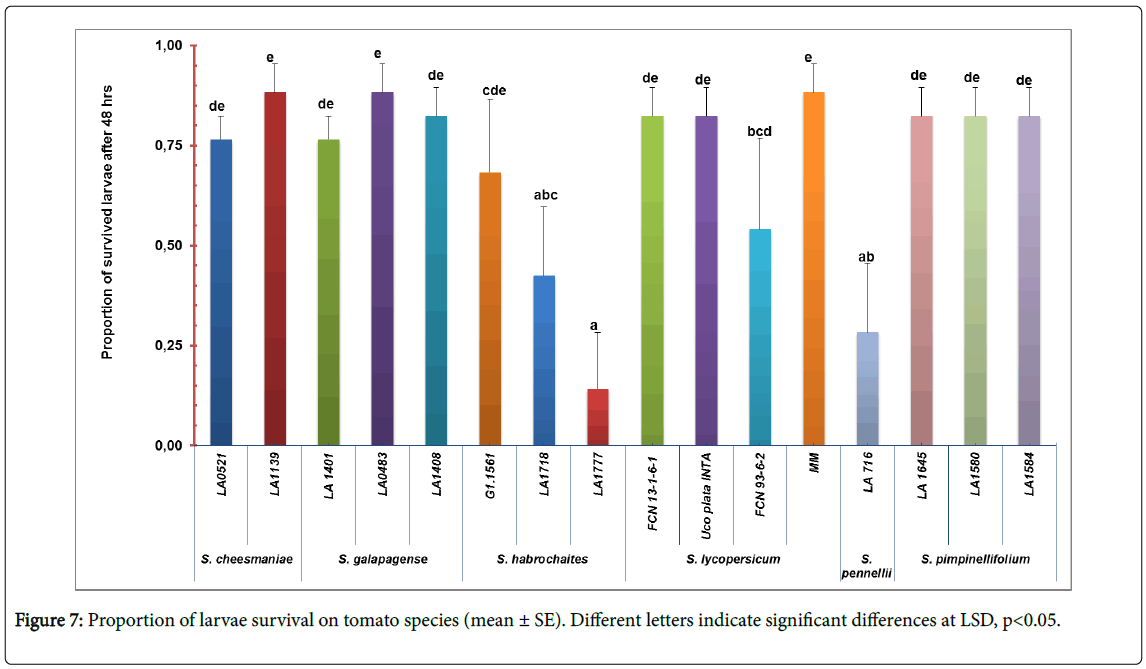
Survival test: To examine the survival ability of the insect two parameters, larvae and adult survival were tested. Significant difference was detected in larvae survival among tomato accession (F=4. 12; df=15, 64, p<0.01) (Figure 7). Accessions LA716 (S. pennellii ), LA1718 and LA1777 (S. habrochaites ) has the lowest number of surviving larvae (0.28 ± 0.39, 0.42 ± 0.39 and 0.14 ± 0.32, respectively) (Figure 1, appendix). No statistically significant differences were observed in the adult survival test (F=0. 68; df=15, 64; p=0.79).
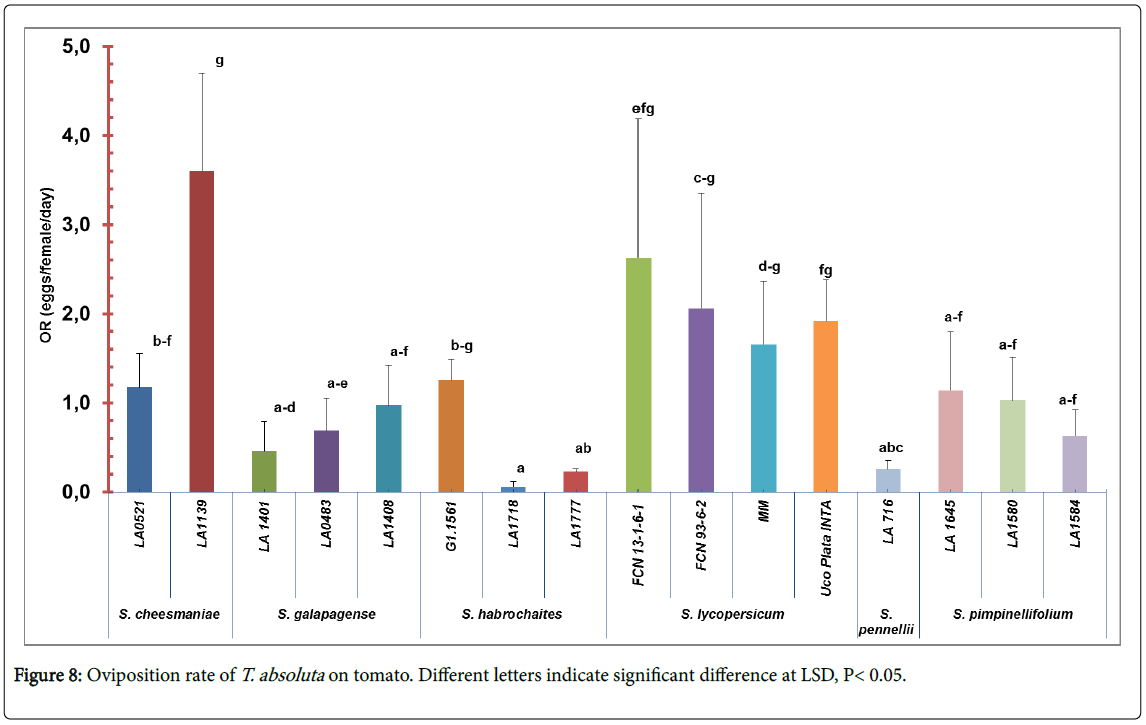
Oviposition rate and emerged larvae: The oviposition rate of T. absolute were significantly different among accessions (F=2. 57; df=15, 64; p<0.01) (Figure 8). The lowest oviposition rate was found in accessions LA1718 (0.06 ± 0.13) and LA1777 (0.23 ± 0.08) and in the accession LA716 (0.26 ± 0.21). On the other hand, accession LA1139 of S. cheesmaniae (3.6 ± 2.45) and all S. lycopersicum accessions had a high number of eggs per female per day (8.26 ± 8.99 average in total) (Figure 1, appendix).
There were no statistically significant differences among tomato accessions for emerging larvae (F=0. 87; df=15, 64; p=0.60).
Choice experiments: Larvae preference: In this experiment each accession were tested to the control cultivar Moneymaker (Figure 9) and significant difference of binomial test detected. The larvae did not choose the accession LA 1645 (S. pimpinellifolium ), LA716 (S. pennellii ), LA0483 and LA1408 (S. galapagense ), and accession G1.1561 and LA1718 (S. habrochaites ) when compared to cv. Moneymaker.
Adult settlement preference: Though most of the adults did not make a choice, statistically significant difference from the binomial test was observed. Adult preference of each wild tomato accession was compared with the reference CV. Moneymaker Tuta absoluta did not settle on accession LA1777 and G1.1561 of (S. habrochaites ) (Figure 10).
Correlation of trichomes with choice and no-choice tests
Significant differences were detected among accessions in density of each trichome type at 0.785 mm2 (ANOVA, PTable 1). From the glandular trichomes, Type IV not able to be found in all accessions of S. lycopersicum and S. cheesmaniae , and from LA1645 of S. pimpinellifolium . Trichome Type I was also absent in accessions LA1139 of S. cheesmaniae , LA1645 of S. pimpinellifolium and in all of S. lycopersicum accession except cv. Uco plata INTA. Trichome Type VI grew in all the studied accessions.
| Mean ± SD | ||||||
|---|---|---|---|---|---|---|
| Species Name | Accession Name | Type I | Type III | Type IV | Type V | Type VI |
| S. cheesmaniae | LA0521 LA1139 |
0.1 ± 0.32 ab 0 ± 0.00a |
0 ± 0.00a 0 ± 0.00a |
0 ± 0.00a 0 ± 0.00a |
7.5 ± 2.32c 32.1 ± 5.59e |
10.5 ± 5.32cdef 2.1 ± 1.20a |
| S. galapagense | LA 1401 LA0483 LA1408 |
0.6 ± 1.07abc 0.6 ± 1.07abc 0.6 ± 1.35abc |
0.6 ± 1.35abcd 0 ± 0.00a 0 ± 0.00a |
18.2 ± 4.49d 10.9 ± 2.85c 27.1 ± 4.31e |
10.5 ± 3.92c 4.7 ± 1.89b 0 ± 0.00a |
10.4 ± 4.74cdef 7.6 ± 2.12cd 3.6 ± 1.90b |
| S. habrochaites | G1.1561 LA1718 LA1777 |
1 ± 1.41c 0.5 ± 0.71abc 3.7 ± 1.34d |
1.6 ± 1.35e 0.9 ± 1.10cde 1.5 ± 1.58e |
16 ± 3.92d 25.7 ± 7.04e 19.7 ± 4.55d |
0.6 ± 1.58a 0 ± 0.00a 0 ± 0.00a |
6.6 ± 1.84c 8.7 ± 3.37cde 9.4 ± 2.63cdef |
| S. lycopersicum | FCN13-1-6-1 FCN93-6-2 MM Uco Plata INTA |
0 ± 00a 0 ± 0.00a 0 ± 0.00a 0.6 ± 0.70abc |
0.1 ± 0.32e 1.6 ± 1.26e 1 ± 1.70bcde 0.8 ± 0.92cde |
0 ± 0.00a 0 ± 0.00a 0 ± 0.00a 0 ± 0.00a |
20.2 ± 4.49d 21.2 ± 4.26d 32.9 ± 6.12e 19.2 ± 5.51d |
12.8 ± 3.08f 4.4 ± 2.01b 10.4 ± 2.80def 9.4 ± 2.37cdef |
| S. pennellii | LA 716 | 0.3 ± 0.67abc |
0.2 ± 0.42abc | 18.6 ± 3.44d | 0 ± 0.00a | 4.4 ± 1.43b |
| S. pimpinellifolium | LA 1645 LA1580 LA1584 |
0 ± 0.00a 0.2 ± 0.42ab 0.7 ± 0.95bc |
0 ± 0.00a 1.1 ± 1.45de 1 ± 0.94de |
0 ± 0.00a 2.8 ± 2.90b 16.7 ± 2.83d |
40.5 ± 4.12e 17.6 ± 8.18d 9.7 ± 6.17c |
3.1 ± 1.29ab 22.4 ± 9.29g 11.3 ± 3.30ef |
Table 1: Mean number (± SD) of trichome types on leaflets of wild and cultivated cv. Moneymaker in 0.785 mm2 area. Different letters indicates significant differences at LSD, p<0.01
On the other hand, from the non-glandular trichomes; Type III is not present in all of S. cheesmaniae accessions, in accession LA1645 of S. pimpinellifolium and in all S. galapagense accession except LA 1401. Trichome Type V grow up in LA 716 (S. pennellii ), LA1408 (S. galapagense ) and from all accessions of S. habrochaites except G1.1561.
The significant Pearson correlation was detected between larvae survival and Type I trichomes (r=0.61), and oviposition rate with Type IV and V (r=0.72, and +0.66, respectively) (Table 2). No significant correlation was observed in the damaged leaf area, adult survival, emerged larvae, larvae and adult preference.
| Parameters of no-choice test | ||
|---|---|---|
| Trichome Type | OR | LS |
| Type_I | -0,44 | -0,61* |
| Type_III | -0,18 | -0,33 |
| Type_IV | -0,72* | -0,48 |
| Type_V | 0,66* | 0,43 |
| Type_VI | -0,17 | 0,05 |
* : indicates parameters which have a significant difference at the 5 % significance level of Pearson correlation
Table 2: Correlation of oviposition rate (OR) and larvae survival (LS) with trichome types at Pearson correlation coefficient (r), p<0.05
No significant Pearson correlation detected in the correlation analysis of Liquid chromatography-mass spectrometry (LC-MS) metabolites with trichome density, no-choice and choice tests (Table 1, appendix).
Discussion
Resistance accessions to Tuta absoluta
The results of non-significant differences among tomato accessions such as adult survival, emerged larvae, and non-significant correlated parameters did not present in a graph or a table.
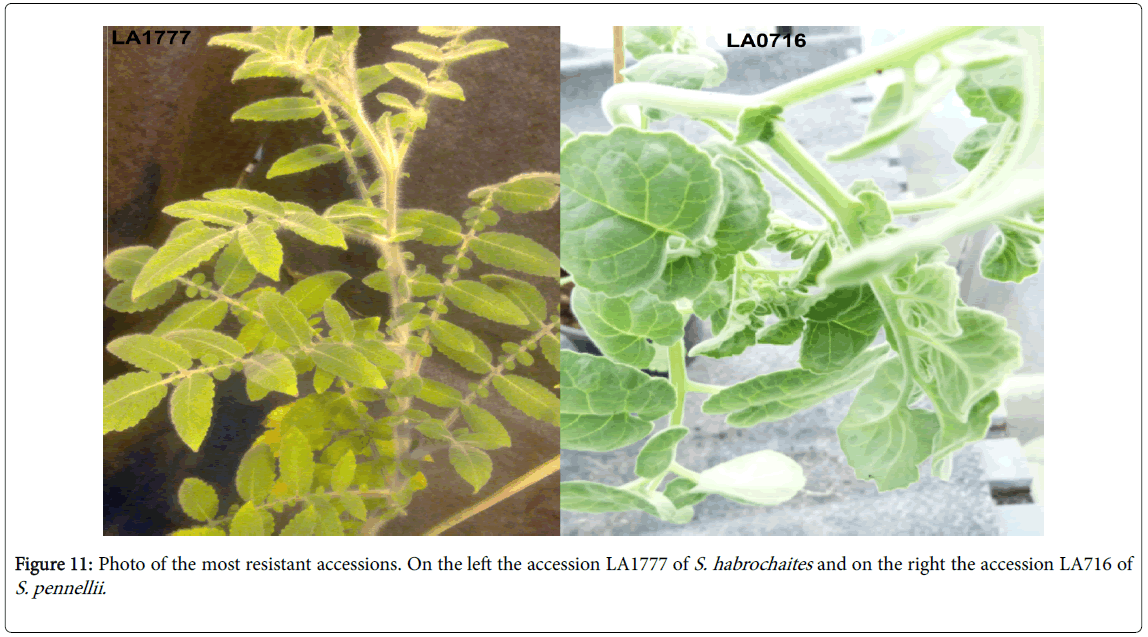
This results shown that the accessions LA1777 (S. habrochaites ) and LA716 (S. pennellii ) were overall the most resistant. Andvery small amount of larvae survival and oviposition rate in accession LA716 (S. pennellii), LA1777 and LA1718 (S. habrochaites ) indicates that those accessions were able to reduce damage caused by T. absoluta. Solanum habrochites and LA716 (S. pennellii ) were already reported to be resistant to T. absoluta as well to other pest herbivores, i.e., spider mites Tetranychus urticae and Silverleaf whitefly Bemisia tabaci . These two accessions possess high densities of glandular and non-glandular trichomes that cover the total adaxial and abaxial leaves (Figure 11).
It was reported that accession of S. galapagense is resistant to a broad range of pest, and that resistance is associated with the cooccurrence of high densities of trichomes type IV and acyl-sugars [24]. However, in our study, the accession LA1401 (S. galapagense ) reported as highly resistantce to B. tabaci was fully susceptible to T. absoluta , agreed to this result. This accession, together with the accessions LA1139 (S. cheesmaniae ) and LA1645 (S. pimpinellifolium ) showed the highest damage leaf area, indicated were not resistance. Hence, resistance could be related with the presence of insect feeding deterrents, not present in some as resistance relative to other which have it.
Tomato leaf volatiles are essential cue's finding and oviposition in tomato leafminor and mated female discriminats between cultivated and wild tomato accessions; they laid more eggs in the cultivated S. lycopersicum . The research shown that T. absoluta adults did not settle to lay eggs on the accession LA1777 and G1.1561 of S. habrochaites. However, ability of host plant selection was not restricted to T. absoluta adults. Larvae can make choices on wild tomato accession. Accessions; LA 1645, LA716, LA 1408, LA0483, G1.1561 and LA1718 were not preferred by T. absoluta larvae. The fact that there was no correlation between damage leaf area and larva preference, allowed to assume that different resistance mechanisms are acting at this level.
Importance of tomato trichomes for T. absoluta resistance
Correlation between trichomes I, IV and V with resistance (oviposition rate and larva survival) was noticed. Trichomes are epidermal structures that originate from the epidermal cells of above ground plant tissue, have been implicated in protection against various biotic and biotic attacks, extreme temperature and excessive light. As well, the glandular trichomes are an important source of essential oils, i.e., natural fragrance or products that can be used by the pharmaceutical industry and most of these substances involved in plant defence to herbivorous.
The diversity of trichome types and chemical composition among tomato species made different species of tomato respond in a different way against herbivore attack (Valverde et al., 2001). For instance, it was reported that wild tomato accessions with a high density of trichome type IV (i.e. S. habrochaites, S. pennellii, S. galapagense ) are resistant to silverleaf whitefly [25-27]. Glandular trichomes are the major sites of different phytochemical production that prevents herbivore attack than non-glandular trichromes. Although a negative correlation between glandular trichome Type, I and IV with the oviposition rate and the larva survival was observed, not all the accessions with trichomes type IV, or I was resistant (Table 2). In addition, no correlation was found between the presence of a trichome type IV and the damaged leaf area or the adult survival. These findings reinforce the hypothesis that the presence of specific types of trichomes is not the only way, but would be the combination of trichomes and metabolites. Similar results were found during the screening of several accessions where the presence of trichomes type IV without high accumulation of acylsugars and vice-versa did not confer resistance to whiteflies [28-30].
Though prevous researches have reported about the relation of different metabolites, acyl sugars, with the trichomes types and density no correlation was found in this work.
Previous studies, have related T. absoluta resistance of tomato to the presence of principal compounds, as cuticular lipids such as tricosane presented a negative correlation with the number of mines unlike tacosane and hexacosane presented a positive correlation. However, we only had acyl sugar LC-MS data and no detected correlation to T. absoluta resistance. This leads a suggestion, there may be other methylketones and sesquiterpanes involved for resistance mechanism. High level of allelochemicals, sesquiterpenesb (Zingiberene) and acylsugars found in S. habrochaites and S. pennellii , respectively are responsible for arthropod resistance, such as spider mites (Tetranychus utricae) and silverleaf whitefly (Bemisia argentifolii ) including T. absoluta.
Overall, the accession LA1777 of S. habrochaites was the most resistant accession. In addition, the accessions LA1718 (S. habrochaites ) and LA716 (S. pennellii ) reduced 55% of larvae survival when compared to the other accessions.
Different resistance mechanisms affecting different developmental stages of the insect were observed. So that these independent mechanisms could be incorporated to get a fully resistant genotype in a breeding program [31,32].
Oviposition rate and larvae survival were negatively correlated with the presence of tricomes type IV and I, respectively indicating a role of those trichome types in resistance. Although, trichomes type I and IV are the place of synthesis and storage of acyl sugars, and the known role of acyls sugars in insect resistance, no correlation was found to this study. This indicates that other metabolites are involved in T. absoluta resistance.
This result suggests that commercial varieties of tomato having LA1777 (S. habrochaites ) and LA716 (S. pennellii ) as a source of resistance to T. absolute will be successful in the future breeding program. As well, it is essential to study the genetic basis of resistance by combining resistance factors from the glandular trichomes type, I and IV in combination with methylketons and sesqueterpenes that could be identified using GC-MS analysis and other molecular anylysis; RNA-Seq and SNPs study.
References
- Lai A, Santangelo E (2007) Analysis of the main secondary metabolites produced in tomato (Lycopersicon esculentum, Mill.) epicarp tissue during fruit ripening using fluorescence techniques. Postharvest Biology and Technology 43: 335-342.
- Darwin CS, Knapp S, Peralta EI (2003) Taxonomy of tomatoes in the Galápagos Islands: Native and introduced species of Solanum section Lycopersicon (Solanaceae). Systematics and Biodiversity, 1: 29-53.
- Peralta EI, Spooner MD, Knapp S (2008) Taxonomy of wild tomatoes and their relatives (solanum sect. lycopersicoides, sect.juglandifolia, sect. lycopersicon; solanaceae). Systematic botany monographs, 84: 1-192.
- Peralta IE, Spooner DM (2005) Morphological characterization and relationships of wild tomatoes (Solanum L. Sect. Lycopersicon). Monographs in Systematic Botany, 104-227.
- Desneux, N, Wajnberg E, Wyckhuys GAK, Burgio (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. Journal of Pest Science, 83: 1-19.
- Muniappan R (2010) Tuta absoluta: the tomato leaf minor. Collaborative research on Integrated Pest Management (IPM IL).
- Vargas H (1970) Observaciones sobre la biologia enemigos naturales de las polilla del tomate, Gnorimoschema absoluta (Meyrick). Depto. Agricultura, Universidad del Norte-Arica, 1: 75-110.
- USDA–APHIS (2011) New Pest Response Guidelines: Tomato Leafminer (Tuta absoluta). USDA–APHIS–PPQ–EDP-Emergency Management, Riverdale, Maryland, 1-176.
- Cocco C, Delliper, Delrio G (2012) Control of Tuta absoluta Meyrick)(Lepidoptera:Gelechiidae) in greenhouse tomato crops using the mating disruption technique. Journal of Applied Entomology 137: 16-28.
- EPPO (2009) First report of Tuta absoluta in France (2009/003). In EPPO Reporting Services 1:003
- Estay P (2000) Polilla del tomate Tuta absoluta (Meyrick). Instituto de Investigationes Agropecuarias, Centro Regional de Investigacion La Platina, Ministerio de Agricultura Santiago Chile.
- EPPO (2005) EPPO Datasheets on quarantine pests: Tuta absoluta. In EPPO Bulletin 35:434-435.
- Moreno CS, Carvalho AG, Picanco CM, Morias GFE, Pereira R (2011) Bioactivity of compounds from Acmella oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and selectivity to two non-target species. Wiley online Library, 1-8.
- Deliperi ACS, Delrio G (2012) Control of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in greenhouse tomato crops using the mating disruption technique. Journal of Applied Entomology 137: 16-28.
- Russell IPM Ltd. (2010) Tuta absoluta information network. The Russell IPM Integrated Pest Management.
- Oliveira AF, de Silva HJD, Leite DLG, Jham NG, Picanco M (2009) Resistance of 57 greenhouses-grown accessions of Lycopersicon esculentum and three captives to Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Scientia Horticulturae, 119: 182-187.
- Mc Dowell ET, Kapteyn J, Schmidt A, Li C, Kang J, et al. (2011) Comparative Functional Genomic Analysis of Solanum Glandular Trichome Types. Plant Physiology, 155: 524-539.
- Schilmiller A, Shi F, Kim J, Charbonnaeu LA, Holmes D, et al. (2010) The Plant Journal. 62:391-403.
- Kang HJ, Shi F, Jones DA, Marks DM, Howe AG (2009) Journal of Experimental Botany. 1-12.
- Tissier A (2012) Glandular trichomes: what comes after expressed sequence tags? The Plant Journal, 70: 51-68.
- Glas JJ, Schimmel JC, Alba MJ, Bravo ER, Schuurink CR, et al. (2012) Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. International Journal of Molecular Sciences, 13:17077-17103.
- Bas N, Mollema C, Lindhout P (1992) Resistance in Lycopersicoum f. glabratum to the greenhouse whitefly (Trialeurodes vaporariorum) increases with plant age. Euphytica, 64: 189-195.
- Channarayappa C, Shivashankar G, Muniyappa V, Frist R (1992) Resistance of Lycopersicon species to Bemisia tabaci a tomato leaf curl virus vector. Canadian Journal of Botany, 11: 2184-2192.
- Firdaus S, Heusden AW, Hidayati N, Supena EDJ, Mumm R, et al. (2013) Identification and QTL mapping of whitefly resistance components in Solanum galapagense. Theoretical and Applied Genetics, 126: 1487-1501.
- Maluf W, Fátima Silva V, Graças Cardoso M, Gomes L, Neto Ã, et al. (2010) Resistance to the South American tomato pinworm Tuta absoluta in high acylsugar and/or high zingiberene tomato genotypes. Euphytica, 176:113-123.
- Firdaus S, van Heusden AW, Hidayati N, Supena EDJ, Visser RGF, et al. (2012) Resistance to Bemisia tabaci in tomato wild relatives. Euphytica, 187: 31-45.
- Lucatti FA, Heusden WA, de Vos HCR, Visser FGR (2013) Differences in insect resistance between tomato species endemic to the Galapagos Islands. Netherlands Metabolomics Centre, 1-31.
- Garzia TG, Siscaro G, Biondi A, Zappala L (2012) Tuta absoluta, a South American pest of tomato now in the EPPO region: biology, distribution and damage. Bulletin OEPP/EPPO Bulletin, 42: 1-6.
- Kennedy GG (2003) Tomato, Pests, Parasitoids, and Predators: Tritrophic interactions involving the Genus Lycopersicon. Annual Review Entomology, 48: 51-72.
- Proffit M, Birgersson G, Bengtsson M, Witzgall P, Lima E (2011) Attraction and oviposition of Tuta absoluta Females in Response to Tomato Leaf Volatiles. Journal of Chemical Ecology, 37: 565-574.
- Valverde LP, Fornonei J, Farfan NJ (2001) Defence Defensive role of leaf trichomes in resistance to herbivorous insect in Datura stramonium. Journal of Evolutionary biology, 14:424-432.
Citation: Bitew MK (2018) Significant role of wild Genotypes of tomato trichomes for Tuta absoluta resistance. J Plant Genet Breed 2: 104.
Copyright: © 2018 Bitew MK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 6945
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 5841
- PDF downloads: 1104